Open Access Article
Open Access ArticleVisible light amination/Smiles cascade: access to phthalazine derivatives†
Etienne
Brachet
 *a,
Leyre
Marzo
*a,
Leyre
Marzo
 b,
Mohamed
Selkti
c,
Burkhard
König
b,
Mohamed
Selkti
c,
Burkhard
König
 b and
Philippe
Belmont
b and
Philippe
Belmont
 *a
*a
aUniversité Sorbonne Paris Cité (USPC), Université Paris Descartes, Faculté de Pharmacie de Paris, UMR CNRS 8638 (COMETE), 4 avenue de l'Observatoire, 75006 Paris, France. E-mail: etienne.brachet@parisdescartes.fr; philippe.belmont@parisdescartes.fr
bUniversity of Regensburg Faculty of Chemistry and Pharmacy, Institute of Organic Chemistry, Universitätsstraße 31, 93053 Regensburg, Germany
cUniversité Sorbonne Paris Cité (USPC), Université Paris Descartes, Faculté de Pharmacie de Paris, UMR CNRS 8015 (LCRB), 4 avenue de l'Observatoire, 75006 Paris, France
First published on 6th May 2016
Abstract
We report the synthesis of various phthalazines via a new cascade reaction, initiated by visible light photocatalysis, involving a radical hydroamination reaction followed by a radical Smiles rearrangement. Phthalazine derivatives are obtained in high yields and from a broad scope readily accessible ortho-alkynylsulfonohydrazone precursors. The mild photoredox conditions ensure an excellent functional group tolerance. Application of this strategy to a one-pot protocol starting from the corresponding carbonyl compounds, and subsequent functionalization allow the rapid synthesis of structurally diverse structures.
Introduction
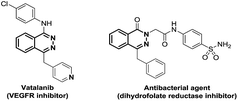
Since the early beginning of organic chemistry, the synthesis of nitrogen-containing heterocycles constantly attracted interest from the chemistry community, due to their ubiquitous presence in natural products.1 Until now, despite many synthetic efforts, phthalazine structures have been less explored and therefore synthetic methods are still desirable. Moreover, these heterocycles are found in many important biological relevant compounds possessing properties, such as antibacterial2 or antitumor3 agents (Fig. 1).Current strategies for the synthesis of these heterocycles are limited to the use of hydrazine on diaryldiketones or ortho alkynyl-phenylketones4 and Diels–Alder reactions.4a,5 These reactions require sometimes harsh reaction conditions and/or use toxic hydrazine reagents, limiting the access to phthalazine structures.4,5 Therefore, looking for a general strategy to build phthalazine derivatives is still highly requested. We were thus interested in developing a milder and general protocol focusing on the formation of the C–N bond via a photoredox strategy. Carbon–nitrogen bonds are ubiquitous and essential in countless important organic compounds.1 Thus, chemists have developed a plethora of strategies to synthesize C–N bonds leading to diversified structures.6,7 Tremendous improvements were made over the last decades especially thanks to transition metal catalysis, such as Buchwald–Hartwig amination6 or oxidative amination reactions.7 Nevertheless, C–N bond formation reactions remain of great interest, particularly in developing more general, milder and ecofriendly conditions.
In this context, carbo-amination and hydro-amination reactions, especially via radical mechanisms, are currently under investigation.8 By taking advantage of photocatalyzed reactions,9 we propose an unprecedented photo-induced radical cascade reaction combining a hydro-amination reaction step followed by a Smiles rearrangement.
