Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Re-orienting coupling of organocuprates with propargyl electrophiles from SN2′ to SN2 with stereocontrol†
Barry M.
Trost
* and
Laurent
Debien
Department of Chemistry Stanford University, Stanford, California 94305-5080, USA. E-mail: bmtrost@stanford.edu
First published on 10th May 2016
Abstract
Diorganocuprate(I) reagents derived from lithiated heterocycles and CuCN react with enantioenriched secondary propargyl bromides to give the corresponding propargylated heterocycles. While propargyl electrophiles typically undergo SN2′ displacement, this transformation represents the first example of the reaction of hard carbanions with propargyl electrophiles in an SN2 fashion and occurs with excellent levels of stereoinversion. The new method was applied to the formal synthesis of (+)-frondosin B.
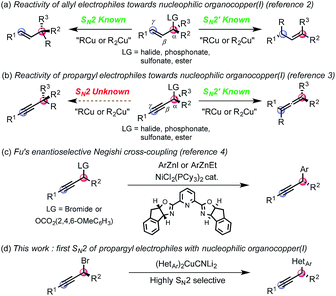
Nucleophilic organocopper(I) reagents are unambiguously invaluable synthetic tools for the chemo-, regio- and stereo-selective delivery of hard carbanions in a number of transformations such as carbocuprations, alkenylations or allylations.1 Regio- and stereo-selective organocopper(I) mediated substitutions of allylic electrophiles have been successfully developed to obtain either the SN2 or SN2′ adduct (eqn (a)).2 In contrast, substituted propropargylic electrophiles are only known to react in an SN2′ fashion3 to form the corresponding allene, regardless of the nature of the leaving group (R2 or R3 ≠ H, eqn (b)).4 Fu has pioneered a direct SN2 substitution of propargyl electrophiles by developing enantioselective nickel-catalyzed Negishi cross-couplings of propargyl bromides and carbonates with aromatic organozinc reagents (eqn (c)).5 However, the reactions with heteroaromatic nucleophiles have not been reported to date. Furthermore, in our hands, attempts to apply Fu's protocol to heterocyclic nucleophiles did not prove efficacious. The ubiquity of bioactive compounds bearing at least one heterocycle encouraged us to delineate a complementary protocol allowing the use of heteroaromatic nucleophiles that would solve this unanswered synthetic problem. Thus, we wondered whether a heteroaryl organocopper(I) reagent could be directed to react with propargyl electrophiles in an SN2 rather than the known SN2′ mode (eqn (d)).
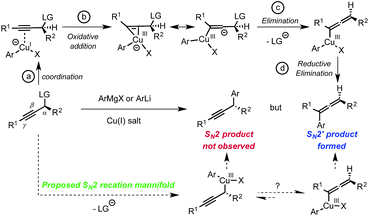
At the outset of our study, based on current knowledge about reaction mechanisms involving nucleophilic organocopper(I) reagents, we considered the mechanistic manifold depicted in Scheme 1 as a working hypothesis.6 We reasoned that the SN2′ selectivity observed for reactions between organocopper(I) reagents and propargyl electrophiles may be explained by the first step of the mechanism where coordination of the copper center to the triple bond triggers the oxidative addition step into the latter (Scheme 1, LG = leaving group, steps a and b). We thus hypothesized that blocking alkyne coordination could prevent oxidative addition via the π-system and direct it to occur at the Cα–LG; ultimately opening-up a mechanistic path for the formation of the desired SN2 adduct (Scheme 1, bottom). Such a distinction may occur by adjusting the HOMO–LUMO energies since π-coordination requires favorable interactions with both the HOMO and the LUMO of the copper(I) complex while oxidative addition into the Cα–LG only involves interaction with the HOMO. In practice, we anticipated that this differentiation could be achieved using coordinatively saturated organocuprates(I). The targeted transformation is nevertheless very challenging since, after oxidative addition into the Cα–LG bond, the propargylcopper(III) species generated could rapidly isomerize to the arguably more stable allenylcopper(III).7 This would ultimately result in the formation of the undesired SN2′ allene product and/or racemization (Scheme 1).
