Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rationally introduce multi-competitive binding interactions in supramolecular gels: a simple and efficient approach to develop multi-analyte sensor array†
Qi
Lin
*,
Tao-Tao
Lu
,
Xin
Zhu
,
Tai-Bao
Wei
,
Hui
Li
and
You-Ming
Zhang
*
Key Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, 730070, China. E-mail: linqi2004@126.com; zhangnwnu@125.com
First published on 25th April 2016
Abstract
Sensor arrays are a powerful tool for multianalyte sensing and the development of an efficient sensor array has become one of the most intriguing problems. However, sensor arrays often employ lots of receptors which need large amounts of work to synthesise. This study describes an efficient method for the fabrication of a simple sensor array based on the competitive binding in supramolecular gels. By rationally introducing various well-designed competitive binding interactions into the supramolecular gel, which is self-assembled from a naphthylhydrazone-based organogelator, a supramolecular gel-based twenty-two-member sensor array has been created. Interestingly, the sensor array has been shown to accurately identify fourteen kinds of important ions (F−, Cl−, I−, CN−, HSO4−, SCN−, S2−, OH−, Al3+, Fe3+, Zn2+, Hg2+, Pb2+ and H+) in water. It's important to note that this sensor array needs only one synthesized receptor. Moreover, using this method, we also obtained a series of ion response fluorescent supramolecular materials, which could act as security display materials. Therefore, it's a novel and facile way for the design of a simple sensor array as well as ion response fluorescent supramolecular materials.
Introduction
In the last decade, interest in molecular sensing has been slowly shifting from selective sensors toward sensor array-based multianalyte sensing because lots of the sensor arrays have been shown to be highly efficient in various analyte detection.1 In general, a sensor array is based on a series of sensors which could recognize a number of analytes with a high classification accuracy, there are several wonderful reviews presenting the development of sensor arrays.1a–f Up to now, lots of strategies have been developed for the creation of sensor arrays. For instance, Anzenbacher et al. developed a series of sensor arrays for the detection of anions,2 and carboxylate drugs.3 Suslick et al. created a series of sensor arrays for the detection of organic compounds,4 volatile organic compounds (VOCs)5 and nanoparticles.6 Usually, a high-performance sensor array employs lots of individual selective receptors which need large amounts of work to design, synthesise and pre-research their sensing properties.1a–c In order to overcome the difficulties associated with the preparation of various individual receptors, we need to develop a novel strategy to design simple but highly efficient sensor arrays. Could we develop a sensor array which is based on only a single synthesized receptor? It's a really interesting task.Moreover, ions play a fundamental role in chemical, biological and environmental processes,7 however, reliable sensing of different ions with a similar structure in water is a difficult problem.2,7 Although there are numerous chemosensors reported every year the development of an efficient sensor array recognizing a number of ions with a high classification accuracy is still a intriguing challenge.
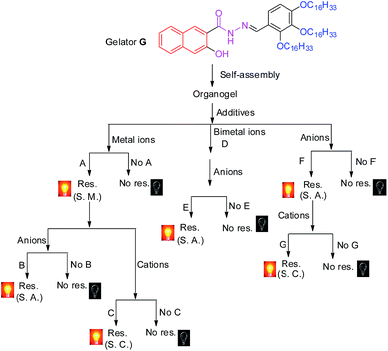
Fortunately, the rapid development of stimuli-responsive supramolecular gels8–11 provides a novel platform for the design of efficient sensor arrays. As we all know, on account of the dynamic and reversible nature of self-assembly in supramolecular gels, stimuli–responsive supramolecular gels can sense, process, and actuate a response to an external change without assistance.8–11 These excellent properties make supramolecular gels a wonderful candidate for chemosensors. However, although many supramolecular gel-based chemosensors have been reported, reports on supramolecular gel-based sensor-arrays are very rare.1a–f,k More interestingly, in our previous work, we found that the stimuli–responsive properties of the supramolecular gels could be efficiently controlled by the competitive coordination of the gelator with the metal ions.11 These fine properties provide the supramolecular gel with good opportunities to act as sensor arrays. In view of these, in this study, by rationally introducing various well-designed competitive binding interactions into a functionalized organogel, we created a novel supramolecular gel-based twenty-two-member sensor array. The sensor array could accurately identify fourteen kinds of ions in water, ions including F−, Cl−, I−, CN−, HSO4−, SCN−, S2−, OH−, Al3+, Fe3+, Zn2+, Hg2+, Pb2+ and H+. It is worth mentioning that the sensor array was constructed using only one receptor. Simply stated, in the twenty-two-member sensor array, the organogelator not only acts as a gelator, but also as a receptor. The selective sensing properties of the sensor array are accurately controlled by the various competitive binding interactions which we rationally induced in the gel.
