Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances and mechanistic insight on the catalytic Mitsunobu reaction using recyclable azo reagents†
Daisuke
Hirose
a,
Martin
Gazvoda
b,
Janez
Košmrlj
*b and
Tsuyoshi
Taniguchi
*c
aGraduate School of Natural Science and Technology, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan
bFaculty of Chemistry and Chemical Technology, University of Ljubljana, Večna pot 113, SI-1000, Ljubljana, Slovenia. E-mail: janez.kosmrlj@fkkt.uni-lj.si
cSchool of Pharmaceutical Sciences, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan. E-mail: tsuyoshi@p.kanazawa-u.ac.jp
First published on 13th April 2016
Abstract
Ethyl 2-arylhydrazinecarboxylates can work as organocatalysts for Mitsunobu reactions because they provide ethyl 2-arylazocarboxylates through aerobic oxidation with a catalytic amount of iron phthalocyanine. First, ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate has been identified as a potent catalyst, and the reactivity of the catalytic Mitsunobu reaction was improved through strict optimization of the reaction conditions. Investigation of the catalytic properties of ethyl 2-arylhydrazinecarboxylates and the corresponding azo forms led us to the discovery of a new catalyst, ethyl 2-(4-cyanophenyl)hydrazinecarboxylates, which expanded the scope of substrates. The mechanistic study of the Mitsunobu reaction with these new reagents strongly suggested the formation of betaine intermediates as in typical Mitsunobu reactions. The use of atmospheric oxygen as a sacrificial oxidative agent along with the iron catalyst is convenient and safe from the viewpoint of green chemistry. In addition, thermal analysis of the developed Mitsunobu reagents supports sufficient thermal stability compared with typical azo reagents such as diethyl azodicarboxylate (DEAD). The catalytic system realizes a substantial improvement of the Mitsunobu reaction and will be applicable to practical synthesis.
Introduction
Many reactions have been utilized as important tools in synthetic organic chemistry. “Name reactions”, such as Wittig, Suzuki–Miyaura, and Mitsunobu, to name just a few, have an outstanding utility that has influenced broad fields of academia and industry.1 In view of economic and environmental concerns however, many of these synthetic methods suffer from serious limitations, diminishing their practical applicability. Therefore, substantial improvements of known synthetic protocols are currently an important subject in chemistry.Indeed, the Mitsunobu reaction is a typical example including both a wide utility and serious drawbacks.2 The reaction is one of the oxidation–reduction condensations reported by Mitsunobu and co-workers in 1967.3 Since then, it has been widely used for the substitution of hydroxyl groups or inversion of the stereochemistry of secondary alcohols. Typically, diethyl azodicarboxylate (DEAD) and triphenylphosphine are employed as the oxidant and reducing agent in the Mitsunobu reaction, but production of a large amount of waste, i.e., diethyl hydrazinedicarboxylate and triphenylphosphine oxide, is unavoidable. These byproducts often contaminate the desired product. In addition, DEAD is hazardous due to its toxicity and potential explosiveness. As a result, the use of the Mitsunobu reaction tends to be avoided in practical synthesis on plant scales.4
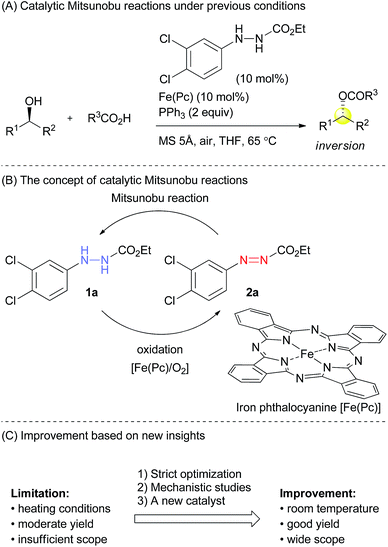
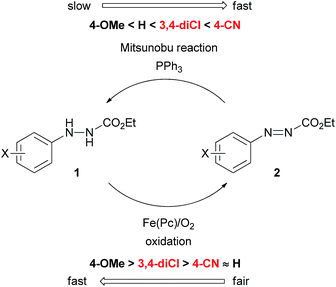
Several modified methods have been developed to facilitate the removal of the waste generated by the Mitsunobu reaction.5 However, there has been no substantial approach to reducing the problematic waste in the Mitsunobu reaction until the report on the catalytic Mitsunobu reaction by Toy in 2006.6 Toy succeeded in reducing DEAD in the Mitsunobu reaction to a catalytic amount (10 mol%) by employing a sacrificial oxidative reagent, i.e., iodobenzene diacetate. Recently, Mitsunobu-type reactions without azo reagents were reported.7 In 2013, we reported the second example of the catalytic Mitsunobu reaction with azo reagents that are recyclable through aerobic oxidation with iron phthalocyanine (Fig. 1A).8 Ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a) has been tentatively identified as the best catalyst. A catalytic concept of this reaction is beneficial from the viewpoint of green chemistry because atmospheric oxygen is economically and environmentally ideal as a sacrificial oxidant to generate a reactive azo form 2a (Fig. 1B). However, the scope of substrates and product yields were still moderate, and the reaction required heating conditions to obtain the products in acceptable yields. Thus, the applicability of the method was still inferior to that of the original Mitsunobu reaction.
