Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Readily accessible multifunctional fluorous emulsions†
Ellen M.
Sletten‡
and
Timothy M.
Swager
*
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Ave, Cambridge, MA 02143, USA. E-mail: tswager@mit.edu
First published on 26th April 2016
Abstract
Strategies for the facile fabrication of nanoscale materials and devices represent an increasingly important challenge for chemists. Here, we report a simple, one-pot procedure for the formation of perfluorocarbon emulsions with defined functionalization. The fluorous core allows for small molecules containing a fluorous tail to be stabilized inside the emulsions. The emulsions can be formed using a variety of hydrophilic polymers resulting in an array of sizes (90 nm to >1 micron) and surface charges (−95 mV to 65 mV) of fluid particles. The surface of the emulsions can be further functionalized, covalently or non-covalently, through in situ or post-emulsion modification. The total preparation time is 30 minutes or less from commercially available reagents without specialized equipment. We envision these emulsions to be applicable to both biological and materials systems.
Introduction
Over the past decade, significant interest has been placed on nanotechnology as the demand for portable electronics,1 mobile diagnostic tools,2 and multifunctional therapeutics3 increases. At the core of the nanotechnology movement is the discovery of materials that act as building blocks for devices and are readily available to a broad range of scientists. It is the chemists' duty to create nanomaterials that can be easily prepared from commercial reagents and readily customized for use in diverse applications.Some of the most successful nanomaterials to date are particles.4 Nanoparticles have been synthesized from an array of materials with Au, Fe3O4, and quantum dots being standouts displaying unique, often size-dependent, properties.5 These inorganic nanoparticles are prepared in multiple synthetic steps beginning with formation of the core followed by a second chemical transformation to modify the surface for enhanced stability, solubility, and functionality of the materials (Fig. 1A).6 These syntheses commonly involve many different reagents and solvent extraction steps, which can be a significant drawback to those not versed in chemical methods and limit the utility of even the most promising materials. Additionally, the use of inorganic nanoparticles, particularly quantum dots, suffers from toxicity concerns, which have prevented their widespread use in biological and environmental devices.7 A class of nanoparticles that overcomes both the toxicity concerns and synthetic challenges of inorganic particles are self-assembled organic nanomaterials such as polymer nanoparticles, micelles, vesicles, liposomes, and emulsions.4 These dynamic structures are extensively employed in the medical and food industries to protect and solubilize active ingredients in aqueous media (Fig. 1B). Additionally, they are ubiquitous in the paint and ink industries.8 However, their expansion into other areas of nanotechnology has been hindered by stability concerns and a limited range of surfactants employed for their formation. Here, we aim to impart additional advantages to organic nanoparticles by diversifying the functionality of perfluorocarbon emulsions in a controlled, yet single-step, procedure.
Our one-step synthesis of functional emulsions is summarized in Fig. 1C. A mixture of fluorous solvent and aqueous buffer is sonicated in the presence of a functionalized polymer surfactant and a fluorous-tagged small molecule to yield emulsions with defined functionalization on the inside and outside of the droplets. Key to our strategy is the use of fluorous solvent as the inner phase of the emulsions, which provides opportunities to direct and control the residence time of small molecules inside the emulsion droplets using fluorous tags9 as well as enhances the overall stability of the emulsions.10 We also expected the extreme hydrophobicity of fluorocarbons to result in emulsions that are more readily stabilized by a variety of surfactants, an important aspect to broadening the scope of these materials.
Initially, fluorous-in-water emulsions were composed of tetrafluoroethylene (TFE). As early as the 1940s, it was realized that surfactants had an important role in dictating the size and shape of the emulsions, as determined by analysis of the polymerized TFE.11 The exceptional properties of poly(tetrafluoroethylene) (PTFE, Teflon™)12 resulted in a large body of work regarding aqueous TFE emulsions,13 which are often stabilized with fluorinated acids. Many other fluorinated polymers are also synthesized by exploiting fluorous emulsions. However, mounting concerns over the safety of fluorinated small molecule surfactants employed for fluoropolymer synthesis14 make new formulations for fluorous emulsions a pressing avenue of research.