Results and discussion
Firstly, as show in Fig. 1, we rationally designed and synthesized a multi-functionalized organogelator G by introducing a coordination site, multi-self-assembly driving forces, and fluorescent signal groups into the gelator molecule. For example, we introduced an acylhydrazone group and a hydroxy group as the coordination, hydrogen-bonding and recognition sites, a naphthyl group as the signal group and π–π stacking site and long alkyl chains as the strong van der Waals forces group. The gelation abilities of G were examined in various solvents by means of the “stable to inversion of a test tube” method (Table S1†). As we expected, G showed excellent gelation abilities in various solvents such as dimethyl formamide (DMF), dimethyl sulfoxide (DMSO), acetone, ethanol, n-propyl alcohol, isopropanol, n-butyl alcohol, isoamyl alcohol, n-hexanol, cyclohexanol, ethyl acetate, CCl4, petroleum ether and so on. Among these solvents, the gelator G showed the lowest critical gelation concentration (CGC) (0.2%, w/v, 10 mg ml−1 = 1%) and the highest gel–sol transition temperature (Tgel) in n-butyl alcohol (Table S1†). Therefore, the G organogel formed in n-butyl alcohol is more stable than the gels in other solutions and we carried out subsequent studies on the G organogel (named OG) formed in n-butyl alcohol.The self-assembly mechanism of OG was investigated using 1H NMR, IR, X-ray and SEM. In the concentration dependent 1H NMR (Fig. 2) of G, the –OH (Ha), –NH (Hb) and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH (Hc) resonance signals showed obvious downfield shifts as the concentration of G rose. These results revealed that in the gelation process, these groups formed hydrogen bonds with the –C
CH (Hc) resonance signals showed obvious downfield shifts as the concentration of G rose. These results revealed that in the gelation process, these groups formed hydrogen bonds with the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –N
O and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups on the adjacent gelators. Moreover, the IR studies also confirmed this result, as shown in Fig. S4a,† in the powder G, the stretching vibration of –OH and –NH appeared as a broad peak at 3202 cm−1, while, owing to the formation of hydrogen bonds, in the organogel OG, these absorptions were shifted to 3380 and 3244 cm−1, respectively. On the other hand, with the gradual increase in concentration, the 1H NMR signal of the naphthyl protons (Hd, He, Hf, Hg, Hh and Ho) showed an obvious upfield shift, indicating that the π–π stacking interactions between the naphthyl groups were involved in the gelation process.12 The X-ray peak (Fig. S5†) at 2θ 24.5° (d = 3.96) also confirms the π–π stacking interactions. Thus, the gelator G self-assembled to organogel OG through the hydrogen bonds and π–π stacking as well as the vdW existing in the long alkyl chains. The morphological features of the organogel OG were studied using SEM (Fig. S6a†) with its xerogels and showed a rugate layer structure.
C groups on the adjacent gelators. Moreover, the IR studies also confirmed this result, as shown in Fig. S4a,† in the powder G, the stretching vibration of –OH and –NH appeared as a broad peak at 3202 cm−1, while, owing to the formation of hydrogen bonds, in the organogel OG, these absorptions were shifted to 3380 and 3244 cm−1, respectively. On the other hand, with the gradual increase in concentration, the 1H NMR signal of the naphthyl protons (Hd, He, Hf, Hg, Hh and Ho) showed an obvious upfield shift, indicating that the π–π stacking interactions between the naphthyl groups were involved in the gelation process.12 The X-ray peak (Fig. S5†) at 2θ 24.5° (d = 3.96) also confirms the π–π stacking interactions. Thus, the gelator G self-assembled to organogel OG through the hydrogen bonds and π–π stacking as well as the vdW existing in the long alkyl chains. The morphological features of the organogel OG were studied using SEM (Fig. S6a†) with its xerogels and showed a rugate layer structure.