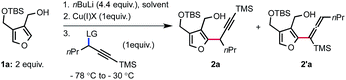
With these considerations in mind, we began to explore the targeted reaction using furan 1a in which the pendant alcohol would serve both as a directing group for site selective deprotonation and as a chelating group to saturate the coordination sphere of the copper. Previous studies on the displacement of aliphatic electrophiles with nucleophilic organocopper(I) reagents suggest that the SN2 reaction is more efficient in a polar solvent.8 Our investigation thus started by identifying the preferred leaving group using DME as a solvent and CuCN as the copper(I) salt (Table 1, entries 1–7). Commonly used leaving groups in the SN2′ displacement of propargyl electrophiles with organocopper(I) nucleophiles gave disparate results, both in terms of reactivity and selectivity. Thus, propargyl sulfonates were found to be superior substrates compare to propargyl phosphates to achieve the desired transformation (entries 1 and 5 versus 2 and 6). Aryl sulfonates and phosphates are more reactive than their aliphatic counterparts (entries 1 and 2 versus 5 and 6). Interestingly, the observed regio-selectivity seems to depend upon the nature of the leaving group (entries 1 to 6). The use of a perfluoroalkylated ester introduced by Dieter et al.4c resulted in partial conversion of the starting electrophile and formation of the desired product 2a in moderate yield and selectivity (entry 4). In addition, a Troc leaving group performed poorly leading to a complex mixture with no desired product observed (entry 3). We then became intrigued about the possibility of using a bromide as leaving group. We found that a bromide acts as a good leaving group but also greatly impacts the selectivity towards the formation of the desired alkyne 2a (entry 7). Various copper(I) salts were also rapidly surveyed and, amongst others, CuI was found to be as efficient as CuCN albeit with a drop in selectivity (entries 8 versus 7). On the other hand, CuSPh failed to deliver the desired alkyne (entry 9).
| Entry | Leaving group | Solvent | 2a/2′a | % conv.a | % yieldb |
|---|---|---|---|---|---|
| a All reactions were performed on 0.5 mmol scale using CuCN (0.5 mmol). b The reaction was run using CuI (0.5 mmol). c The reaction was run using CuSPh (0.5 mmol). d The reaction was performed in the absence of copper(I) salts. | |||||
| 1 | OMs | DME | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
82 | 44 |
| 2 | OP(O)(OEt)2 | DME | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
42 | 27 |
| 3 | OTroc | DME | — | n.d. | 0 |
| 4 | OCOC6F5 | DME | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
66 | 22 |
| 5 | OSO2Ph | DME | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
100 | 61 |
| 6 | OP(O)(OPh)2 | DME | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
100 | 58 |
| 7 | Br | DME | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
100 | 77 |
| 8b | Br | DME | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
100 | 68 |
| 9c | Br | DME | — | 100 | 0 |
| 10 | Br | THF | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
100 | 63 |
| 11 | Br | Et2O | — | 0 | 0 |
| 12d | Br | DME | — | 0 | 0 |
Since the nature of the solvent is known to possess a strong effect on reactivity and selectivity in organocopper(I) chemistry, we evaluated alternative solvents. Running the displacement reaction in THF produced 2a in diminished yield and selectivity. Conversely, using Et2O as a solvent resulted in the absence of reactivity (entry 11). Whitesides and co-workers previously observed that Et2O as a solvent can negatively impact the reactivity of organocopper(I) reagents towards alkyl electrophiles.8 Not surprisingly, reacting the organolithium reagent with the propargyl bromide in the absence of copper(I) salt resulted in the degradation of the reactants (entry 12).