The reported examples of C–N bond formation using photoredox catalysis mainly differ in nitrogen radical precursors (hydrazone, azide, N-acyloxyphthalimides, N-oxyl sulfonylcarbamates…).10 Among them, hydrazone precursors were initially converted into radicals using stoichiometric quantities of TEMPO11 or azodicarboxylate reagents12 yielding pyrazoline derivatives, via an intramolecular 5-exo-trig cyclisation process. In these cases, the heterocyclic products were obtained predominantly as adducts with the radical initiator13 (Scheme 1A). Last year, Chen, Xiao and coworkers were able to replace stoichiometric oxidation with catalytic photo-oxidation, using visible light, leading to pyrazolines, thanks to the base-mediated photocatalytic conversion of the N–H bond into an N-centered radical.14 In both cases, the nitrogen radical, generated on a hydrazone precursor, makes an intramolecular attack on an alkenyl residue (Scheme 1A). It is noteworthy that all reported photo-induced cyclization reactions of nitrogen-based radicals on unsaturated moieties happen on alkenyl residues.9f Intriguingly, cyclization reactions of nitrogen-based radicals on alkynyl residues have never been investigated under visible light catalysis.9f,10g–j Therefore, our findings are of particular interest since we designed new alkynyl-substituted arylsulfonohydrazone precursors (Scheme 1C) involved in intramolecular photo-induced hydro-amination to alkynes. To our delight, we were thrilled to isolate benzhydryl-substituted phthalazine structures through a subsequent radical Smiles rearrangement. Indeed, only a few recent examples of radical Smiles rearrangements (aryl migration15) from Gérard/Sapi16 and Nevado,17 using sulfonylamides precursors, demonstrated that this process could happen in a radical fashion (Scheme 1B), but no report included an arylsulfonohydrazone scaffold. Therefore, we describe here the behavior of novel phenylsulfonohydrazone moieties in an unprecedented photo-induced reaction cascade.18 We found that they were able to undergo an intramolecular hydroamination reaction followed by a Smiles rearrangement, providing a new strategy to build phthalazine derivatives, under visible light catalysis (Scheme 1C).
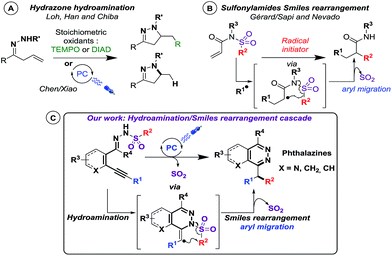 | ||
| Scheme 1 State of art in radical hydro- and oxo-amination reactions and in the radical Smiles rearrangement (A and B); our photocatalyzed cascade (C). | ||
Results and discussion
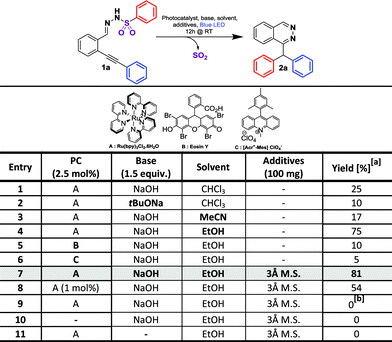
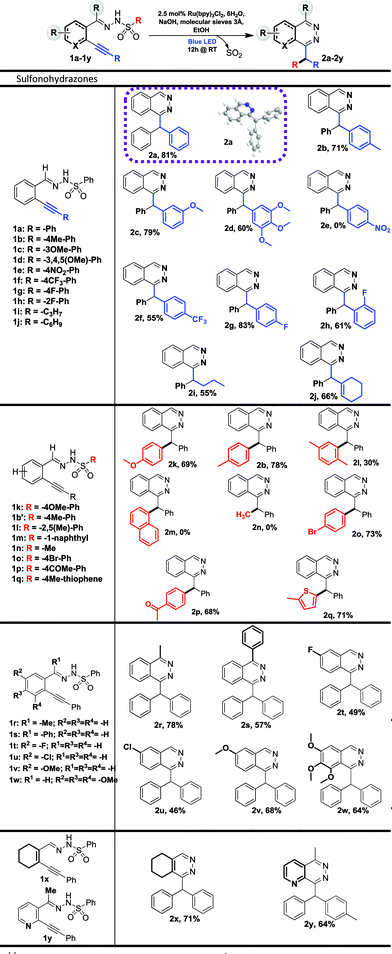