The effect of substituents on the aromatic ring of the hydrazine catalysts was drastic. Clearly, electronic properties of catalysts affected both the Mitsunobu reactivity of the azo form as well as the aerobic oxidation of the hydrazine form. At first glance, these seem incompatible because electron-withdrawing groups would promote the addition reaction of triphenylphosphine to the azo form but would suppress oxidation of the hydrazine form to the azo form. In the case of electron-donating groups there is the same dilemma, though the situation is interchanged. We presumed that the 3,4-dichlorophenyl group had an electronic property that made the two processes moderately compatible.
Quite recently, we have reported a detail of the aerobic oxidation process of 2-arylhydrazinecarboxylates with iron phthalocyanine, indicating two important observations.9 First, the oxidation process was promoted in apolar solvents such as toluene or dichloromethane, and second, electron-withdrawing substituents at the aryl group did not suppress the hydrazine-to-azo compound oxidation. Interestingly, halogen atoms at the para-position rather promoted the reaction. Thus, this study provided us important insights to improve the catalytic Mitsunobu reaction.
Providing the serious limitations indicated in Fig. 1C are avoided, the catalytic Mitsunobu reaction will gain a large potential in practical synthesis.10 In this paper, we describe new advances in our catalytic Mitsunobu reaction including substantial improvement of the reaction and insights into the reaction mechanism.
Results and discussion
Strict optimization of the reaction conditions
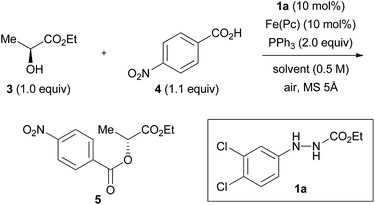
We previously found that the combination of ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a) and iron phthalocyanine [Fe(Pc)] formed an optimum catalytic system (both 10 mol%), and that addition of activated molecular sieves was required to induce the reaction (vide infra).8 We tentatively improved the yields of the products by using 3,5-dinitrobenzoic acid as a nucleophile when secondary alcohols were used as substrates.8 We employed a model reaction between (S)-ethyl lactate (3, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er) and 4-nitrobenzoic acid (4) using this catalytic system to strictly optimize the conditions. The reaction between 3 and 4 in heating THF (65 °C) gave ester product 5 in 50% yield and in 97% inversion (Table 1, entry 1).
1 er) and 4-nitrobenzoic acid (4) using this catalytic system to strictly optimize the conditions. The reaction between 3 and 4 in heating THF (65 °C) gave ester product 5 in 50% yield and in 97% inversion (Table 1, entry 1).
| Entry | Solvent | Temp. (°C) | Time (h) | Yield (%) | Er |
|---|---|---|---|---|---|
| a Reaction conditions: 3 (1.0 mmol), 4 (1.1 mmol), catalyst 1a (0.10 mmol), Fe(Pc) (0.10 mmol), PPh3 (2.0 mmol), solvent (2 mL), MS 5 Å (500 mg) under air atmosphere. MS 5 Å was activated by heating using a heat gun (ca. 450 °C) in vacuo (ca. 0.1 mmHg) for 5 min. b Under reflux. | |||||
| 1 | THF | 65 | 24 | 50 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 2 | 1,4-Dioxane | 65 | 24 | 46 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 3 | CPME | 65 | 24 | 70 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 4 | MTBE | 55b | 24 | 76 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | DME | 65 | 24 | 40 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
| 6 | MeCN | 65 | 24 | 14 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 81 |
| 7 | n-Hexane | 65 | 24 | 69 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Toluene | 65 | 24 | 74 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 9 | Toluene | 110b | 12 | 78 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
| 10 | Toluene | rt | 29 | 88 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | CPME | rt | 36 | 75 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | CHCl3 | 62b | 24 | 80 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
| 13 | CH2Cl2 | rt | 48 | 75 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88 |
| 14 | PhCl | 65 | 24 | 70 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
| 15 | PhCF3 | 65 | 24 | 75 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
In the previous study, the effect of solvents was investigated at a very preliminary stage using unoptimized catalysts.11 We could not find a large effect of the solvents at that time, and thereby, the effects of solvents and temperature were re-investigated using the optimum catalytic system (Table 1).12 Ether solvents such as 1,4-dioxane, cyclopentyl methyl ether (CPME)13 and tert-butyl methyl ether (MTBE), except for dimethoxyethane (DME), provided product 5 in a high inversion ratio (entries 2–5), whereas acetonitrile gave a contrasting result (entry 6).14 Reactions in hydrocarbon solvents such as n-hexane and toluene at 65 °C afforded good results (entries 7 and 8). However, chlorinated solvents gave product 5 in a low inversion ratio, though the total product yield was good (entries 12–14). This drastic change in the results was attributed to the presence of chlorine atoms in the solvent, and is based on the fact that the reaction in α,α,α-trifluorotoluene15 provided similar results to those in toluene (entry 15). The enantiomeric ratio was sensitive to temperature in the reaction in toluene (entries 8–10). To our delight, the reaction in toluene at room temperature provided product 5 in an excellent yield (88%) and in a perfect inversion ratio. CPME also gave a relatively good result for the reaction at room temperature. The reactions were basically clean. In the case of low yields of the product, the starting materials remained unconsumed.
