Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helices†
Taehoon
Kim
ab,
Tadashi
Mori
c,
Takuzo
Aida
ab and
Daigo
Miyajima
 *a
*a
aRIKEN Center for Emergent Matter Science, 2-1 Hirosawa, Saitama 351-0198, Wako, Japan. E-mail: daigo.miyajima@riken.jp
bDepartment of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
cDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, Osaka 565-0871, Japan
First published on 12th July 2016
Abstract
An unprecedentedly high degree of chiral amplification of supramolecular helices in a sergeants and soldiers system was realized using a propeller-shaped molecule, triphenylamine (TPA), as the monomer. One sergeant controlled the handedness of 500 soldiers in supramolecular helices. We further demonstrated that a TPA derivative could switch its role from sergeant to soldier and vice versa depending on its partners. These achievements could be realized using the dynamic propeller conformation of TPA and provide new insights into supramolecular assemblies and the supramolecular chiral amplification of helices.
Introduction
The helix has attracted scientists owing to its structural beauty, its vital roles in biological systems,1 and its potential for practical applications, such as chiral separation2 or asymmetric catalysis.3 The helix is intrinsically chiral and is always either left- or right-handed. To fully exploit the potential of helices,1–3 it is important to selectively synthesize or isolate helices with a single handedness. However, left- and right-handed helices are enantiomers, and their thermodynamic stabilities are identical. Hence, to selectively form one-handed helices, it is necessary to differentiate their stabilities kinetically or thermodynamically. For this purpose, a well-established method is the employment of chiral auxiliaries for racemic helices at the molecular and supramolecular levels.4 Owing to the introduction of other chiral sources, left- and right-handed helices become diastereomers of each other, and consequently, one of the helical structures becomes thermodynamically preferred. However, the presence of chiral auxiliaries inevitably alters the conformation of the helices and their physical properties.4e,5 Therefore, it is desirable to control the handedness of helices using a minimal amount of chiral auxiliaries via chiral amplification.As represented by the pioneering works of Okamoto6 and Yashima,7 one-handed helices consisting of only achiral monomers have been successfully isolated, even in the absence of a chiral source.8 The helical polymers memorize the induced handedness by the action of chiral sources, even after the removal of the chiral sources. However, most reported helical polymers and supramolecular helices are dynamic and undergo racemization after removal of the chiral sources.4 In 1989, Green and his colleagues demonstrated the first chiral amplification system for helices in which a one-handed helical conformation of dynamic helices could be obtained by copolymerization of the achiral monomers with a small fraction of the chiral monomers.9 Because the chiral monomers dictate the handedness of the helices, which mainly consist of achiral monomers, this system is referred to as the sergeants (chiral monomers) and soldiers (achiral monomers) principle. This concept was later proven to be valid even for supramolecular helices.10a Thanks to the systematic study by Meijer and coworkers using benzene-1,3,5-tricarboxamides (BTA) derivatives, now we have deep insights on the mechanism and important parameters for chiral amplifications in supramolecular helices.10 However, the degree of chiral amplification in the sergeants and soldiers system is still at most approximately 100 (one sergeant per 100 soldiers) for both dynamic helical polymers and supramolecular helices.9,10d,11 Because the concept of sergeants and soldiers is widely used for various helices,4 it is desirable to further extend this limit for both fundamental science and practical applications.
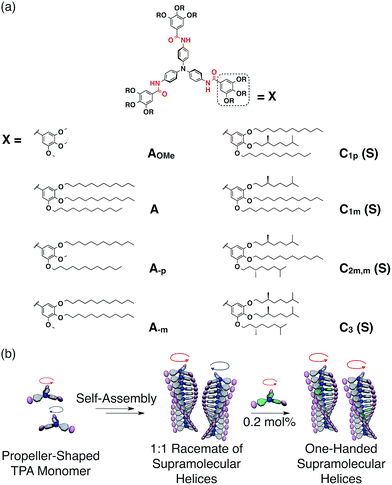
Here, we report an unprecedentedly high degree of amplification of supramolecular chirality in a sergeants and soldiers system using a propeller-shaped aromatic core in which only one sergeant controls the helicity of 500 soldiers in a supramolecular helix, on average (Fig. 1). Furthermore, we have substantiated the hierarchy of the sergeant molecules for the sergeants and soldiers systems.