We started our investigation on a model substrate, N′-(2-(phenylethynyl)benzylidene)benzenesulfonohydrazone 1a, easily prepared from the corresponding carbonyl19a and sulfonohydrazide moieties (Table 2). We optimized the reaction conditions (see ESI† for full details) modifying the nature of the base, of the solvent, of the photocatalyst and the crucial presence of a drying agent. Starting from reaction conditions using Ru(bpy)3Cl2·6H2O as the photocatalyst, and NaOH as the base in CHCl3, we were pleased to isolate the desired product 2a with an encouraging yield of 25% (Table 1, entry 1). The X-ray analysis of 2a (Table 2) unambiguously confirmed the structure. Changing the base from NaOH to tBuONa leads to a lower 10% yield (entry 2). Investigation on the nature of the solvent (Entries 3 and 4) allowed us to increase the isolated yield from 25% to 75% with EtOH (entry 4). The influence of the photocatalyst was then evaluated with Eosin Y9 and Fukuzumi’s catalyst.9 However, the reaction was inefficient in both cases, leading to a 10% or 5% product yield, respectively, for entries 5 and 6. Additives were then screened and we were pleased to find that, with the use of 3 Å molecular sieves, we were able to avoid the formation of a side product, thus improving the yield of the desired product 2a to 81% (see ESI†). Decreasing the amount of the ruthenium catalyst from 2.5 mol% to 1 mol% led to a lower yield of 54% (entry 8). The presence of the photocatalyst, a base and light proved to be crucial for the reaction since the lack of one of them prevented the formation of product 2a (entries 9–11). Finally, the optimized reaction conditions are: 2.5 mol% of Ru(bpy)3Cl2·6H2O, 1.5 equivalents of NaOH and 100 mg of 3 Å molecular sieves in EtOH (0.08 M) under blue light irradiation (∼450 nm). With these conditions in hand, we explored the reaction scope. At first, we examined the impact of alkyne substitution by evaluating various aryls with ortho, meta or para substituents, exhibiting electron-donating or electron-withdrawing properties (1b–h, Fig. 2 and 2b–h, Table 2). Electron-donating groups are well tolerated; para-methyl or meta-methoxy substituted derivatives 2b and 2c provided good product yields, 71 and 79%, respectively. 3,4,5-Trimethoxybenzene derivative 1d gave the expected product 2d in 60% yield (Table 2). Electron-withdrawing groups are also well tolerated, producing para- and ortho-fluoro or para-trifluoromethyl derivatives 2g/2h/2f with moderate to good isolated yields of 83%, 61% or 55%, respectively. However, para-nitro derivative 1e was unreactive under these conditions due to low solubility in ethanol. Cyclohexenyl derivative 1j reacted chemoselectively, yielding 2j in 66% yield (Table 2). Starting from pentyne derivative 1i, we obtained product 2i in a moderate 55% yield.| a Reaction conditions: 1a–y (0.15 mmol, 1 eq.), 3 Å M.S. (100 mg), Rubpy3Cl2·6H2O (3.5 μmol, 2.5 mol%) and NaOH (0.225 mmol, 1.5 eq.) in dry EtOH (0.08 M) was irradiated for 12 h under blue light (450 nm) at 20 °C. |
|---|
|
Then, we investigated the reaction scope for the sulfonyl part (1b′, 1k–r, Fig. 2) of the starting materials, thus modifying the migrating moiety.