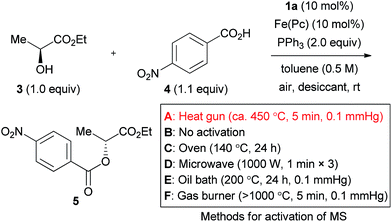
The effect of molecular sieves was drastic, and no reaction was induced in their absence (Table 2, entry 1).16 This is likely due to the high moisture sensitivity of the intermediate generated from the azo form of catalyst 1a and triphenylphosphine. Molecular sieves would serve for removing residual moisture as well as water generated by the iron-catalyzed aerobic oxidation of the hydrazine catalyst. The use of at least 500 mg MS 5 Å (1.0 mmol scale), activated by heating with a heat gun (ca. 450 °C) under reduced pressure (ca. 0.1 mmHg), was desirable to obtain product 5 in a good yield (entries 2–5). MS 4 Å and MS 3 Å were ineffective in the present reaction (entries 6 and 7).
| Entry | Desiccant | Amount (mg mmol−1) | Yield (%) | Er |
|---|---|---|---|---|
| a Reaction conditions: 3 (1.0 mmol), 4 (1.1 mmol), catalyst 1a (0.10 mmol), Fe(Pc) (0.10 mmol), PPh3 (2.0 mmol), toluene (2 mL), desiccant (0–1000 mg) for 24–48 h at room temperature under air atmosphere. Methods for activation of molecular sieves: A: heated using a heat gun (ca. 450 °C) in vacuo (ca. 0.1 mmHg) for 5 min; B: not activated; C: heated in an oven (140 °C) for 24 h; D: heated using a microwave (1000 W for 1 min, three times); E: heated using an oil bath (200 °C) in vacuo (ca. 0.1 mmHg) for 24 h; F: heated using a gas burner (>1000 °C) in vacuo (ca. 0.1 mmHg) for 5 min. | ||||
| 1 | None | — | 0 | — |
| 2 | MS 5 Å (A) | 100 | 32 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | MS 5 Å (A) | 300 | 77 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | MS 5 Å (A) | 500 | 88 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | MS 5 Å (A) | 1000 | 95 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | MS 4 Å (A) | 500 | 32 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 7 | MS 3 Å (A) | 500 | 26 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 8 | MS 5 Å (B) | 500 | 13 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 9 | MS 5 Å (C) | 500 | 16 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 10 | MS 5 Å (D) | 500 | 25 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 11 | MS 5 Å (E) | 500 | 67 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 12 | MS 5 Å (F) | 500 | 94 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | Na2SO4 | 500 | 0 | — |
| 14 | CaSO4 | 500 | 0 | — |
| 15 | MgSO4 | 500 | 0 | — |
Various “traditional methods” for the activation of molecular sieves are used in many laboratories. Representative activation methods were tested to assure a reliable experimental procedure. The use of MS 5 Å without activation gave the product in a very poor yield (entry 8). MS 5 Å heated for 24 h at 140 °C in an oven were also ineffective (entry 9). Although heating using a microwave is sometimes used for activation of molecular sieves, this method did not afford a good result in the present reaction (entry 10). When the reaction was tested with MS 5 Å activated through heating at 200 °C with an oil bath under reduced pressure (ca. 0.1 mmHg), the product yield was still insufficient (entry 11). Heating using a flame under reduced pressure would be a strict method for activation of molecular sieves, and this method provided product 5 in an excellent 94% yield (entry 12). As a result, and from the viewpoints of safety and convenience, we consider the activation with a heat gun as the method of choice. Incidentally, sulfate salts did not work as a desiccant in the reaction (entries 13–15).