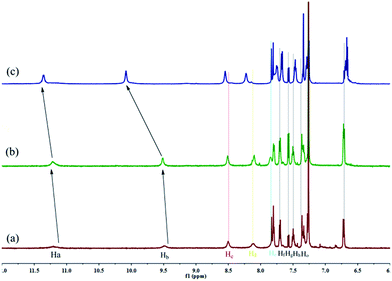 | ||
| Fig. 2 Partial 1H NMR spectra of G in CDCl3 with different concentrations, (a) 10 mg ml−1; (b) 20 mg ml−1; (c) 30 mg ml−1. | ||
Interestingly, OG shows aggregation-induced fluorescence emission (AIE)13 in the organogel state in various solvents. For example, as shown in Fig. 3, G has no fluorescence in hot n-butyl alcohol solution (T > Tgel). However, with the temperature of hot n-butyl alcohol solution dropping below the Tgel of OG, the emission intensity at 475 nm showed a sudden increase and reached a steady state, which indicated that the turquoise fluorescence of OG was AIE. Meanwhile, this emission shows a large Stokes shift ca. 175 nm (Fig. S7†).
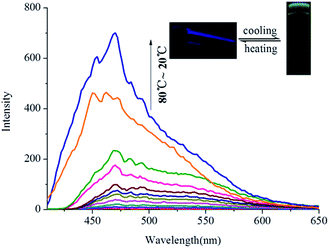 | ||
| Fig. 3 Temperature-dependent fluorescence spectra of OG (in n-butyl alcohol, 0.8%) during the gelation process (λex = 300 nm). | ||
Secondly, in order to develop an OG-based sensor array, as shown in Fig. 1, we rationally induced seven kinds of well-designed competitive binding interactions ((A–G) in Fig. 1) into organogel OG to control the ion response capabilities of the gel. These competitive binding interactions were introduced by adding various metal ions, bimetal ions, anions or alternatively adding metal ions and anions into OG according to Fig. 1, respectively. Interestingly, via the gelator and ions taking place through competitive coordination or binding, the supramolecular gel could show a selective response for the target ions (Fig. 4).
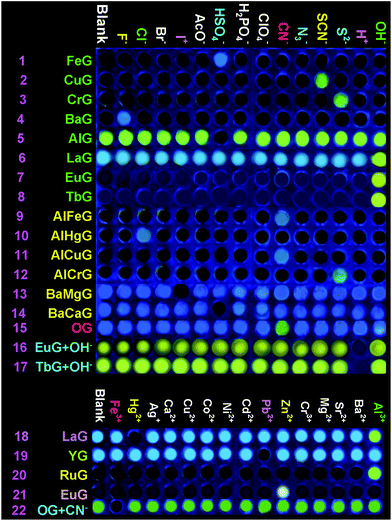 | ||
| Fig. 4 Fluorescence responses of the supramolecular gel-based sensor array to the presence of various anions and cations. | ||
At the beginning, we introduced competitive binding interactions between the metal ions and gelators (competitive binding interaction (A) in Fig. 1) by adding various metal ions into OG. The addition and diffusion of various metal ions (Mg2+, Ca2+, Cr3+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Hg2+, Pb2+, Ba2+, Sr2+, Al3+, La3+, Y3+, Ru3+, Eu3+ and Tb3+) to OG generated the corresponding metallogels (named MGs, for example MgG, CaG, CrG and so on) respectively (Fig. S8†). Interestingly, as shown in Fig. S8 and S9,† upon the addition of 0.5 equiv. of Cu2+, Ba2+, Fe3+, Cr3+, Ru3+, Eu3+ or Tb3+ to OG, the AIE of OG was quenched and a corresponding no fluorescence metallogel formed; while the addition of 0.5 equiv. of Ca2+, Al3+, La3+, Y3+ and so on could induce the AIE of OG taking place with obvious shifts. For instance, the metallogel AlG emitted a strong green fluorescence, while, LaG and YG emitted a strong blue fluorescence.