Result and discussion
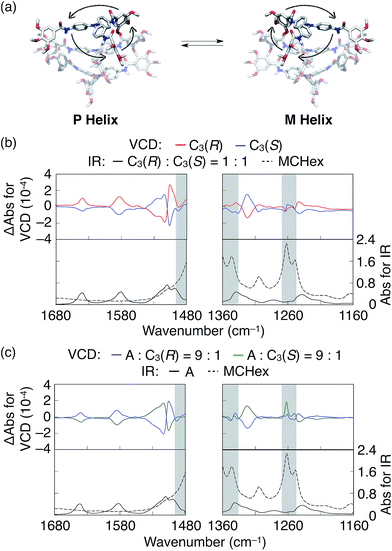
Recently, Giuseppone and coworkers have intensively investigated the self-assembling behaviours of triphenylamine (TPA) derivatives.12 In particular, they have suggested that TPA derivatives having amide groups in its side chains self-assemble into supramolecular helices and TPA derivatives selectively adopt (P)- or (M)-propeller chirality according to the handedness of the supramolecular helices in which they are assembled.12c Inspired by these pioneering works, we wondered how such propeller chirality of TPA would affect the degree of chiral amplification. We then first performed a density functional theory (DFT) computational study13 to predict the optimal conformation of trimeric AOMe (Fig. 1a), a simplified model compound consisting of TPA as the core and three methoxylated benzamide groups. As expected,12c the DFT calculation results indicated that AOMe forms a helical trimer via intermolecular hydrogen bonding of the amides and that all TPA cores adopt the same propeller chirality (Fig. 2a). We then synthesized similar TPA derivatives (Fig. 1a) and investigated their self-assembly behavior in cyclohexane. Dynamic light scattering (DLS) measurements suggested that A was molecularly dispersed at 75 °C and formed supramolecular aggregates at low temperature (25 °C, Fig. S19a†). Although the absorption spectra of A at 75 °C and 25 °C were similar (Fig. S19b†), a characteristic valley in the absorption spectra at approximately 315 nm appeared at 55 °C during the cooling process, suggesting the formation of supramolecular aggregates of A started at 55 °C (Fig. S19c†). Tapping-mode atomic force microscopy (AFM) measurements of the air-dried sample on a silicon wafer revealed that A forms one-dimensional supramolecular polymers (Fig. S20†), possibly by connecting via intermolecular hydrogen bonding and π–π interactions. The CD spectrum of C3(S) in cyclohexane exhibited characteristic exciton splitting at 312 nm and 328 nm (Fig. 3a, solid blue line), whereas C3(R) displayed the mirror-image CD spectrum under the same conditions, indicating the formation of one-handed supramolecular helices (Fig. 3a, dashed blue line). Based on the DFT calculations and the similarity between the absorption spectra of A and C3(S) in their assembled states (Fig. 3a and S21a†), we concluded that A also formed supramolecular helices, although they were a racemic mixture of left- and right-handed helices. Note that some TPA derivatives having amide groups are known to form lateral interaction between supramolecular helices.12d,g However, due to the presence of multiple long alkyl-chains around amide groups, such lateral interactions are supposed to hardly happen in our system.To determine whether the TPA core selectively adopts a supramolecular helices, we performed vibrational circular dichroism (VCD) measurements of methylcyclohexane solutions of C3(R) and C3(S).14 As shown in Fig. 2b, C3(R) and C3(S) exhibited clear mirror-image spectra, with the exception of a few wavenumber ranges highlighted in gray in which the solvent absorption interference was not negligible. The VCD peaks between 1639 and 1584 cm−1 correspond to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and the bending vibration of the N–H bond, respectively (Fig. S22†),14b,15 suggesting that the amide groups adopt a helical array in the assembled state. Moreover, the absorption bands with a strong Cotton effect at approximately 1500 cm−1 correspond to the stretching vibration and the side chains.15,16 The peaks at approximately 1322 and 1242 cm−1 correspond to the C–N stretching vibrational bands of the outer amine linked with TPA and the central amine present in TPA, respectively.15,16 The VCD data strongly suggest that the propeller chiralities of the TPA monomers are linked with the handedness of the supramolecular helices. However, based on these VCD data alone, it is difficult to exclude the possibility that the propeller chirality of TPA is decided by the side-chain chirality, independent of the handedness of the helices. Hence, we performed VCD experiments of a sergeants and soldiers system of A/C3(R) and A/C3(S) at a mole ratio of 9 to 1. We obtained VCD spectra nearly identical to those of C3(R) or C3(S) alone, indicating that TPA adopts a single propeller chirality according to the helicity of the supramolecular helices (Fig. 2b and c).