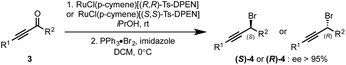
With optimized conditions for controlling the regioselectivity of the heterocycle transfer (entry 7), we initiated the study of the enantioselectivity of the reaction by developing a facile synthesis of previously inaccessible enantioenriched propargyl bromides 4. These are readily obtained in good yields via highly enantioselective Noyori reduction of ynones 3 followed by the one-pot activation and displacement of the alcohol in the presence of commercially available bromo(triphenyl)phosphonium bromide (Scheme 2).5b Noteworthy, under these conditions, the bromide substitution reactions occur with high levels of inversion of configuration. To our delight, under the conditions previously established (Table 1, entry 7), the reaction of the diorganocuprate(I) derived from 1a with (S)-(3-bromohex-1-yn-1-yl)trimethylsilane 4a (98% ee) delivered (R)-2a in 96% ee and a re-producible 77% yield. Further extension to a variety of enantioenriched propargyl bromides 4 was conducted to deliver enantioenriched propargyl heteroaryls 2 in good yields and with good levels of stereoinversion.
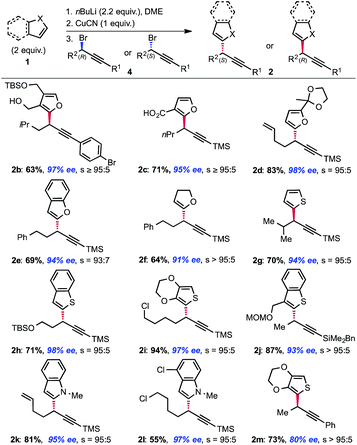
Our study focused on diorganocuprates derived from readily accessible α-lithiathed heterocycles obtained via direct lithiation of the corresponding heterocyles. The results, compiled in Fig. 1 demonstrate the generality of the method. We initially observed that a carboxylate may be used in place of the alkoxide as a directing group without losing selectivity (example 2c). We later found that no chelating group was necessary to achieve the desired transformation and that the displacement occurs with almost complete selectivity (s = SN2/SN2′ products ratio typically >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) when the nucleophile is metalated α- to the heteroatom. Thus, α-metalated furans (2d), benzofurans (2e), dihydrofurans (2f), thiophenes (2g, i, m), benzo-thiophenes (2h, j) and indoles (2k, l) are all competent nucleophiles in this transformation. It was intriguing to find that, despite the fact that thiophene typically acts as a “dummy” ligand in nucleophilic diorganocuprate(I) reactions, thiophenes and benzothiophenes are amongst the most efficient and most selective nucleophiles in this transformation (no allene formation observed). It is also worth noting that the excess nucleophile used in the transformation may be recovered after purification.9 Additionally, while using copper for inducing chirality practically requires the copper to be used catalytically, stoichiometric copper is not a limitation in processes involving achiral copper complexes.10 As for the electrophilic partner 4, the reaction is particularly efficient with TMS-protected alkynes,11 which is ideal in view of subsequent functionalization. The reaction is also efficient with R1 substituents being aromatic (2b, m) and BDMS groups (2j). As recently illustrated in our synthesis of leustrodusin B,12 the BDMS alkynes can serve as useful vinyl cross-coupling partners after semi-hydrogenation of the triple bond; even in complex settings. A number of useful functional groups such as alkenes (2d, k), arenes (2b, e, f, m), protected alcohols (2h) and chlorides (2i, l) are perfectly tolerated substituents. Interestingly an aryl bromide (2b) is compatible with the mild reaction conditions. In addition, this stereo-selective displacement is also efficient when the starting bromide 4 possess a secondary center adjacent to the reactive carbon center such as an iso-propyl group (2g). Overall, the scope of the transformation compares favorably to the Negishi cross-couplings developed by Fu.5 As illustrated in Fig. 1, both enantiomers are of equal ease to access using our strategy and the levels of enantioselectivity achieved are good. Furthermore, while the reactions were routinely run on a 1 mmol scale, we successfully utilized our protocol on up to 50 mmol scale.