Fluorinated surfactants and emulsions also play a large role in the paint and coating industry.15 Fluorinated surfactants are more effective at reducing surface tensions of colloidal systems than their organic congeners,16 rendering them the molecule of choice for many coatings. The robust properties of fluorinated polymers have also resulted in their use in paints. In particular, poly(vinylidene fluoride) (PVDF) has been found to be an advantageous component in many outdoor paints.17
Kinetically stable emulsions of perfluorinated compounds in water can be achieved without employing fluorinated small molecule surfactants. Nanoemulsions composed of a fluorous inner phase stabilized by Pluronic polymers and/or phospholipids were developed in the 1970s due to their use as artificial blood.18 The following decades yielded much excitement over these materials and numerous formulations, some of which received clinical approval,19 were studied in efforts to enhance oxygen content, stability, and pharmacokinetic properties.20 Despite their biomedical potential, the use of fluorous nanoemulsions in medical applications has been limited, with most reports focusing on 19F-MRI-based cell-tracking and imaging.21 Other notable biological applications include a cellular pH sensor22 and the delivery of fluorinated anaesthetics.23
Results and discussion
For demonstration of multifunctional perfluorocarbon nano-emulsions, we employed Pluronic-F68 (1) as a surfactant and a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of perfluorodecalin (PFD, 2)/perfluorotripropylamine (PFTPA, 3) as the inner phase (Fig. 2A). This formulation represents a simplified version of Fluosol-DA, one of the early perfluorocarbon nanoemulsions approved by the FDA.24 Using this preparation, we analysed the scope of small molecules that could be directed to the centre of the nanoemulsions. The fluorous environment of the droplets made for a clear strategy of directing and stabilizing molecules inside the emulsions using fluorous tags. Horvath and Rabai introduced the use of fluorous tags to solubilize catalysts in perfluorinated solvents in 1994.25 Since this seminal report, many applications for localizing and purifying small-molecules in the fluorous phase have emerged.26 Consequently, compounds with an array of functionalities can be purchased with fluorous tails.27 We purchased fluorous-tagged aniline 4 (63 wt% F), triazine 5 (72 wt% F), trityl 6 (41 wt% F), and benzimidazole 7 (57 wt% F) (Fig. 2B), which contain varying degrees of fluorination and incorporated them into our emulsions at three different concentrations by first dissolving 4–7 in a 7
3 ratio of perfluorodecalin (PFD, 2)/perfluorotripropylamine (PFTPA, 3) as the inner phase (Fig. 2A). This formulation represents a simplified version of Fluosol-DA, one of the early perfluorocarbon nanoemulsions approved by the FDA.24 Using this preparation, we analysed the scope of small molecules that could be directed to the centre of the nanoemulsions. The fluorous environment of the droplets made for a clear strategy of directing and stabilizing molecules inside the emulsions using fluorous tags. Horvath and Rabai introduced the use of fluorous tags to solubilize catalysts in perfluorinated solvents in 1994.25 Since this seminal report, many applications for localizing and purifying small-molecules in the fluorous phase have emerged.26 Consequently, compounds with an array of functionalities can be purchased with fluorous tails.27 We purchased fluorous-tagged aniline 4 (63 wt% F), triazine 5 (72 wt% F), trityl 6 (41 wt% F), and benzimidazole 7 (57 wt% F) (Fig. 2B), which contain varying degrees of fluorination and incorporated them into our emulsions at three different concentrations by first dissolving 4–7 in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of PFD/PFTPA and then subjecting these solutions to sonication in an aqueous solution containing 2.8 wt% Pluronic-F68 (1). We analysed the size of the resulting emulsions and found that no significant changes in hydrodynamic diameter were evident when loaded with 0.4 μmol mL−1 of any of the compounds studied here and only slight changes in diameter were observed for emulsions with higher loadings of 4 μmol mL−1 of 4 and 6 (Fig. 2B). These results indicate that ∼105 to 106 molecules can be encapsulated within the droplets without significant changes in size.28
3 mixture of PFD/PFTPA and then subjecting these solutions to sonication in an aqueous solution containing 2.8 wt% Pluronic-F68 (1). We analysed the size of the resulting emulsions and found that no significant changes in hydrodynamic diameter were evident when loaded with 0.4 μmol mL−1 of any of the compounds studied here and only slight changes in diameter were observed for emulsions with higher loadings of 4 μmol mL−1 of 4 and 6 (Fig. 2B). These results indicate that ∼105 to 106 molecules can be encapsulated within the droplets without significant changes in size.28