O bond and the bending vibration of the N–H bond, respectively (Fig. S22†),14b,15 suggesting that the amide groups adopt a helical array in the assembled state. Moreover, the absorption bands with a strong Cotton effect at approximately 1500 cm−1 correspond to the stretching vibration and the side chains.15,16 The peaks at approximately 1322 and 1242 cm−1 correspond to the C–N stretching vibrational bands of the outer amine linked with TPA and the central amine present in TPA, respectively.15,16 The VCD data strongly suggest that the propeller chiralities of the TPA monomers are linked with the handedness of the supramolecular helices. However, based on these VCD data alone, it is difficult to exclude the possibility that the propeller chirality of TPA is decided by the side-chain chirality, independent of the handedness of the helices. Hence, we performed VCD experiments of a sergeants and soldiers system of A/C3(R) and A/C3(S) at a mole ratio of 9 to 1. We obtained VCD spectra nearly identical to those of C3(R) or C3(S) alone, indicating that TPA adopts a single propeller chirality according to the helicity of the supramolecular helices (Fig. 2b and c).
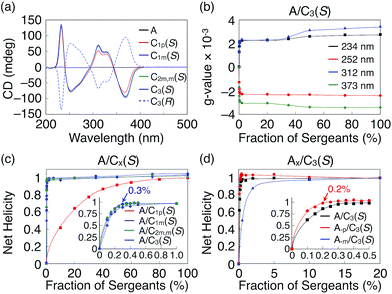
Next we investigated the degree of chiral amplification using A and C3(S) and found that one-handed helices of A could be obtained by mixing an unusually small fraction of C3(S) with A. In other words, the chiral information of the side chains of C3(S) was efficiently amplified into the handedness of the supramolecular helices of A. To accurately estimate the degree of chiral amplification of the mixed system of A/C3(S), we performed the sergeants and soldiers experiments using various fractions of C3(S) (Fig. S23†). As shown in Fig. S23c,† the observed g-values varied depending on the fraction of C3(S) without noticeable changes in the CD spectral shapes. By plotting the g-values of the peak tops at 234, 252, 312, and 373 nm, we detected minor discontinuous changes of the g-values at an approximately 40 mol% fraction of C3(S) (Fig. 3b). The same feature was observed in the plots of the electronic absorption at the same wavelengths (Fig. S23d†). Although the electronic absorption spectra of A and C3(S) were nearly identical, the peak tops of C3(S) were slightly red-shifted by 1–2 nm on average (Fig. S21a†), indicating that the helical conformations of the supramolecular helices of A and C3(S) are slightly different. Hence, we assumed that co-assemblies of A and C3(S) adopt a helical conformation similar to that of either the homo-assembly of A or that of C3(S) and that the transition of this helical conformation occurs at an approximately 40 mol% fraction of C3(S), resulting in the observed discontinuous changes in the g-values. Therefore, in the following studies, we used the g-value at a 20 mol% fraction of C3(S) as the value for the one-handed supramolecular helices of A. To clarify the magnitude of the changes in the g-values, we used net helicity as a probe for the degree of chiral amplification.17 As shown in the inset of Fig. 3c, the net helicity was saturated by addition of 0.3 mol% fraction of the sergeant. A single C3(S) manipulated the handedness of the supramolecular helices of 333 molecules of A on average. At this high degree of chiral amplification, the average length of the supramolecular helices may affect the result. However, the degree of chiral amplification of this system exhibited no change, even at a 30-fold diluted concentration (Fig. S24†), suggesting that the average length of the supramolecular helices are enough long not to limit the degree of chiral amplification.10a Previously, 1 mol% sergeants was the minimum fraction to fully bias the handedness of supramolecular helices of soldiers.4b,e,10d,11 Hence, our TPA system exceeds the limit of the conventional sergeants and soldiers system.