5) when the nucleophile is metalated α- to the heteroatom. Thus, α-metalated furans (2d), benzofurans (2e), dihydrofurans (2f), thiophenes (2g, i, m), benzo-thiophenes (2h, j) and indoles (2k, l) are all competent nucleophiles in this transformation. It was intriguing to find that, despite the fact that thiophene typically acts as a “dummy” ligand in nucleophilic diorganocuprate(I) reactions, thiophenes and benzothiophenes are amongst the most efficient and most selective nucleophiles in this transformation (no allene formation observed). It is also worth noting that the excess nucleophile used in the transformation may be recovered after purification.9 Additionally, while using copper for inducing chirality practically requires the copper to be used catalytically, stoichiometric copper is not a limitation in processes involving achiral copper complexes.10 As for the electrophilic partner 4, the reaction is particularly efficient with TMS-protected alkynes,11 which is ideal in view of subsequent functionalization. The reaction is also efficient with R1 substituents being aromatic (2b, m) and BDMS groups (2j). As recently illustrated in our synthesis of leustrodusin B,12 the BDMS alkynes can serve as useful vinyl cross-coupling partners after semi-hydrogenation of the triple bond; even in complex settings. A number of useful functional groups such as alkenes (2d, k), arenes (2b, e, f, m), protected alcohols (2h) and chlorides (2i, l) are perfectly tolerated substituents. Interestingly an aryl bromide (2b) is compatible with the mild reaction conditions. In addition, this stereo-selective displacement is also efficient when the starting bromide 4 possess a secondary center adjacent to the reactive carbon center such as an iso-propyl group (2g). Overall, the scope of the transformation compares favorably to the Negishi cross-couplings developed by Fu.5 As illustrated in Fig. 1, both enantiomers are of equal ease to access using our strategy and the levels of enantioselectivity achieved are good. Furthermore, while the reactions were routinely run on a 1 mmol scale, we successfully utilized our protocol on up to 50 mmol scale.
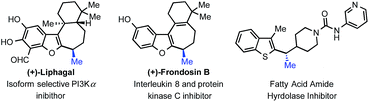
As nicely highlighted in Jarvo and co-workers' recent work;13 in the context of drug discovery, the strategic introduction of a methyl group can improve drug potency through various mechanisms in the hit to lead approach. It is thus interesting to observe that tertiary benzylic stereocenters bearing a methyl group are common motifs in medicines. In particular, such a motif is present in a number of heteroaromatic bioactive compounds with varied activities (Fig. 2). Development of stereoselective methods to obtain these compounds are relevant both from a synthetic and a practical point of view since the different enantiomers are known to possess differential bioactivity. In addition, methods to access these motifs are still scarce. We decided to probe further the scope of the transformation towards the synthesis of such privileged building blocks. Example 2j and 2m (Fig. 1), already hinted to the possibility of using our newly designed strategy for their synthesis.