An important aspect of our approach is the ability to modify the functionality inside the nanoemulsion droplets without affecting the surface properties. To assay this, we performed zeta potential measurements on the nanoemulsions containing different concentrations of 4–7. No significant changes in surface charges were observed at concentrations of 0.4 μmol mL−1 and lower suggesting the fluorous-tagged compounds were completely localized inside the emulsions (Fig. 2C). However, at the highest concentration tested, 4 μmol mL−1, triazine 5 and trityl 6 displayed surface charge differences when compared to particles lacking payloads, thereby indicating an upper threshold for orthogonally modifying the functionality inside and outside of the emulsion droplets. It is interesting to note that for both the size and surface charge changes, we do not see a trend that correlates to fluorine content. Both “light” (less than 40 wt% F), “heavy” (greater than 60 wt% F), and “in between” compounds can all be incorporated into the nanoemulsions.29
Having demonstrated that molecules can be directed and stabilized inside the perfluorocarbon nanoemulsions, we sought to prepare emulsions with different surface properties. Initial approaches to surface functionalization involving the introduction of amphiphilic small molecules or triblock oligomers containing a fluorous segment to Pluronic-F68 stabilized emulsions were unsuccessful as a result of the poor solubility of the custom surfactant and/or inadequate stability of the resulting emulsions. These results prompted us to look toward changing the polymer surfactant altogether. We envisioned that a variety of functional polymers would stabilize the fluorous emulsions (Fig. 3A). Gratifyingly, a screen of polymers revealed that a vast majority of the hydrophilic polymers investigated promoted emulsion formation to give an array of emulsions with different sizes and surface charges (Fig. 3B). From Fig. 3B, it is clear that the easiest emulsions to prepare are 100–350 nm in diameter with negative zeta potentials (lower left portion of plot) and positively charged emulsions are generally larger in size and display greater polydispersity (upper right portion of plot). Emulsions were stabilized by commercially available hydrophilic polymers (8–13), biomolecules (14–19), as well as custom conjugated polymers (20, 21).30 We analysed the stability of the emulsions over two months, and found that the long-term stability varies considerably (Fig. 3C–H and S1†). Emulsions stabilized by 11, 15, 16, and 17 had reasonable stabilities over time. Emulsions made from 8, 9, 14, 20 displayed steady Ostwald ripening31 over two months, while emulsions formed from 10, 18, and 21 only remained stable for a few days. The array of emulsions in Fig. 3B shows that for applications where emulsions can be immediately prepared and utilized, the options for obtaining functionalized perfluorocarbon emulsions from commercially available materials are plentiful.
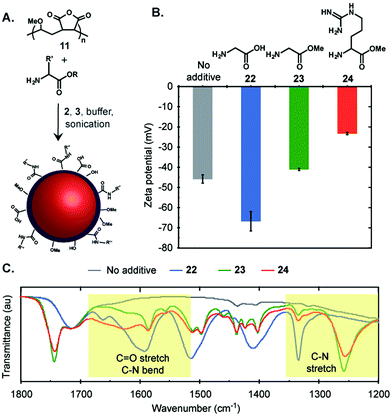
To further customize the surface functionality of the emulsions, we focused on poly(methyl vinyl ether-alt-maleic anhydride) (11) as a surfactant. If partially hydrolysed prior to emulsion formation, polymer 11 yielded stable, negatively charged emulsions that did not display significant Ostwald ripening over two months (Fig. 3C). We envisioned that the anhydride moiety could be opened by amine nucleophiles in situ to modify the surface composition and/or charge of the emulsions (Fig. 4A). This was demonstrated by the addition of glycine (22), methyl glycine (23), and methyl arginine (24) to the mixture of PFD, PFTPA, PBS, and 11 prior to sonication.32 We measured the changes in surface charge of the resulting emulsions and found that the emulsions formed in the presence of glycine were significantly more negatively charged (blue, Fig. 4B), methyl arginine were significantly more positively charged (red, Fig. 4B), and methyl glycine were only minimally changed (green, Fig. 4B). To further verify the covalent modification, the emulsions were dried and the remaining polymer surfactants were analysed by infrared spectroscopy (Fig. 4C). New absorbance peaks in the amide bond I and II region (1680 to 1515 cm−1)33 were observed corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches and C–N bends. Additionally, the amide bond III region34 from 1350 to 1200 cm−1 also exhibited new absorbance peaks resulting from the presence of C–N stretches. Collectively, these data suggest covalent modification of the fluorous emulsion surface. Further support of covalent modification is obtained when analogous experiments are performed with poly(acrylic acid) (9) and smaller changes in zeta potential and IR spectra are observed (Fig. S2†). The ability to modify poly(methyl vinyl ether-alt-maleic anhydride) nanoemulsions through in situ modification allows for facile custom functionalization without additional chemical steps, an advantage over many nanomaterials.