Systematic studies of chiral amplification10,18 have revealed that two important parameters, mismatch penalty (MMP) and helical reversal penalty (HRP), govern the degree of chiral amplification in sergeant and soldier systems. The MMP is an energetic penalty incurred by a system when chiral sergeants are incorporated into non-preferred helices, whereas the HRP is the energetic barrier that maintains the handedness of supramolecular helices. In general, as these two parameters increase, one-handed helices can be obtained with a smaller amount of sergeants. As expected from the above results, a mixed system of A and C3(S) exhibited the highest MMP and HRP values ever reported (Fig. S25 and Table S1†) and the large degree of polymerization (Fig. S35†),10e,i,18b as estimated using the amplification model developed by Meijer and coworker.10i In contrast to conventional supramolecular helices, our TPA system has no characteristic features, except for the propeller conformation of TPA. Hence, we assume that TPA has an intrinsic potential for an anomalously high degree of chiral amplification in the sergeants and soldiers system. For example, the propeller chirality of TPA is uniquely decided by the handedness of the supramolecular helices. Consequently, handedness inversion requires extra energy to invert the propeller chirality of TPA. Hence, it is reasonable that supramolecular TPA helices exhibit high HRP values.
On the other hand, the MMP primarily originates from the mismatch between the stereogenic centres of the sergeant and the handedness of the supramolecular helices. To reveal the relationship between TPA and the large MMP value, we prepared various sergeants and soldiers based on the molecular designs of A and C3(S) (Fig. 1a). All analogues exhibited nearly identical absorption spectra at 25 °C in cyclohexane (Fig. 3a and S21†). Hence, the following discussion is based on the assumption that the TPA derivatives form supramolecular helices with the same conformations as A and C3(S). We investigated the degree of chiral amplification in the mixed systems with A and its chiral analogues C1m(S), C1p(S), and C2m,m(S), respectively, whose names indicate the number and positions of the stereogenic centers in their side chains (Fig. S26–28†). As summarized in Fig. 3c, the use of C1p(S) resulted in the lowest chiral amplification, and C1m(S) and C2m,m(S) resulted in nearly identical degrees of chiral amplification to that of C3(S), suggesting that the role of side-chain chirality is completely different at the para- and meta-positions. Because side chains at the meta-positions sterically hinder the rotation of the phenyl groups to which the side chains are attached, side chains at these positions must affect the propeller conformation of TPA more than those at the para-position. Consequently, the MMP of C1m(S) originating from the mismatch between the chiral side chains and the propeller chirality might be larger than that of C1p(S). An identical trend was observed for soldiers A-m and A-p, whose long alkyl chains at the meta- and para-positions are replaced by methyl groups (Fig. S29 and 30†). When the achiral TPA derivatives were mixed with C3(S), A-p resulted in a nearly identical degree of chiral amplification to that of A, whereas A-m exhibited a much lower degree of chiral amplification (Fig. 3d). From the observed unique effects of the side chains at the meta-position, we concluded that propeller conformation of TPA enabled effective peripheral packing and realized the large MMP. Interestingly, a mixed system of A-p and C3(S) had a higher degree of chiral amplification than that of A and C3(S) (inset in Fig. 3d); the handedness of the supramolecular helices of A-p was fully biased by the addition of only 0.2 mol% C3(S). Because A-p starts to self-assemble at a higher temperature during the cooling process than A and A-m (Fig. S31†), molecules of A-p interact better with each other in the resultant helices, potentially underlying the increase in the HRP and MMP values.