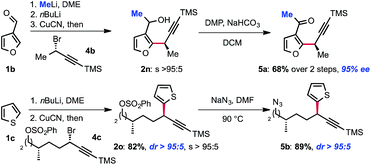
Interestingly, molecular complexity may be rapidly built by combining our displacement reaction with other transformations. As shown in Scheme 3, methyllithium can be added to 3-furan-carboxaldehyde and the corresponding alkoxide can be used as a directing group for metallation. In spite of the proximal presence of the racemic alkoxide, no effect on the enantioselectivity was observed. Thus, transmetallation to CuCN and treatment with (S)-(3-bromobut-1-yn-1-yl)trimethylsilane 4b followed by Dess–Martin oxidation of the corresponding alcohol 2n delivered the doubly functionalized methylketone 5a in 68% yield and 95% ee. Alternatively, we found that the displacement reaction is selective for propargyl bromides over other electrophiles that can in turn be functionalized. Thiophene 2o, was obtained using this strategy. This approach allows for further functionalization of the SN2 adduct via the subsequent transformation of the sulfonate as illustrated by the preparation of azide 5b (Scheme 3). The selectivity of the displacement reaction for the propargyl bromide over other electrophiles such as primary alkylchlorides and arylsulfonates is particularly noteworthy. Indeed, even secondary alkylhalides and arylsulfonates are known to undergo SN2 displacement with diorganocuprate(I) reagents at low temperatures.14
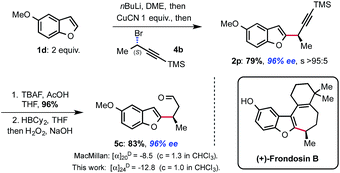
Having established this methodology, we wanted to demonstrate its potential for natural product synthesis. The synthetic utility of the novel displacement was thus further demonstrated by the rapid assembly of aldehyde 5c that was used as a key intermediate in the synthesis of (+)-frondosin B.15 Thus, applying our protocol, alkyne 2p was obtained in 79% yield, 96% ee and with full SN2 selectivity. Deprotection of the TMS-alkyne with TBAF/AcOH followed by hydroboration and oxidation of the intermediate vinylborane, delivered the targeted enantioenriched aldehyde 5c in good yield over 3 steps, completing the formal synthesis of (+)-frondosin B (Scheme 4). The absolute configuration of intermediate 5c was confirmed by measurement of its specific rotation, which was comparable to the value obtained previously.15 This means that the displacement occurs with Walden inversion at the electrophilic carbon bearing the bromide.16
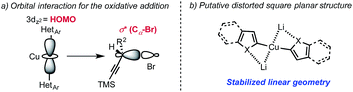
While the origin of the observed SN2 selectivity and high stereoinversion have not yet been elucidated, a scenario in which: (i) the diorganocopper(I) oxidatively adds into the Cα–Br bond and (ii) the resulting copper(III) species is undergoing rapid reductive elimination is plausible. In this context, it also worth noting that our attempt to react diarylcopper(I) nucleophiles with propargyl bromide 4a was poorly regioselective and resulted in the obtention of mixtures of the desired alkynes 2 together with allenes 2′.17 These additional observations suggest that the presence of the heteroatom α- to the copper center is important to achieve the desired selectivity. In 2012, Nakamura published guidelines for the structure/reactivity relationship of nucleophilic organocopper(I) reagents based on orbital analysis.6 Following this analysis, a linear geometry at copper would inhibit alkyne coordination and favor oxidative addition into the Cα–Br bond (Scheme 5). The presence of lithium cations could additionally increase the rate of oxidative addition into the Cα–Br bond by coordination of the lithium to the bromine atom.18 Overall, we propose that the high affinity of the heteroatoms (O, S, N) for lithium invoked to explain the behavior of “dummy” ligands,19 might favor the linear geometry for the diorganocuprate(I) intermediates and would ultimately account for the observed reactivity. A putative simplified structure for the diheteroarylcuprate(I) reagents is distorted square planar (Scheme 5). It is also interesting to note that, in the case of adduct 2f, the organo-cuprate resulting from oxidative addition undergoes reductive elimination faster than the Kocieński style migratory insertion.20
In summary, we have developed the first example of a selective SN2 displacement of propargyl electrophiles with hard carbanions employing readily accessible diorganocuprate(I) reagents and enantioenriched propargyl bromides. This transformation is particularly selective with heterocyclic nucleophiles, occurs with stereoinversion at the Cαsp3 center, and leads to versatile enantioenriched alkynes. The success of the copper mediated reaction in generating highly enantioenriched propargylic centers represents a useful complement to the nickel-catalyzed protocol that failed when attempted using the reported optimized conditions. In addition, the reaction scope compares favorably to the nickel catalyzed Negishi coupling. The synthetic utility of this novel transformation was illustrated by a short formal synthesis of (+)-frondosin B. We expect this transformation to offer new synthetic opportunities not hitherto conceivable. Extension of this method to other types of nucleophiles and in particular to standard aromatic nucleophiles is underway and will be reported in due course.