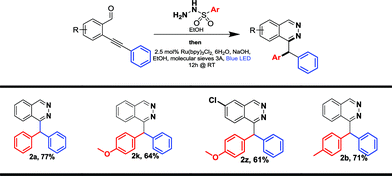
Derivatives bearing electron donating-groups, such as para-methoxy or para-tolyl on the phenyl ring of sulfonohydrazone, gave the desired products 2k and 2b in 69% and 78% yields, respectively (Table 2). Steric congestion is detrimental to the reaction: xylene substitution provided the desired product 2l in only 30% yield; 1-naphthalene substitution (1m) is unreactive under these conditions and the starting material is almost fully recovered. Bromo-substituted phenylsulfonohydrazone reacted nicely to 2o in 73% yield; this derivative could be further functionalized using cross-coupling reactions. Derivatives bearing electron-withdrawing groups, such as para-acetyl on the phenylsulfonohydrazone moiety, yield product 2p in 68% yield. Alkyl-substituted sulfonohydrazones are not tolerated under our conditions. Indeed methylsulfonohydrazone 1n (or benzylsulfonohydrazone, data not shown) did not yield any reaction product. Finally, heteroarene-derived sulfonohydrazone, 2-methylthiophene 1q, was successfully transferred giving the desired product 2q in 71% yield. Differently substituted benzaldehyde units on the carbamoyl or aromatic part were synthesized and evaluated in the reaction (1r–y, Table 2). Thus, 1-substituted phthalazines became accessible via our new process and compounds 2r and 2s were obtained with yields of 78% and 57%, respectively. Then, the aromatic part was substituted by electron-donating or -withdrawing groups and we isolated products (2t–w) in moderate to good yields (46–68%). Finally, we applied the method to the synthesis of other heterocycles and obtained pyridazine 2x and pyridopyridazine 2y in 71% and 64% yield, respectively. The reaction was also attempted in a sequential one-pot process. This one-pot strategy was successfully applied to the synthesis of four compounds with yields ranging from 61 to 77%, which are similar compared to the two-steps procedure for compounds 2a, 2b and 2k, (Tables 2 and 3) and one new disubstituted analog 2z. The one-pot process could be particularly attractive for the synthesis of phthalazine or pyridazine compound libraries (Table 3).
| a Reaction conditions: benzaldehyde (0.15 mmol, 1 eq.) and sulfonylhydrazine (0.15 mmol, 1 eq.) were mixed at room temperature in EtOH (1 mL) for 1 hour, then we followed our optimized conditions. |
|---|
|
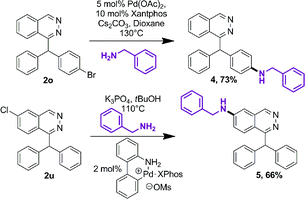
Further functionalization of phthalazine derivatives is possible, since the presence of a halogen atom is compatible with our cascade process, either on a phthalazine substituent (2o, Table 3) or directly on the phthalazine core (2u). As an example, the Buchwald–Hartwig cross-coupling reaction of compounds 2o and 2u with benzylamine provided products 4 and 5 in 73% and 66% yield, respectively (Scheme 2).19
Mechanistic study
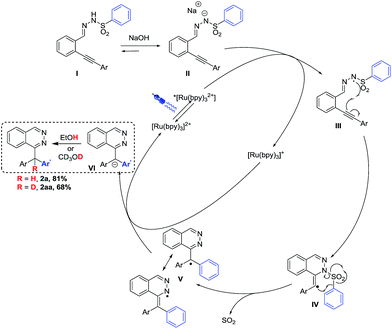
A proposed mechanism based on the observations of several control experiments and measurements is depicted in Scheme 3. The reaction in MeOH-d4 gave deuterated product 2aa in 68% yield with more than 85% incorporation of deuterium, confirming that the solvent is the proton source. Cyclic voltammetry measurements suggest that only a deprotonated sulfonohydrazone II (EIII/II = 0.54 eV vs. SCE) can be photo-oxidized by the excited ruthenium-based photocatalyst (ERuII*/RuI = 0.77 eV vs. SCE), confirmed by further fluorescence experiments (see ESI† for data). Then, the N-centered radical III may add to the triple bond and the loss of sulfur dioxide in intermediate IV yields the phthalazine radical structure V (C-centered and N-centered radical species in equilibrium). The reduction of intermediate V through the regeneration of the catalyst yields anion VI, which can be protonated or deuterated by the solvent (2a, 2aa). The quantum yield of the reaction was determined to be Φ = 0.33 indicating that the mechanism does not contain significant radical chain pathways.20Conclusions
In summary, we have developed a new photoredox cascade process combining a hydroamination reaction followed by a Smiles rearrangement. This unprecedented transformation provides an efficient access to highly diversified benzhydrylphthalazine structures. Moreover, mechanistic investigations confirmed the radical pathway displayed in our proposed mechanism. Halogen substituents are tolerated, allowing, through subsequent cross-coupling reaction, further functionalization of the phthalazines. The in situ preparation of the sulfonohydrazones from the corresponding carbonyl substrates allows a one-pot procedure. We currently investigate further applications of the photoredox Smiles reaction in our laboratory.Acknowledgements
EB and PB are thankful for the CNRS/INCa ATIP fellowship and research encouragement from “Université Paris Descartes” and “Faculté de Pharmacie de Paris”. We are grateful for the mass spectrometry facilities at “Faculté de Pharmacie de Paris” (P. Leproux). Special thanks for helpful scientific discussions are extended to the team Heterocycles and Peptides (UMR 8638). LM and BK thank the German Science Foundation (GRK 1626) for the financial support of our work.Notes and references
- (a) Heterocycles in Natural Product Synthesis, ed. K. C. Majumdar and S. K. Chattopadhyay, Wiley, Hoboken, NJ, 2012 Search PubMed; (b) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed; (c) A. Deiters and S. F. Martin, Chem. Rev., 2004, 104, 2199 CrossRef CAS PubMed; (d) F. R. de Sá Alves, E. J. Barreiro and C. A. Fraga, Mini-Rev. Med. Chem., 2009, 9, 782 CrossRef; (e) A. Brancale and R. Silvestri, Med. Res. Rev., 2007, 27, 209 CrossRef CAS PubMed; (f) G. Zeni and R. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed.