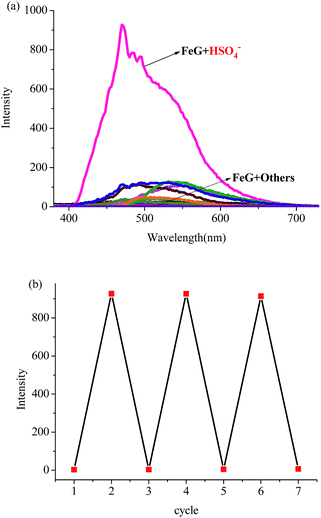
Then, we introduced competitive binding interactions between the metal ions, anions and gelators (competitive binding interaction (B) in Fig. 1) by adding various anions into the OG-based metallogels. As shown in Fig. 4 (line 1) and Fig. 5a, with the addition of water solutions of various anions (F−, Cl−, Br−, I−, AcO−, H2PO4−, HSO4−, N3−, SCN−, S2−, ClO4−, CN−, and OH−) into the metallogel FeG, only HSO4− could induce the metallogel FeG emitting a turquoise fluorescence at 470 nm immediately. While other anions such as F−, Cl−, Br−, I−, AcO−, H2PO4−, N3−, CN−, SCN−, ClO4−, S2− and OH− could not induce any significant emission changes. Therefore, FeG showed selective fluorescence “turn-on” sensing of HSO4− in water. The detection limits of FeG for HSO4− are 1.0 × 10−6 M (Fig. S11a and Table S2†).
Similar tests were applied to the other no fluorescence metallogels such as CuG, CrG, BaG, EuG, TbG and the fluorescence metallogels such as AlG, LaG and so on. From the results, CuG, CrG, BaG, EuG, and TbG could selectively fluorescence “turn-on” sense SCN−, S2−, F− and OH− (Fig. 4) (line 2, 3, 4, 7, 8; and Fig. S10a–c, f and g†) while AlG and LaG could selectively sense HSO4− and OH− (Fig. 4 (line 5 and 6), Fig. S10d and e†) respectively.
Then we introduced competitive binding interactions between the metal ions and gelators (competitive binding interaction (C) in Fig. 1) by adding water solutions of various metal ions (Mg2+, Ca2+, Cr3+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Hg2+, Pb2+, Ba2+, Sr2+, Al3+, La3+, Y3+, Ru3+, Eu3+ and Tb3+) into the metallogels. As a result, LaG and YG could selectively sense Hg2+ and Pb2+ (Fig. 4 (line 18 and 19), Fig. S11l and n†) while RuG and EuG could selectively fluorescence “turn-on” sense Al3+ and Zn2+ (Fig. 4 (line 20 and 21), Fig. S11m and o†) respectively.
In light of the above results that the addition of metal ions such as Cu2+, Ba2+, Fe3+, Cr3+ or Ru3+ could quench the AIE of OG while the addition of Ca2+, Al3+, La3+, and Y3+ could induce the AIE of OG taking place with obvious shifts or enhancement, we rationally induced two different kinds of metal ions into OG to control the AIE of the gel. Due to the different coordination capabilities of the bimetal ions, there is competitive coordination existing in the gelator and the two kinds of metal ions (competitive binding interaction (D) in Fig. 1), therefore, the fluorescence properties of the gel could be well controlled using this competition. For instance, through the synchronous addition of 0.5 equiv. of Al3+ and Fe3+ into OG, we could obtain the corresponding bimetallogel AlFeG. Using a similar method, we could obtain a series of bimetallogels such as AlHgG, AlCuG, AlCrG, BaMgG, BaCaG and so on. Interestingly, as we expected, the fluorescence properties of the bimetallogel depends on the metal ion which has the stronger coordination capability than the other metal ion. For example, because the coordination capability of Fe3+ is stronger than Al3+, the fluorescence properties of AlFeG are similar to those of FeG. Therefore, AlFeG is a no fluorescence metallogel. Similarly, AlHgG, AlCuG and AlCrG are no fluorescence metallogels while BaMgG and BaCaG are fluorescence metallogels.