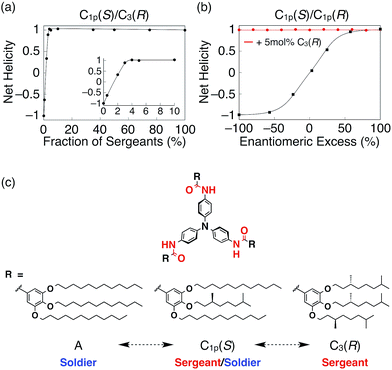
The constituents of supramolecular helices are in equilibrium between the monomeric and aggregate (helix) states.4b,e Hence, to obtain a high degree of chiral amplification in these dynamic systems, sergeants and soldiers must co-aggregate efficiently. The MMP of the supramolecular helices increases after introducing larger numbers of chiral side chains per sergeant. However, this approach is incompatible with efficient co-aggregation because the chiral side chains decrease the association constant of the sergeants for soldiers because of steric hindrance.10m,18b However, in the TPA system, because the sergeants and soldiers are both propeller-shaped, the presence of propeller chirality does not lead to the segregation of the sergeants and soldiers, which may be no less important than the MMP and HRP values of TPA systems for chiral amplification. Furthermore, the degree of chiral amplification can be compared over the various TPA derivatives because their helical conformations are always nearly identical, in contrast to supramolecular helices based on planar π-conjugated molecules, such as perylene bisimide.5b In addition, it is rare that the degree of chiral amplification drastically changes just by altering the position of the stereogenic centers. By utilizing these unique features, we demonstrated that C1p(S) can switch its role from sergeant to soldier if the partner was changed from A to C3(R). As shown in Fig. 4a, the g-value of the mixed system of C1p(S) and C3(R) was saturated with the addition of a 4 mol% fraction of C3(R) (Fig. S32†). By contrast, in the majority rule experiments10e,19 for C1p(R)/C1p(S) (Fig. 4b and S33†), the net helicity was constant, independent of the enantiomeric excess of C1p(R) or C1p(S), if 5 mol% C3(R) was present. Because C1p(S) behaves as a sergeant in the mixed system with C1p(S) and A (Fig. 3c and S26†), the observed results suggest hierarchy in the sergeants and soldiers systems of our TPA helices (Fig. 4c). Note that, Meijer and coworkers have reported the similar majority rule experiments using two BTA derivatives with different numbers of stereocenters with opposite stereoconfiguration.10j However, in their case, the majority rule was simply dictated by the number of stereocenters, suggesting that propeller conformation of TPA plays an important role for the observed anomalous chiral amplifications.
Conclusions
In conclusion, we have demonstrated unprecedented chiral amplification of supramolecular helices consisting of TPA derivatives in which the handedness of the helices was fully controlled using a 0.2 mol% fraction of sergeants. Such an unprecedentedly high degree of chiral amplification was achieved via the dynamic propeller conformation of TPA. Therefore, this study provides valuable insights into the construction of helical structures and a novel design strategy for supramolecular chiral amplification.Acknowledgements
This work was financially supported by a Grant-in-Aid for Scientific Research (Grant Number; JP15H05487 to D. M., JP15H03779, JP15K13642, JP15H01087 to T. M.) from JSPS/MEXT and the Matching Planner Program (Grant Number MP27215667549) from JST. T. K. thanks the Fellowship of JSPS for Young Scientists.Notes and references
- (a) F. H. C. Crick and J. D. Watson, Nature, 1956, 177, 473 CrossRef CAS PubMed; (b) K. Cahill, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 72, 062901 CrossRef PubMed; (c) V. Hunyadi, D. Chrétien, H. Flyvbjerg and I. M. Jánosi, Biol. Cell, 2007, 99, 117 CrossRef CAS PubMed; (d) A. A. Kornyshev, D. J. Lee, S. Leikin and A. Wynveen, Rev. Mod. Phys., 2007, 79, 943 CrossRef CAS.