Acknowledgements
We thank the NSF (CHE-1360634), the NIH (GM-033049) and Syngenta for their generous support of our programs.Notes and references
- For leading references on organocuprate(I) reactions, see: (a) Modern Organocopper Chemistry, ed. N. Krause, Wiley-VCH, Weinheim, 2002 Search PubMed; (b) Organocopper Reagents, A Practical Approach, ed. R. J. K. Taylor, Oxford University Press, Oxford, 1994 Search PubMed; (c) B. H. Lipshutz and S. Sengupta, Org. React., 1992, 41, 135 CAS; (d) Y. Yamamoto, Angew. Chem., Int. Ed. Engl., 1986, 25, 947 CrossRef; (e) G. H. Posner, Org. React., 1975, 22, 253 CAS.
- For SN2 selective reactions of allylic electrophiles, see: (a) S. P. Tanis, Tetrahedron Lett., 1982, 23, 3115 CrossRef CAS; (b) S. Suzuki, M. Shiono and Y. Fujita, Synthesis, 1983, 804 CrossRef CAS; (c) J.-E. Bäckvall and M. Sellén, J. Chem. Soc., Chem. Commun., 1987, 827 RSC; (d) J. E. Bäckvall, M. Sellen and B. Grant, J. Am. Chem. Soc., 1990, 112, 6615 CrossRef; (e) E. S. M. Persson, M. Vanklaveren, D. M. Grove, J. E. Bäckvall and G. van Koten, Chem.–Eur. J., 1995, 1, 351 CrossRef CAS; (f) C. C. Tseng, S.-J. Yen and H. L. Goering, J. Org. Chem., 1986, 51, 2892 CrossRef.
- Primary propargyl bromides (R2 = R3 = H and LG = Br) can react via an SN2 path: (a) J. Robertson and S. Naud, Org. Lett., 2008, 10, 5445 CrossRef CAS PubMed; (b) Y. Wang and J. M. Ready, Org. Lett., 2012, 14, 2308 CrossRef CAS PubMed; (c) P. J. Parsons, J. Board, D. Faggiani, P. B. Hitchcock, L. Preece and A. J. Waters, Tetrahedron, 2008, 66, 6526 CrossRef.
- These reactions were shown to be efficient with halides, epoxydes, sulfonates, phosphonates and esters as leaving groups: (a) M. R. Uehling, S. T. Marionni and G. Lalic, Org. Lett., 2012, 14, 362 CrossRef CAS PubMed; (b) H. Li, D. Müller, L. Guenee and A. Alexakis, Org. Lett., 2012, 14, 5880 CrossRef CAS PubMed; (c) R. K. Dieter, N. Chen and V. K. Gore, J. Org. Chem., 2006, 71, 8755 CrossRef CAS PubMed; (d) M. Purpura and N. Krause, Eur. J. Org. Chem., 1999, 1, 267 CrossRef; (e) P. Rona and P. Crabbé, J. Am. Chem. Soc., 1968, 90, 4733 CrossRef CAS; (f) K. S. Feldman, C. C. Mechem and L. Nader, J. Am. Chem. Soc., 1982, 104, 4011 CrossRef CAS; (g) H. Ohmiya, U. Yokobori, Y. Makida and M. Sawamura, Org. Lett., 2011, 13, 6312 CrossRef CAS PubMed; (h) M. Yang, N. Yokokawa, H. Ohmiya and M. Sawamura, Org. Lett., 2012, 14, 816 CrossRef CAS PubMed; (i) R. K. Neff and D. E. Frantz, ACS Catal., 2014, 4, 519 CrossRef CAS.
- (a) A. J. Oelke, J. Sun and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 2966 CrossRef CAS PubMed; (b) S. W. Smith and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 12645 CrossRef CAS PubMed.