Then, we introduced competitive binding interactions among the bimetal ions, anions, and gelators (competitive binding interaction (E) in Fig. 1) by adding water solutions of various anions into these bimetallogels. As show in Fig. 4 (line 9–14), Fig. S10h–m,†AlFeG, AlHgG, AlCuG and AlCrG could selectively fluorescence “turn-on” sense CN− (by AlFeG and AlCuG), Cl− (by AlHgG) and S2− (by AlCrG) respectively; while, BaMgG and BaCaG could selectively fluorescence “turn-off” sense I− and HSO4− respectively.
Moreover, we also introduced competitive binding interactions between the anions and gelators (competitive binding interaction (F) in Fig. 1) by adding the water solutions of various anions into OG. As show in Fig. 4 (line 15) and Fig. S10o,† with the addition of water solutions of various anions (F−, Cl−, Br−, I−, AcO−, H2PO4−, N3−, SCN−, S2−, ClO4−, CN−, and OH−) into OG respectively, only CN− could induce the AIE emission of OG to take place a significant red shift from 470 nm to 520 nm immediately. Meanwhile, turquoise fluorescence of OG changed to yellow. While other anions such as F−, Cl−, Br−, I−, AcO−, H2PO4−, N3−, SCN−, ClO4−, S2− and OH− could not induce any significant emission or color changes. Therefore, OG could selectively fluorescence and colorimetric sense CN− in water.
Finally, we introduced competitive binding interactions among the anions, metal ions and gelators (competitive binding interaction (G) in Fig. 1) by adding various metal ions into the organogel OG + CN, as a result, as shown in Fig. 4 (line 22) and Fig. S10o,†OG + CN can selectively sense Fe3+.
The detection limits of this supramolecular gel-based sensor array for the corresponding ions were investigated via fluorescence titrations (Fig. S11†). As a result, the sensor array shows a high sensitivity for target ions. For example, the bimetallogel sensor AlCuG shows a very low detection limit for CN− (1.0 × 10−7 M), which is lower than the WHO guideline of 1.9 × 10−6 M.14 The other detection limits of the sensor array for the corresponding ions are listed in Table S2.†
We investigated the sensing mechanism of the above mentioned metallogels and bimetallogels via IR and SEM. For instance, in the FT-IR of OG (Fig. S4b†), the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –N
O and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– vibration absorption peaks appeared at 1641 and 1594 cm−1 respectively. While, with the addition of Fe3+ into OG and the formation of FeG, these two peaks shifted to 1647 and 1588 cm−1 respectively, which was attributed to the coordination of Fe3+ with the gelator via the acylhydrazone group of the gelator. As we expected, after the addition of HSO4− into metallogel FeG, the –C
CH– vibration absorption peaks appeared at 1641 and 1594 cm−1 respectively. While, with the addition of Fe3+ into OG and the formation of FeG, these two peaks shifted to 1647 and 1588 cm−1 respectively, which was attributed to the coordination of Fe3+ with the gelator via the acylhydrazone group of the gelator. As we expected, after the addition of HSO4− into metallogel FeG, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –N
O and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– vibration absorption peaks returned to 1641 and 1594 cm−1 respectively, which indicated that the HSO4− competitively binds with Fe3+ and releases the acylhydrazone group of the gelator. This result indicated that the ion sensing mechanism of the above mentioned metallogels and bimetallogels is based on the competitive coordination which took place among the gelators, metal ions or anions in the supramolecular gel system. In the corresponding SEM (Fig. S6†), owing to the competitive binding of the Fe3+ with gelator G, the micromorphology of the supramolecular gels shows obvious changes.