- H. S. Ibrahim, W. M. Eldehna, H. A. Abdel-Aziz, M. M. Elaasser and M. M. Abdel-Aziz, Eur. J. Med. Chem., 2014, 85, 480 CrossRef CAS PubMed.
- E. N. Scott, G. Meinhardt, C. Jacques, D. Laurent and A. L. Thomas, Expert Opin. Invest. Drugs, 2007, 16, 367 CrossRef CAS PubMed.
- (a) M. Asif, Curr. Med. Chem., 2012, 19, 2984 CrossRef CAS PubMed; (b) C. Dong, Z. Liao, X. Xu and H. Zhou, J. Heterocycl. Chem., 2014, 51, 1282 CrossRef CAS; (c) J. Munína, L. Santanab, E. Uriarteb, F. Borgesc and E. Quezada, Tetrahedron Lett., 2015, 56, 828 CrossRef; (d) Y. Maki, T. Furuta and M. Suzuki, J. Chem. Soc., Perkin Trans. 1, 1979, 553 RSC; (e) G. Heinisch and T. Huber, J. Heterocycl. Chem., 2009, 26, 1787 CrossRef.
- S.-E. Suh, S. A. Barros and D. M. Chenoweth, Chem. Sci., 2015, 6, 5128–5132 RSC.
- (a) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13 RSC; (b) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27 RSC; (c) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS PubMed; (d) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed.
- (a) M. L. Louillat and F. W. Patureau, Chem. Soc. Rev., 2014, 43, 901 RSC; (b) J. L. Jeffrey and R. Sarpong, Chem. Sci., 2013, 4, 4092 RSC; (c) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (d) F. Collet, C. Lescot and P. Dauban, Chem. Soc. Rev., 2011, 40, 1926 RSC.
- (a) M. T. Pirnot, Y.-M. Wang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 48 CrossRef CAS PubMed; (b) M. Villa and A. Jacobi von Wangelin, Angew. Chem., Int. Ed., 2015, 54, 11906 CrossRef CAS PubMed.
- Selected reviews on photocatalysis: (a) J. Xuan and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (c) M. N. Hopkinson, B. Sahoo, J.-L. Li and F. Glorius, Chem. - Eur. J., 2014, 20, 3874 CrossRef CAS PubMed; (d) D. A. Nicewicz and T. M. Nguyen, ACS Catal., 2014, 4, 355 CrossRef CAS; (e) D. P. Hari and B. König, Angew. Chem., Int. Ed., 2013, 52, 4734 CrossRef CAS PubMed; (f) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2016, 45, 2044–2056 RSC; (g) J.-R. Chen, X.-Y. Yu and W.-J. Xiao, Synthesis, 2014, 47, 604 CrossRef.