- (a) Y. Okamoto and E. Yashima, Angew. Chem., Int. Ed., 1998, 37, 1020 CrossRef; (b) T. Nakano and Y. Okamoto, Chem. Rev., 2001, 101, 4013 CrossRef CAS PubMed; (c) M. Teraguchi, J. Suzuki, T. Kaneko, T. Aoki and T. Masuda, Macromolecules, 2003, 36, 9694 CrossRef CAS; (d) Y. Okamoto and T. Ikai, Chem. Soc. Rev., 2008, 37, 2593 RSC; (e) Y. Okamoto, Adv. Polym. Sci., 2013, 261, 391 CrossRef CAS.
- (a) M. Reggelin, M. Schultz and M. Holbach, Angew. Chem., Int. Ed., 2002, 41, 1614 CrossRef CAS; (b) M. Reggelin, S. Doerr, M. Klussmann, M. Schultz and M. Holbach, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5461 CrossRef CAS PubMed; (c) G. Roelfes and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 3230 CrossRef CAS PubMed; (d) E. A. C. Davie, S. M. Mennen, Y. Xu and S. J. Miller, Chem. Rev., 2007, 107, 5759 CrossRef PubMed; (e) T. Yamamoto and M. Suginome, Angew. Chem., Int. Ed., 2009, 48, 539 CrossRef CAS PubMed; (f) T. Miyabe, Y. Hase, H. Iida, K. Maeda and E. Yashima, Chirality, 2009, 21, 44 CrossRef CAS PubMed; (g) M. Raynal, F. Portier, P. W. N. M. van Leeuwen and L. Bouteiller, J. Am. Chem. Soc., 2013, 135, 17687 CrossRef CAS PubMed; (h) E. Huerta, B. van Genabeek, B. A. G. Lamers, M. M. E. Koenigs, E. W. Meijer and A. R. A. Palmans, Chem.–Eur. J., 2015, 21, 3682 CrossRef CAS PubMed.
- For review; (a) M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3138 CrossRef; (b) A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8948 CrossRef CAS PubMed; (c) E. Yashima, K. Maeda, H. Iida, Y. Furusho and K. Nagai, Chem. Rev., 2009, 109, 6102 CrossRef CAS PubMed; (d) E. Yashima, Polym. J., 2010, 42, 3 CrossRef CAS; (e) M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304 CrossRef CAS PubMed.
- (a) A. Brizard, R. Oda and I. Huc, Top. Curr. Chem., 2005, 256, 167 CrossRef CAS PubMed; (b) S. Ghosh, X.-Q. Li, V. Stepanenko and F. Würthner, Chem.–Eur. J., 2008, 14, 11343 CrossRef CAS PubMed; (c) M. B. Baker, L. Albertazzi, I. K. Voets, C. M. A. Leenders, A. R. A. Palmans, G. M. Pavan and E. W. Meijer, Nat. Commun., 2015, 6, 6234 CrossRef PubMed.
- (a) Y. Okamoto, K. Suzuki, K. Ohta, K. Hatada and H. Yuki, J. Am. Chem. Soc., 1979, 101, 4763 CrossRef CAS; (b) T. Nakano, Y. Okamoto and K. Hatada, J. Am. Chem. Soc., 1992, 114, 1318 CrossRef CAS.