CH– vibration absorption peaks returned to 1641 and 1594 cm−1 respectively, which indicated that the HSO4− competitively binds with Fe3+ and releases the acylhydrazone group of the gelator. This result indicated that the ion sensing mechanism of the above mentioned metallogels and bimetallogels is based on the competitive coordination which took place among the gelators, metal ions or anions in the supramolecular gel system. In the corresponding SEM (Fig. S6†), owing to the competitive binding of the Fe3+ with gelator G, the micromorphology of the supramolecular gels shows obvious changes.
The further response mechanism of OG for CN− was investigated via1H NMR titration experiments and IR. In the 1H NMR (Fig. S12†), the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH protons on G appeared at δ 9.51 ppm. After adding CN−, this signal faded away, while, two new signals appeared at δ 3.10 and 5.64 ppm, which were attributed to the formation of the NC–CH– and –NH groups, respectively. Meanwhile, in the FT-IR of OG (Fig. S4c†), the –N
CH protons on G appeared at δ 9.51 ppm. After adding CN−, this signal faded away, while, two new signals appeared at δ 3.10 and 5.64 ppm, which were attributed to the formation of the NC–CH– and –NH groups, respectively. Meanwhile, in the FT-IR of OG (Fig. S4c†), the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH vibration absorption at 1594 nm−1 disappeared and a new –C
CH vibration absorption at 1594 nm−1 disappeared and a new –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N vibration absorption appeared at 2176 cm−1. These results indicated that the CN− was added to the imines group on OGvia a nucleophilic addition reaction and formed a new organogelator OG + CN. During this process, the emission band of OG underwent a red shift, which is attributed to the ICT process.
N vibration absorption appeared at 2176 cm−1. These results indicated that the CN− was added to the imines group on OGvia a nucleophilic addition reaction and formed a new organogelator OG + CN. During this process, the emission band of OG underwent a red shift, which is attributed to the ICT process.
The competitive binding mechanism was also supported by the reversibility of the sensing process. For example, the metallogel FeG could selectively fluorescence “turn-on” sense HSO4−, while, upon the addition of Fe3+ into the HSO4−-containing FeG, the fluorescence of FeG was quenched, which was attributed to the Fe3+ coordination with the gelator again. These properties make FeG act as a HSO4− and Fe3+ controlled “OFF–ON–OFF” fluorescent switch. By alternating the addition of HSO4− and Fe3+, the switch could be reversibly performed for at least three cycles with little fluorescence efficiency loss (Fig. 5b). Moreover, EuG and TbG could selectively fluorescence “turn-on” sense OH−, while, upon the addition of H+ into the OH−-containing EuG or TbG, the fluorescence of EuG or TbG was quenched (Fig. 4 (line 16 and 17)), which was attributed to H+ competitively binding with OH− and the Eu3+ or Tb3+ coordination with the gelator again. Meanwhile, EuG and TbG could act as H+ sensors.
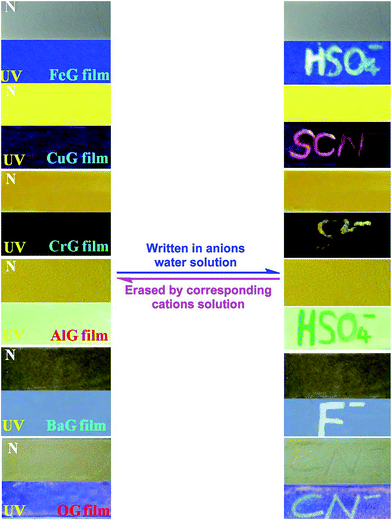
Moreover, these supramolecular gels could act as ion response fluorescent materials. For example, by pouring a heated solution of these gels onto a clean glass surface and drying in the air, we could obtain the corresponding ion response supramolecular films. As shown in Fig. 6, the FeG film has no fluorescence emission, when writing on the film with a writing brush dipped in HSO4− water solution, a brilliant blue fluorescent writing appeared. This fluorescent image could be erased by brushing Fe3+ on the film again. Other supramolecular gels (Fig. 6) show similar properties also. Therefore, these supramolecular films could act as security display materials.