- The mechanism depicted in Scheme 1 is proposed by analogy with the mechanism generally accepted for carbocuprations and 1,4-additions to ynones. For references on the mechanisms of nucleophilic organocopper(I) reactions, see: N. Yoshikai and E. Nakamura, Chem. Rev., 2012, 112, 2339 CrossRef CAS PubMed and references therein.
- For studies of the equilibrium between propargylcopper(I) and allenylcopper(I) and their reactivity see: Comprehensive Organic Synthesis, ed. P. Knochel and G. A. Molander, Elsevier, 2014, vol. 1 Search PubMed and references cited therein.
- G. M. Whitesides, W. F. Fischer Jr, J. San Filippo Jr, R. W. Bashe and H. O. House, J. Am. Chem. Soc., 1969, 91, 4871 CrossRef CAS.
- For example, excess alcohol 1a was recovered in up to 95% yield after reaction with propargyl bromides.
- A gram scale synthesis of prostaglandins was achieved using stoichiometric CuI: (a) M. Suzuki, A. Yanagisawa and R. Noyori, J. Am. Chem. Soc., 1988, 110, 4718 CrossRef CAS. The commercial route to Fulvestrant was optimized starting from a reaction using stoichiometric CuCl: (b) E. J. Brazier, P. J. Hogan, C. W. Leung, A. O'Kearny-McMullan, A. K. Norton, L. Powell, G. E. Robinson and E. G. Williams, Org. Process Res. Dev., 2010, 14, 544 CrossRef CAS; (c) M. Sury and F. Glorius, Org. Synth., 2014, 91, 211 CrossRef; (d) M. P. C. Yeh, H. G. Chen and P. Knochel, Org. Synth., 1992, 70, 195 CrossRef CAS.
- We observed that, while fully SN2 selective, the reactions with TIPS and TES alkynes resulted in decreased yields.
- B. M. Trost, B. Biannic, C. S. Brindle, B. M. O'Keefe, T. J. Hunter and M.-Y. Nga, J. Am. Chem. Soc., 2015, 137, 11594 CrossRef CAS PubMed.
- H. M. Wisnieswska, E. C. Swift and E. R. Jarvo, J. Am. Chem. Soc., 2013, 135, 9083 CrossRef PubMed.
- See, ref. 8 and: (a) C. R. Johnson and G. A. Dutra, J. Am. Chem. Soc., 1973, 95, 7783 CrossRef CAS; (b) C. Y. Guo, M. L. Brownawell and J. Sanfilippo, J. Am. Chem. Soc., 1985, 107, 6028 CrossRef CAS.
- M. Reiter, S. Torssell, S. Lee and D. W. C. MacMillan, Chem. Sci., 2010, 1, 37 RSC.
- In all instances, the reaction was assumed to occur with Walden inversion and the absolute configuration of the alkyne products 2 was assigned based on this observation.
- The alkyne/allenes ratios observed varied between 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 depending on the arene nucleophile.
1 depending on the arene nucleophile. - Work by Pr. Knochel on the beneficial effect of lithium halides supports such proposal: T. Klatt, J. T. Markiewicz, C. Sämann and P. Knochel, J. Org. Chem., 2014, 79, 4253 CrossRef CAS PubMed.
- The high affinity of “dummy” ligands for lithium has been invoked to justify that their significantly slower rate of transfer: (a) The Chemistry of Organocopper Compounds, ed. Z. Rappoport and I. Marek, Wiley-VCH, Weinheim, 2009 Search PubMed; (b) E. Nakamura and M. Yamanaka, J. Am. Chem. Soc., 1999, 121, 8941 CrossRef CAS.
- P. R. Blakemore, W. J. Cole, P. J. Kocieński and A. Morley, Synlett, 1998, 26 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc01086e |
| This journal is © The Royal Society of Chemistry 2016 |