- (a) E. Yashima, K. Maeda and Y. Okamoto, Nature, 1999, 399, 449 CrossRef CAS; (b) K. Shimomura, T. Ikai, S. Kanoh, E. Yashima and K. Maeda, Nat. Chem., 2014, 6, 429 CrossRef CAS PubMed.
- (a) T. J. Deming and B. M. Novak, J. Am. Chem. Soc., 1992, 114, 7926 CrossRef CAS; (b) T. Aoki, T. Kaneko, N. Maruyama, A. Sumi, M. Takahashi, T. Sato and M. Teraguchi, J. Am. Chem. Soc., 2003, 125, 6346 CrossRef CAS PubMed.
- M. M. Green, M. P. Reidy, R. D. Johnson, G. Darling, D. J. O'Leary and G. Willson, J. Am. Chem. Soc., 1989, 111, 6452 CrossRef.
- (a) A. R. A. Palmans, J. A. J. M. Vekemans, E. E. Havinga and E. W. Meijer, Angew. Chem., Int. Ed. Engl., 1997, 36, 2648 CrossRef CAS; (b) L. Brunsveld, A. P. H. J. Schenning, M. A. C. Broeren, H. M. Janssen, J. A. J. M. Vekemans and E. W. Meijer, Chem. Lett., 2000, 292 CrossRef CAS; (c) L. Brunsveld, B. G. G. Lohmeijer, J. A. J. M. Vekemans and E. W. Meijer, Chem. Commun., 2000, 2305 RSC; (d) L. Brunsfeld, B. G. G. Lohmeijer, J. A. J. M. Vekemans and E. W. Meijer, J. Inclusion Phenom. Macrocyclic Chem., 2001, 41, 61 CrossRef CAS; (e) J. van Gestel, J. Vekemans, A. R. A. Palmans, B. Titulaer, J. A. J. M. Vekemans and E. W. Meijer, J. Am. Chem. Soc., 2005, 127, 5490 CrossRef CAS PubMed; (f) A. J. Wilson, J. van Gestel, R. P. Sijbesma and E. W. Meijer, Chem. Commun., 2006, 4404 RSC; (g) M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2008, 130, 606 CrossRef CAS PubMed; (h) P. J. M. Stals, J. F. Haveman, R. Martín Rapún, C. F. C. Fitié, A. R. A. Palmans and E. W. Meijer, J. Mater. Chem., 2009, 19, 124 RSC; (i) M. M. J. Smulders, I. A. W. Filot, J. M. A. Leenders, P. van der Schoot, A. R. A. Palmans, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2010, 132, 611 CrossRef CAS PubMed; (j) M. M. J. Smulders, P. J. M. Stals, T. Mes, T. F. E. Paffen, A. P. H. J. Schenning, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2010, 132, 620 CrossRef CAS PubMed; (k) P. J. M. Stals, J. C. Everts, R. de Bruijn, I. A. W. Filot, M. M. J. Smulders, R. Martín Rapún, E. A. Pidko, T. F. A. De Greef, A. R. A. Palmans and E. W. Meijer, Chem.–Eur. J., 2010, 16, 810 CrossRef CAS PubMed; (l) T. Metzroth, A. Hoffmann, R. Martín Rapún, M. M. J. Smulders, K. Pieterse, A. R. A. Palmans, J. A. J. M. Vekemans, E. W. Meijer, H. W. Spiess and J. Gauss, Chem. Sci., 2011, 2, 69 RSC; (m) A. J. Markvoort, H. M. M. ten Eikelder, P. A. J. Hilbers, T. F. A. De Greef and E. W. Meijer, Nat. Commun., 2011, 2, 509 CrossRef PubMed; (n) P. J. M. Stals, P. A. Korevaar, M. A. J. Gillissen, T. F. A. De Greef, C. F. C. Fitié, R. P. Sijbesma, A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2012, 51, 11297 CrossRef CAS PubMed.