Conclusions
In summary, by rationally introducing competitive binding interactions into a well designed supramolecular gel, a twenty-two member sensor array has been successfully developed. This sensor array could sense fourteen kinds of important ions such as CN−, F−, SCN−, Hg2+, Pb2+etc. with high selectivity and sensitivity in a water solution. This sensor array needed only one synthesized receptor. Moreover, using this method, we also obtained a series of ion response fluorescent supramolecular materials, which could act as erasable security display materials. Therefore, this is an efficient and simple way to develop a sensor array as well as stimuli–responsive supramolecular materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 21574104; 21161018; 21262032) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 15R56).Notes and references
- (a) K. L. Diehl and E. V. Anslyn, Chem. Soc. Rev., 2013, 42, 8596 RSC; (b) J. R. Askim, M. Mahmoudi and K. S. Suslick, Chem. Soc. Rev., 2013, 42, 8649 RSC; (c) P. Anzenbacher Jr., P. Lubal, P. Buček, M. A. Palaciosa and M. E. Kozelkova, Chem. Soc. Rev., 2010, 39, 3954 RSC; (d) K. J. Albert, N. S. Lewis, C. L. Schauer, G. A. Sotzing, S. E. Stitzel, T. P. Vaid and D. R. Walt, Chem. Rev., 2000, 100, 2595 CrossRef CAS PubMed; (e) J. W. Grate, Chem. Rev., 2000, 100, 2627 CrossRef CAS PubMed; (f) P. C. Jurs, G. A. Bakken and H. E. McClelland, Chem. Rev., 2000, 100, 2649 CrossRef CAS PubMed; (g) L. Mosca, S. K. Behzad and P. Anzenbacher Jr., J. Am. Chem. Soc., 2015, 137, 7967 CrossRef CAS PubMed; (h) G. V. Zyryanov, M. A. Palacios and P. Anzenbacher Jr., Angew. Chem., Int. Ed., 2007, 46, 7849 CrossRef CAS PubMed; (i) T. Minami, Y. L. Liu, A. Akdeniz, P. Koutnik, N. A. Esipenko, R. Nishiyabu, Y. Kubo and P. Anzenbacher Jr., J. Am. Chem. Soc., 2014, 136, 11396 CrossRef CAS PubMed; (j) P. Anzenbacher Jr., F. Li and M. A. Palacios, Angew. Chem., Int. Ed., 2012, 51, 2345 CrossRef; (k) Q. Lin, T.-T. Lu, X. Zhu, B. Sun, Q. P. Yang, T.-B. Wei and Y.-M. Zhang, Chem. Commun., 2015, 51, 1635 RSC.
- M. A. Palacios, R. Nishiyabu, M. Marquez and P. Anzenbacher Jr., J. Am. Chem. Soc., 2007, 129, 7538 CrossRef CAS PubMed.
- Y. L. Liu, T. Minami, R. Nishiyabu, Z. Wang and P. Anzenbacher Jr, J. Am. Chem. Soc., 2013, 135, 7705 CrossRef CAS PubMed.
- C. Zhang and K. S. Suslick, J. Am. Chem. Soc., 2005, 127, 11548 CrossRef CAS PubMed.
- (a) H. W. Lin, M. Jang and K. S. Suslick, J. Am. Chem. Soc., 2011, 133, 16786 CrossRef CAS PubMed; (b) H. W. Lin and K. S. Suslick, J. Am. Chem. Soc., 2010, 132, 15519 CrossRef CAS PubMed.
- M. Mahmoudi, S. E. Lohse, C. J. Murphy and K. S. Suslick, ACS Sens., 2016, 1, 17 CrossRef CAS.
- (a) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC; (b) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564 CrossRef CAS PubMed; (c) S. V. Krivovichev, O. Mentré, O. I. Siidra, M. Colmont and S. K. Filatov, Chem. Rev., 2013, 113, 6459 CrossRef CAS PubMed.
- (a) M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086 RSC; (b) Z.-Y. Li, Y. Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. W. Tan, Y. H. Yu, X. P. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; (c) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC; (d) J. M. Hu, G. Q. Zhang and S. Y. Liu, Chem. Soc. Rev., 2012, 41, 5933 RSC; (e) Z. H. Qi and C. A. Schalley, Acc. Chem. Res., 2014, 47, 2222 CrossRef CAS PubMed; (f) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed; (g) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed.