O stretches and C–N bends. Additionally, the amide bond III region34 from 1350 to 1200 cm−1 also exhibited new absorbance peaks resulting from the presence of C–N stretches. Collectively, these data suggest covalent modification of the fluorous emulsion surface. Further support of covalent modification is obtained when analogous experiments are performed with poly(acrylic acid) (9) and smaller changes in zeta potential and IR spectra are observed (Fig. S2†). The ability to modify poly(methyl vinyl ether-alt-maleic anhydride) nanoemulsions through in situ modification allows for facile custom functionalization without additional chemical steps, an advantage over many nanomaterials.
Another simple strategy for modification of the surface is exploiting the affinity of β-cyclodextrin and adamantane (Ka ∼ 105 M−1).35 Emulsions stabilized by poly(β-cyclodextrin) (8) have size and charge properties similar to Pluronic-F68 nanoemulsions, although they are more prone to Ostwald ripening (Fig. 3D). Nanoemulsions prepared from 8 were subjected to different adamantyl reagents (Fig. 5A). Poly(β-cyclodextrin) stabilized perfluorocarbon nanoemulsions were treated with varying amounts of 1-adamantylamine (25) or 1-adamantane carboxylic acid (26) and the surface charge was measured (Fig. 5B). A dose-dependent increase in zeta potential with 26 and decrease with 25 suggested successful non-covalent modification of the poly(β-cyclodextrin) stabilized emulsions. Only minimal changes in zeta potential are observed when the analogous experiment is performed with Pluronic-F68 nanoemulsions (Fig. S3†).
To convincingly conclude that non-covalent complexation was a robust strategy for perfluorocarbon emulsion surface functionalization, we conjugated 1-adamantanecarbonyl chloride to Lucifer yellow cadaverine to yield 27 (Fig. 5 and Scheme S1†). Fluorous emulsions formed from poly(β-cyclodextrin) as well as Pluronic-F68 were treated with varying amounts of 27, thrice washed by centrifugation and resuspension in buffer, and the photoluminescence of the nanoemulsions was measured (Fig. 5C). The poly(β-cyclodextrin) stabilized emulsions displayed dose-dependent fluorescence while the Pluronic-F68 control only displayed minimal background fluorescence. The bulk fluorescence data was confirmed by confocal microscopy (Fig. 5D). We expect the ability to non-covalently modify the surface of perfluorocarbon emulsions will be valuable for elaboration of the surface with sensitive small molecules, biomolecules, or targeting agents that are not amenable to sonication.
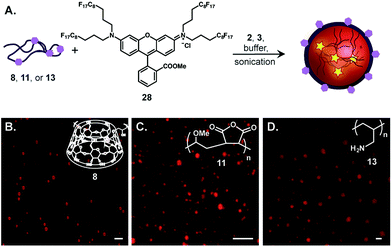
Finally, we combined our core and surface functionalization strategies to prepare multifunctional emulsions in one-pot. Emulsions loaded with fluorous rhodamine 2836 and stabilized by poly(β-cyclodextrin) (8), poly(methyl vinyl ether-alt-maleic anhydride) (11), and poly(allylamine) (13) were prepared by predissolving 28 in 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 PFD/PFTPA, introducing the polymer surfactant in PBS and emulsifying via sonication (Fig. 6A). The resulting perfluorocarbon emulsions were imaged by confocal microscopy (Fig. 6B–D). As evident from the microscopy, emulsion formation was observed for each polymer surfactant, demonstrating that the properties of the polymer do not influence the ability to encapsulate fluorous-tagged small molecules. Thus, we have devised a generalizable and modular strategy for differentially functionalized emulsion synthesis.