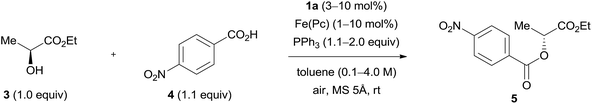
The concentration of the reactants is likely to affect the product yield (Table 3, entries 1–4 and 7). The reaction was promoted and gave improved yields of product 5 in high concentrations (2.0 M or 4.0 M) (entries 4 and 7). When the amount of triphenylphosphine was decreased to 1.5 equivalent in the reaction in high concentration (2.0 M or 4.0 M), a good yield was maintained in this model reaction (entries 5 and 8). However, the use of a lower amount (1.1 equiv.) of triphenylphosphine diminished the yield of product 5 (entries 6 and 9). High concentration conditions would be beneficial to a practical synthesis because the solvent can be saved. The good result was reproducible in a scale-up experiment (10 mmol), though the reaction time was somewhat prolonged (entry 7, results in parentheses). Triphenylphosphine is sometimes replaced with trialkylphosphines because they often provide good results due to their high nucleophilicity.17 We tested a representative reaction with tri-n-butylphosphine, but the result was very poor (entry 8, results in parentheses). TLC analysis of the reaction mixture implied decomposition of the iron phthalocyanine presumably through strong coordination with the tri-n-butylphosphine. When most of the triphenylphosphine was consumed in the reaction, the Mitsunobu catalyst was detected as the azo form using TLC. The latter was easily recovered in 80–90% yield using silica gel chromatography due to its low polarity. The hydrazine form of the catalyst, if it remained in the reaction mixture, usually did not cause problems in the purification of the product. Finally, iron phthalocyanine could be easily removed using filtration of the reaction mixture through a pad of Celite® or filter paper. The impact of decreasing the amount of hydrazine catalyst 1a seemed to be larger than that of decreasing the amount of iron phthalocyanine (entries 10–15). It is noteworthy that good results were maintained with as low as 1 mol% of iron phthalocyanine (entries 11 and 12) indicating that its amount can be flexibly changed depending on the substrates or situations of the reactions. No reaction was induced in the absence of the iron catalyst.8,9
| Entry | 1a (mmol%) | Fe(Pc) (mmol%) | PPh3 (equiv.) | Conc. (M) | Time (h) | Yield (%) | Er |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 3 (1.0 mmol), 4 (1.1 mmol), 1a (0.10, 0.050 or 0.030 mmol), Fe(Pc) (0.10, 0.050, 0.030 and 0.010 mmol), PPh3 (2.0, 1.5 or 1.1 mmol), toluene (10, 2, 1, 0.5 or 0.25 mL), MS 5 Å (500 mg) at room temperature under air atmosphere unless otherwise noted. MS 5 Å was activated by heating using a heat gun (ca. 450 °C) in vacuo (ca. 0.1 mmHg) for 5 min. b The reaction was performed on the 10 mmol scale. c PBu3 was used instead of PPh3. | |||||||
| 1 | 10 | 10 | 2.0 | 0.1 | 52 | 80 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | 10 | 10 | 2.0 | 0.5 | 29 | 88 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 10 | 10 | 2.0 | 1.0 | 18 | 91 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 4 | 10 | 10 | 2.0 | 2.0 | 14 | 97 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | 10 | 10 | 1.5 | 2.0 | 14 | 91 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 10 | 10 | 1.1 | 2.0 | 12 | 68 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 10 | 10 | 2.0 | 4.0 | 12 (24)b | 93 (88)b | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (99 1 (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)b 1)b |
| 8 | 10 | 10 | 1.5 | 4.0 | 12 (48)c | 92 (10)c | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (46 1 (46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54)c 54)c |
| 9 | 10 | 10 | 1.1 | 4.0 | 12 | 76 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 10 | 5 | 1.5 | 4.0 | 21 | 84 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 10 | 1 | 1.5 | 4.0 | 18 | 81 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 10 | 1 | 2.0 | 4.0 | 36 | 89 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 5 | 10 | 1.5 | 4.0 | 38 | 78 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 5 | 5 | 1.5 | 4.0 | 24 | 76 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 3 | 3 | 1.5 | 4.0 | 39 | 68 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Kinetic properties of the ethyl 2-arylazocarboxylates