- (a) J. G. Sun, Y. C. Liu, L. Y. Jin, T. Chen and B. Z. Yin, Chem. Commun., 2016, 52, 768 RSC; (b) Y. C. Liu, Y. Wang, L. Y. Jin, T. Chen and B. Z. Yin, Soft Matter, 2016, 12, 934 RSC; (c) X. W. Du, J. Zh, J. F. Shi and B. Xu, Chem. Rev., 2015, 115, 13165 CrossRef CAS PubMed; (d) A. Prri, G. Cabriella, P. Metrabgolo and G. Resbati, Acc. Chem. Res., 2013, 46, 2686 CrossRef PubMed; (e) J. Seo, J. W. Chung, J. E. Kwon and S. Y. Park, Chem. Sci., 2014, 5, 4845 RSC; (f) M. M. Zhang, D. H. Xu, X. Z. Yan, J. Z. Chen, S. Y. Dong, B. Zheng and F. H. Huang, Angew. Chem., Int. Ed., 2012, 51, 7011 CrossRef CAS PubMed; (g) X. Z. Yan, D. H. Xu, X. D. Chi, J. Z. Chen, S. Y. Dong, X. Ding, Y. H. Yu and F. H. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed; (h) S. Y. Dong, Y. Luo, X. Z. Yan, B. Zheng, X. Ding, Y. H. Yu, Z. Ma, Q. L. Zhao and F. H. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed; (i) B. B. Shi, K. C. Jie, Y. J. Zhou, J. Zhou, D. Y. Xia and F. H. Huang, J. Am. Chem. Soc., 2016, 138, 80 CrossRef CAS PubMed; (j) B. B. Shi, K. C. Jie, Y. J. Zhou, D. Y. Xia and Y. Yao, Chem. Commun., 2015, 51, 4503 RSC.
- (a) A. Y.-Y. Tam and V. W.-W. Yam, Chem. Soc. Rev., 2013, 42, 1540 RSC; (b) M. J. Mayoral, C. Rest, V. Stepanenko, J. Schellheimer, R. Q. Albuquerque and G. Fernández, J. Am. Chem. Soc., 2013, 135, 2148 CrossRef CAS PubMed; (c) Y. Zhang, B. Zhang, Y. Kuang, Y. Gao, J. F. Shi, X. X. Zhang and B. Xu, J. Am. Chem. Soc., 2013, 135, 5008 CrossRef CAS PubMed; (d) D. Yuan, J. F. Shi, X. W. Du, N. Zhou and B. Xu, J. Am. Chem. Soc., 2015, 137, 10092 CrossRef CAS PubMed; (e) S. Dong, B. Zheng, F. Wang and F. Huang, Acc. Chem. Res., 2014, 47, 1982 CrossRef CAS PubMed.
- (a) Q. Lin, T.-T. Lu, J.-C. Lou, G.-Y. Wu, T.-B. Wei and Y.-M. Zhang, Chem. Commun., 2015, 51, 12224 RSC; (b) Q. Lin, B. Sun, Q. P. Yang, Y. P. Fu, X. Zhu, T.-B. Wei and Y.-M. Zhang, Chem.–Eur. J., 2014, 20, 11457 CrossRef CAS PubMed; (c) Q. Lin, Q.-P. Yang, B. Sun, Y.-P. Fu, X. Zhu, T.-B. Wei and Y.-M. Zhang, Soft Matter, 2014, 10, 8427 RSC.
- C. Po, Z. Ke, A. Y.-Y. Tam, H.-F. Chow and V. W.-W. Yam, Chem.–Eur. J., 2013, 19, 15735 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- Guidelines for Drinking-Water Quality, World Health Organization, Geneva, 1996 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data; gelation properties; fluorescence spectra of the supramolecular gels and the supramolecular gel-based sensor array response for guest ions; fluorescence titrations for target ions. See DOI: 10.1039/c6sc00955g |
| This journal is © The Royal Society of Chemistry 2016 |