3 PFD/PFTPA, introducing the polymer surfactant in PBS and emulsifying via sonication (Fig. 6A). The resulting perfluorocarbon emulsions were imaged by confocal microscopy (Fig. 6B–D). As evident from the microscopy, emulsion formation was observed for each polymer surfactant, demonstrating that the properties of the polymer do not influence the ability to encapsulate fluorous-tagged small molecules. Thus, we have devised a generalizable and modular strategy for differentially functionalized emulsion synthesis.
We envision that the multifunctionality and simplicity of emulsion formation will be advantageous for a range of applications. The ability for emulsions to be stabilized by conjugated polymers (e.g.20, 21) make them of interest to the materials community where processing methods such as spray-on electronics are attractive. Both the surface tension and volatility differences between the fluorous and aqueous systems could be exploited to modulate assembly. Additionally, the fluid nature of the emulsions imparts opportunities for aqueous-based sensing schemes, particularly those involving particle interactions and fusions. Toward this end, the ability to make emulsions with complementary (e.g. positive emulsions 12, 13, or 17 and negative emulsions 9, 10, or 14) as well as stimuli-responsive (e.g. phosphorylation of 16 by kinase, degradation of 19 by heparanase) surface functionality is necessary.
The non-toxic nature of fluorous compounds37 coupled with the orthogonality of the fluorous phase provide many opportunities in biotechnology including targeted drug delivery and imaging. We expect emulsions stabilized by anhydride-containing 11 and modified in situ with targeting agents, or proteins containing protease-specific cleavage sequences to be of particular interest for these applications. Preliminary results indicate bovine serum albumin (BSA, 15)-stabilized emulsions undergo trypsin-dependent changes.38 Also highly relevant to biomedical applications is the ability to incorporate significant amounts of fluorophore inside the emulsions, which can rival the brightness of quantum dots in aqueous environments using a non-toxic platform.
For some of these applications, the long-term stability and/or size distribution of the emulsions could be a concern. In particular for biomedical applications, small emulsions, that are stable for months are necessary. Our current method to prepare true nanoscale emulsions39 is to increase the amount of surfactant; however, with this approach, the smaller the nanoemulsion the more Ostwald ripening occurs (Fig. S4†). Thus, a promising future direction is the development of custom polymers that allow for functionalizable, stable, perfluorocarbon nanoemulsions. Another avenue toward modifying the size/stability of the nanoemulsions is to change the method of emulsification to homogenization or microfluidics.8,40 The long-term stability of nanoscale emulsions is less imperative for applications in materials science and sensor development. We envision the emulsions reported herein to see immediate use in these areas.
Conclusions
We have developed methods to differentially functionalize the surface and core of emulsion droplets in one simple procedure. These emulsions can be prepared in ∼20 minutes through simple mixing and sonication of commercially available or custom reagents, depending on the desired application. The choice of fluorous solvent for the inner phase of the emulsions imparts stability and orthogonality in the presence of organic compounds, resulting in enhanced control over the encapsulation and release of small molecules as well as simple surface modification of the emulsions. Work towards the use of customized fluorous emulsions in sensing, bioimaging, and device fabrication is underway.Acknowledgements
E. M. S. was supported by a F32 Ruth L. Kirschstein National Research Service Award (NIH). A portion of this work was supported by the NSF, award ECCS-0939514.Notes and references
- Z. L. Wang, G. Zhu, Y. Yang, S. Wang and C. Pan, Mater. Today, 2012, 15, 532–543 CrossRef CAS
.
- M. J. Cima, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 355–378 CrossRef PubMed
.
-
(a) A. Fernandez-Fernandez, R. Manchanda and A. J. McGoron, Appl. Biochem. Biotechnol., 2011, 165, 1628–1651 CrossRef CAS PubMed
; (b) G. Bao, S. Mitragotri and S. Tong, Annu. Rev. Biomed. Eng., 2013, 15, 253–282 CrossRef CAS PubMed
.