- T. Ishi-i, R. Kuwahara, A. Takata, Y. Jeong, K. Sakurai and S. Mataka, Chem.–Eur. J., 2006, 12, 763 CrossRef CAS PubMed.
- (a) E. Moulin, F. Niess, M. Maaloum, E. Buhler, I. Nyrkova and N. Giuseppone, Angew. Chem., Int. Ed., 2010, 49, 6974 CrossRef CAS PubMed; (b) V. Faramarzi, F. Niess, E. Moulin, M. Maaloum, J.-F. Dayen, J.-B. Beaufrand, S. Zanettini, B. Doudin and N. Giuseppone, Nat. Chem., 2012, 4, 485 CrossRef CAS PubMed; (c) J. J. Armao, M. Maaloum, T. Ellis, G. Fuks, M. Rawiso, E. Moulin and N. Giuseppone, J. Am. Chem. Soc., 2014, 136, 11382 CrossRef CAS PubMed; (d) I. Nyrkova, E. Moulin, J. J. Armao, M. Maaloum, B. Heinrich, M. Rawiso, F. Niess, J.-J. Cid, N. Jouault, E. Buhler, A. N. Semenov and N. Giuseppone, ACS Nano, 2014, 8, 10111 CrossRef CAS PubMed; (e) Y. Domoto, E. Busseron, M. Maaloum, E. Moulin and N. Giuseppone, Chem.–Eur. J., 2015, 21, 1938 CrossRef CAS PubMed; (f) J. J. Armao, Y. Domoto, T. Umehara, M. Maaloum, C. Contal, G. Fuks, E. Moulin, G. Decher, N. Javahiraly and N. Giuseppone, ACS Nano, 2016, 10, 2082 CrossRef CAS PubMed; (g) J. J. Armao, P. Rabu, E. Moulin and N. Giuseppone, Nano Lett., 2016, 16, 2800 CrossRef CAS PubMed.
- (a) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed; (b) S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- (a) T. Buffeteau, L. Ducasse, L. Poniman, N. Delsuc and I. Huc, Chem. Commun., 2006, 2714 RSC; (b) M. M. J. Smulders, T. Buffeteau, D. Cavagnat, M. Wolffs, A. P. H. J. Schenning and E. W. Meijer, Chirality, 2008, 20, 1016 CrossRef CAS PubMed; (c) F. Aparicio, B. Nieto-Ortega, F. Nájera, F. J. Ramírez, J. T. López Navarrete, J. Casado and L. Sánchez, Angew. Chem., Int. Ed., 2014, 53, 1373 CrossRef CAS PubMed; (d) A. Lambrecht, M. Pfeifer, W. Konz, J. Herbst and F. Axtmann, Analyst, 2014, 139, 2070 RSC; (e) J. Kang, D. Miyajima, T. Mori, Y. Inoue, Y. Itoh and T. Aida, Science, 2015, 347, 646 CrossRef CAS PubMed.
- M. F. Chernyshova and N. P. Lushina, J. Appl. Spectrosc., 1969, 11, 1330 CrossRef.
- I. Reva, L. Lapinski, N. Chattopadhyay and R. Fausto, Phys. Chem. Chem. Phys., 2003, 5, 3844 RSC.
- The net helicity is obtained by dividing the g-value
for a certain composition by the g-value for 20 mol% fraction of sergeants at 252 nm. The g-value is the anisotropy factor, which is calculated from the following equation: g = Δε/ε = CD-effect [mdeg]/(32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 × absorbance).
982 × absorbance). - (a) J. van Gestel, P. van der Schoot and M. A. J. Michels, Macromolecules, 2003, 36, 6668 CrossRef CAS; (b) F. Helmich, C. C. Lee, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 12238 CrossRef CAS PubMed.
- W. Jin, T. Fukushima, M. Niki, A. Kosaka, N. Ishii and T. Aida, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10801 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, analytical data and Fig. S1–S34. See DOI: 10.1039/c6sc02814d |
| This journal is © The Royal Society of Chemistry 2016 |