- (a) E. Brachet, T. Ghosh, I. Ghosh and B. König, Chem. Sci., 2015, 6, 987–992 RSC; (b) D. C. Miller, G. J. Choi, H. S. Orbe and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 13492 CrossRef CAS PubMed; (c) N. A. Romero, K. A. Margrey, N. E. Tay and D. A. Nicewicz, Science, 2015, 349, 1326 CrossRef CAS PubMed; (d) Q. Qin and S. Yu, Org. Lett., 2015, 17, 1894 CrossRef CAS PubMed; (e) L. J. Allen, P. J. Cabrera, M. Lee and M. S. Sanford, J. Am. Chem. Soc., 2014, 136, 5607 CrossRef CAS PubMed; (f) G. Cecere, C. M. König, J. L. Alleva and D. W. C. MacMillan, J. Am. Chem. Soc., 2013, 135, 11521 CrossRef CAS PubMed; (g) J.-R. Chen, X.-Y. Yu and W.-J. Xiao, Synthesis, 2014, 47, 604 CrossRef. Nevertheless N-centered radicals addition on alkynes have been already reported using classical radical initiator: (h) U. Wille, G. Heuger and C. Jargstorff, J. Org. Chem., 2008, 73, 1413 CrossRef CAS PubMed; (i) G. Zheng, Y. Li, J. Han, T. Xiong and Q. Zhang, Nat. Commun., 2015, 6, 7011 CrossRef CAS PubMed; (j) U. Wille, Chem. Rev., 2013, 113, 813 CrossRef CAS PubMed.
- (a) X.-Y. Duan, N.-N. Zhou, R. Fang, X.-L. Yang, W. Yu and B. Han, Angew. Chem., Int. Ed., 2014, 53, 3158 CrossRef CAS PubMed; (b) X.-Y. Duan, X. L. Yang, R. Fang, X. X. Peng, W. Yu and B. Han, J. Org. Chem., 2013, 78, 10692 CrossRef CAS PubMed.
- M. K. Zhu, Y. C. Chen and T. P. Loh, Chem. - Eur. J., 2013, 19, 5250 CrossRef CAS PubMed.
- Only one paper reported a radical ring closing of hydrazones on alkenes leading to pyrazolines without trapping of the radical initiator used: X. Zhu, Y.-F. Wang, W. Ren, F.-L. Zhang and S. Chiba, Org. Lett., 2013, 15, 3214 CrossRef CAS PubMed.
- X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, A. M. Beauchemin and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163 CrossRef CAS.
- (a) A. Gheorghe, B. Quiclet-Sire, X. Vila and S. Z. Zard, Org. Lett., 2005, 7, 1653 CrossRef CAS PubMed; (b) A. Studer and M. Bossart, Tetrahedron, 2001, 57, 9649 CrossRef CAS; (c) T. J. Snape, Chem. Soc. Rev., 2008, 37, 2452 RSC.
- M. Pudlo, I. Allart-Simon, B. Tinant, S. Gérard and J. Sapi, Chem. Commun., 2012, 48, 2442 RSC.
- (a) W. Kong, M. Casimiro, E. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480 CrossRef CAS PubMed; (b) W. Kong, N. Fuentes, A. Garcia-Dominguez, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2015, 54, 2487 CrossRef CAS PubMed.
- The photo-induced Smiles rearrangement has been recently reported exclusively on difluorobromo sulfonate substrates needed for the generation of the gem-difluoro radical. J. J. Douglas, H. Albright, M. J. Sevrin, K. P. Cole and C. R. J. Stephenson, Angew. Chem., Int. Ed., 2015, 54, 14898 CrossRef CAS PubMed.
- (a) G. Mariaule, G. Newsome, P. Y. Toullec, P. Belmont and V. Michelet, Org. Lett., 2014, 16, 4570 CrossRef CAS PubMed; (b) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed; (c) E. Brachet, J.-F. Peyrat, J.-D. Brion, S. Messaoudi and M. Alami, Org. Biomol. Chem., 2013, 11, 3808 RSC; (d) N. C. Bruno and S. L. Buchwald, Org. Lett., 2013, 15, 2876 CrossRef CAS PubMed.
- M. A. Cismesia and T. P. Yoon, Chem. Sci., 2015, 6, 5426 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1450316. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc01095d |
| This journal is © The Royal Society of Chemistry 2016 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yields.
Isolated yields.