-
(a)
V. Rotello, Nanoparticles: Building Blocks for Nanotechnology, Springer Science, USA, 2004 Search PubMed
; (b) A. Lopez-Serrano, R. M. Olivas, J. S. Landaluze and C. Camara, Anal. Methods, 2014, 6, 38–56 RSC
; (c) L. E. Euliss, J. A. DuPont, S. Gratton and J. DeSimone, Chem. Soc. Rev., 2006, 35, 1095–1104 RSC
.
-
J. M. de la Fuente and V. Grazu, Nanobiotechnology: Inorganic Nanoparticles vs. Organic Nanoparticles, Elsevier, Oxford, 2012 Search PubMed
.
- K. E. Sapsford, W. R. Algar, L. Berti, K. B. Gemmill, B. J. Casey, E. Oh, M. H. Stewart and I. L. Medintz, Chem. Rev., 2013, 113, 1904–2074 CrossRef CAS PubMed
.
- F. M. Winnik and D. Maysinger, Acc. Chem. Res., 2013, 46, 672–680 CrossRef CAS PubMed
.
- A. Maali and M. T. H. Mosavian, J. Dispersion Sci. Technol., 2013, 34, 92–105 CrossRef CAS
.
-
J. A. Gladysz, D. P. Curran and I. T. Horvath, Handbook of Fluorous Chemistry, Wiley-VHC, Weinheim, 2004 Search PubMed
.
-
(a) T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108–109, 303–318 CrossRef CAS PubMed
; (b) J. E. Brady and P. W. Carr, Anal. Chem., 1982, 54, 1751–1757 CrossRef CAS
.
-
(a)
K. L. Berry, US Pat. 2559750, July 1951
; (b) B. Luhmann and A. E. Feiring, Polymer, 1989, 30, 1723–1732 CrossRef CAS
.
-
(a)
R. J. Plunkett, US Pat. 2230654, Feb. 1941
; (b) M. M. Renfrew and E. E. Lewis, Ind. Eng. Chem., 1946, 38, 870–877 CrossRef CAS
; (c) C. A. Sperati and H. W. Starkweather, Jr, Fortschr. Hochpolym.-Forsch., 1961, 2, 465–495 CrossRef CAS
.
-
(a) C. U. Kim, J. M. Lee and S. K. Ihm, J. Appl. Polym. Sci., 1999, 73, 777–793 CrossRef CAS
; (b) S. D. Kimmins and N. R. Cameron, Adv. Funct. Mater., 2011, 21, 211–225 CrossRef CAS
; (c) H. B. Adel, B. Felix, K. Hintzer, G. L. Ohr, W. D. Mitterberger, US Pat. 5576381, Nov. 1996
; (d) H. S. Wu, US Pat. 5504170, Apr. 1996
.
-
(a) M. P. Krafft and J. G. Riess, Curr. Opin. Colloid Interface Sci., 2015, 20, 192–212 CrossRef CAS
; (b) M. M. Schultz, D. F. Barofsky and J. A. Field, Environ. Eng. Sci., 2003, 20, 487–501 CrossRef CAS
; (c) J. W. Martin, S. A. Mabury, K. R. Solomon and D. C. G. Muir, Environ. Toxicol. Chem., 2013, 32, 2421–2433 CrossRef CAS PubMed
; (d) N. Rich, New York Times Magazine, 2016 Search PubMed
.
-
L. W. McKeen, Fluorinated Coatings and Finishes Handbook, William Andrew, Norwich, 2006 Search PubMed
.
-
(a) V. M. Sadtler, F. Giulieri, M. P. Krafft and J. G. Riess, Chem. – Eur. J., 1998, 4, 1952–1956 CrossRef CAS
; (b) J. G. Riess, S. Pace and L. Zarif, Adv. Mater., 1991, 3, 249–251 CrossRef CAS
.
- R. A. Iezzi, S. Gaboury and K. Wood, Prog. Org. Coat., 2000, 40, 55–60 CrossRef CAS
.
-
(a) H. Sloviter and T. Kamimoto, Nature, 1967, 216, 1634–1638 CrossRef
; (b) R. Geyer, R. Monroe and K. Taylor, Fed. Proc., 1968, 27, 384 Search PubMed
.
- C. I. Castro and J. C. Briceno, Artif. Organs, 2010, 34, 622–634 Search PubMed
.
- S. I. Vorob'ev, Pharm. Chem. J., 2009, 43, 30–40 CrossRef
.
-
(a) J. M. Janjic and E. T. Ahrens, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 492–501 CrossRef CAS PubMed
; (b) E. T. Ahrens, R. Flores, H. Xu and P. A. Morel, Nat. Biotechnol., 2005, 23, 983–987 CrossRef CAS PubMed
; (c) D. Zhoa, L. Jiang, E. W. Hahn and R. P. Mason, Int. J. Radiat. Oncol., Biol., Phys., 2005, 62, 872–880 CrossRef PubMed
; (d) T. K. Hitchens, Q. Ye, D. F. Eytan, J. M. Janjic, E. T. Ahrens and C. Ho, Magn. Reson. Med., 2011, 65, 1145–1154 CrossRef PubMed
; (e) P. Boehm-Strum, L. Mengler, S. Wecker, M. Hoehn and T. Kallur, PLoS One, 2011, 6, 1–9 Search PubMed
; (f) U. Flogel, Z. Ding, H. Hardung, S. Jander, G. Reichmann, C. Jacoby, R. Schubert and J. Schrader, Circulation, 2008, 118, 140–148 CrossRef PubMed
; (g) A. M. Morawski, P. M. Winter, X. Yu, R. W. Fuhrop, M. J. Scott, F. Hockett, J. D. Robertson, P. J. Gaffney, G. M. Lanza and S. A. Wickline, Magn. Reson. Med., 2004, 52, 1255–1262 CrossRef CAS PubMed
; (h) J. M. Janjic, M. Srinivas, D. K. K. Kadayakkara and E. T. Ahrens, J. Am. Chem. Soc., 2008, 130, 2832–2841 CrossRef CAS PubMed
; (i) Y. T. Lim, Y. W. Noh, J. N. Kwon and B. H. Chung, Chem. Commun., 2009, 6952–6954 RSC
; (j) Y. T. Lim, M. Y. Cho, J. H. Kang, Y. W. Noh, J. H. Cho, K. S. Hong, J. W. Chung and B. H. Chung, Biomaterials, 2010, 31, 4964–4971 CrossRef CAS PubMed
; (k) K. C. Partlow, J. Chen, J. A. Brant, A. M. Neubauer, T. E. Meyerrose, M. H. Creer, J. A. Nolta, S. D. Caruthers, G. M. Lanza and S. A. Wickline, FASEB J., 2007, 21, 1647–1654 CrossRef CAS PubMed
; (l) M. Srinivas, L. J. Cruz, F. Bonetto, A. Heerschap, C. G. Figdor and I. J. M. de Vries, Biomaterials, 2010, 31, 7070–7077 CrossRef CAS PubMed
; (m) S. K. Patel, M. J. Patrick, J. A. Pollock and J. M. Janjic, J. Biomed. Opt., 2013, 18, 101312-1–101312-8 CrossRef PubMed
; (n) A. A. Kislukhin, H. Xu, S. R. Adams, K. H. Narsinh, R. Y. Tsien and E. T. Ahrens, Nat. Mater., 2016 DOI:10.1038/nmat4585
.
- M. J. Patrick, J. M. Janjic, H. Teng, M. R. O'Hear, C. W. Brown, J. A. Stokum, B. F. Schmidt, E. T. Ahrens and A. S. Waggoner, J. Am. Chem. Soc., 2013, 135, 18445–18457 CrossRef CAS PubMed
.
- J.-P. Jee, M. C. Parlato, M. G. Perkins, S. Mecozzi and R. A. Pearce, Anesthesiology, 2012, 116, 580–585 CrossRef PubMed
.
- K. C. Lowe, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1987, 87, 825–838 CrossRef CAS
.
- I. T. Horvath and J. Rabai, Science, 1994, 266, 72–75 CAS
.
-
(a) E. de Wolf, G. van Koten and B. J. Deelman, Chem. Soc. Rev., 1999, 28, 37–41 RSC
; (b) D. P. Curran and Z. Luo, J. Am. Chem. Soc., 1999, 121, 9069–9072 CrossRef CAS
; (c) W. Zhang, Chem. Rev., 2009, 109, 749–795 CrossRef CAS PubMed
; (d) L. P. Barthel-Rosa and J. A. Gladysz, Coord. Chem. Rev., 1999, 190–192, 587–605 CrossRef CAS
.
- See ChemFiles https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Fluka/Application_Notes/al_chemfile_v4_no9.pdf for commercially available fluorous compounds.
- Assuming a 200 nm diameter nanoemulsion particle, the volume of each particle is 4.19 × 10−18 L (from V = 4/3πr3). The concentration of small molecule in the fluorous phase is 0.0385 M (for the highest concentration samples). Multiplying these values and Avogadro's number results in the number of molecules per an emulsion particle.
-
(a)
I. T. Horvath, Topics in Current Chemistry, Fluorous Chemistry, Springer Science, Berlin, 2012 Search PubMed
; (b) W. Zhang and D. P. Curran, Tetrahedron, 2006, 62, 11837–11865 CrossRef CAS PubMed
; (c) I. T. Horvath, Acc. Chem. Res., 1998, 31, 641–650 CrossRef CAS
.
-
(a) B. VanVeller, K. Miki and T. M. Swager, Org. Lett., 2010, 12, 1292–1295 CrossRef CAS PubMed
; (b) D. Izuhara and T. M. Swager, J. Am. Chem. Soc., 2009, 131, 17724–17725 CrossRef CAS PubMed
.
- A. S. Kabalnov and E. D. Shchukin, Adv. Colloid Interface Sci., 1992, 38, 69–97 CrossRef CAS
.
- It should be noted that 11 is pre-incubated in buffer with mild (bath) sonication until it is water-soluble. Thus, some anhydride moieties are hydrolysed and others are functionalized with the amine. See ESI† for further details.
-
R. M. Silverstein, F. X. Webster and D. J. Kiemle, Spectrometric Identification of Organic Compounds, John Wiley & Sons, Hoboken, 2005 Search PubMed
.
- K. Koichi, T. Matsui and S. Tanaka, Appl. Spectrosc., 1987, 41, 180–184 CrossRef
.
- R. F. Gomez-Biagi, R. B. C. Jagt and M. Nitz, Org. Biomol. Chem., 2008, 6, 4622–4626 CAS
.
- E. M. Sletten and T. M. Swager, J. Am. Chem. Soc., 2014, 136, 13574–13577 CrossRef CAS PubMed
.
- Only long chain perfluorocarbons are a concern for bioaccumulation. See:
(a) M. M. Schultz, D. F. Barofsky and J. A. Field, Environ. Eng. Sci., 2003, 20, 487–501 CrossRef CAS
; (b) J. W. Martin, S. A. Mabury, K. R. Solomon and D. C. G. Muir, Environ. Toxicol. Chem., 2003, 22, 196–204 CrossRef CAS PubMed
.
- BSA nanoemulsions display a trypsin-dependent increase in size, which we hypothesized to be due to the degradation of the stabilizing protein and the need for more fluorous solvent to be contained in each droplet.
- A. Lopez-Serrano, R. M. Olivas, J. S. Landaluze and C. Camara, Anal. Methods, 2014, 6, 38–56 RSC
.
-
(a) M. Y. Koroleva and E. V. Yurtov, Russ. Chem. Rev., 2012, 81, 21–43 CrossRef CAS
; (b) D. J. McClements, Soft Matter, 2012, 8, 1719–1729 RSC
; (c) A. A. Date, N. Desai, R. Dixit and M. Nagarsenker, Nanomedicine, 2010, 5, 1595–1616 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S4, Scheme S1, detailed experimental procedures, characterization of 27. See DOI: 10.1039/c6sc00341a |
| ‡ Current address: Department of Chemistry and Biochemistry, University of California, Los Angeles, 607 Charles E. Young Dr East, Los Angeles, CA 90095. Email: E-mail: sletten@chem.ucla.edu |
| This journal is © The Royal Society of Chemistry 2016 |