The catalytic cycle between the hydrazines and azo compounds would affect the efficiency of the formation of an alkoxyphosphonium intermediate to provide the final product. We conducted kinetic experiments to investigate the substituent effect of azo compounds 2b–j in the reaction with triphenylphosphine (Fig. 2). The analysis of a mixture of 2b–j and triphenylphosphine (10 equiv.) in CDCl3 using 1H NMR spectroscopy revealed the presence of some starting azo compounds after 10 hours. In contrast, in an independent experiment, 1H NMR analysis showed that DEAD immediately disappeared under the same reaction conditions, indicating an irreversible process in this case.18 Obviously, the addition of triphenylphosphine to ethyl 2-arylazocarboxylates is reversible, and the formation of adducts is less favorable as compared to DEAD. Therefore, reaction rates were estimated from the model reaction of azo compounds 2b–j (50 mM) with excessive amounts (10 equiv.) of triphenylphosphine and water in THF at 25 °C. The reactions were monitored by measuring the absorbance of the azo compounds 2b–j at λ = 419–450 nm. Rate constants were calculated from plots of a pseudo-first-order dependence.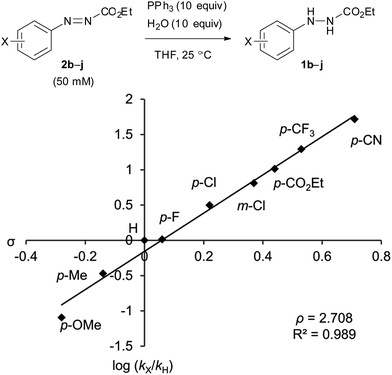 | ||
| Fig. 2 The Hammett plot of the reactions of ethyl 2-arylazocarboxylates with PPh3 in the presence of water. b: p-OMe, c: p-Me, d: H, e: p-F, f: p-Cl, g: m-Cl, h: p-CO2Et, i: p-CF3, j: p-CN. | ||
The Hammett plot for these reactions shows a linear fit with a relatively large positive slope value of ρ = +2.71 (Fig. 2). The value is close to that of the alkaline hydrolysis of benzoate esters (ρ = +2.51).19 The result reflects a dependence of the electronic density at the aromatic ring of azo compounds in the rate of the addition reaction of triphenylphosphine. Ethyl 2-(3,4-dichlorophenyl)azocarboxylate (2a) was also applied to the kinetic experiment, and its reaction rate (kobs = 8.5 × 10−2 min−1) was approximately 13.7 times faster than that of ethyl 2-phenylazocarboxylate (2d, kobs = 6.2 × 10−3 min−1). In addition, it is still 2.3 times faster compared to that of ethyl 2-(3-chlorophenyl)azocarboxylate (2g, kobs = 3.75 × 10−2 min−1). This supports the high reactivity of 2a in the catalytic Mitsunobu reaction.
When benzoic acid or 4-nitrobenzoic acid (each 10 equiv.) were added to the reaction system with 2d, only a minor impact to the reaction rate was noted (2d with benzoic acid: kobs = 7.1 × 10−3 min−1; 2d with 4-nitrobenzoic acid: kobs = 6.8 × 10−3 min−1). This observation supports that the model reaction reflects the reactivity of azo compounds toward triphenylphosphine and indicates that acids do not kinetically affect the reaction.
The kinetics of the catalytic aerobic oxidation of ethyl 2-arylhydrazinecarboxylates (1) with iron phthalocyanine basically show zero-order dependence, but the substituent effect is of irregular tendency probably due to the participation of radical species in the mechanism.9 The reaction rates of aerobic oxidation of ethyl 2-(4-chlorophenyl)hydrazinecarboxylate (1f) and ethyl 2-(4-bromophenyl)hydrazinecarboxylate to the corresponding azo compounds are approximately 1.5 times faster than that of ethyl 2-phenylhydrazinecarboxylate (1d).9 In the model reaction, in dichloromethane as a solvent, the aerobic oxidation of ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a) with iron phthalocyanine is completed within 2 hours. This is clearly faster than the oxidation (4 hours)9 of ethyl 2-phenylhydrazinecarboxylate (1d), though the kinetics of the reaction of 1a do not show a clear zero-order dependence (Fig. S14 in the ESI†). Thus, the 4-chlorine atom on the aromatic ring of 1a promotes oxidation to the corresponding azo form 2a by stabilization of the intermediary radical species, whereas the 3-chlorine atom of azo compound 2a contributes to an increased electrophilicity by its inductive effect. This is the reason why azo compound 2a operates as a good catalyst in the catalytic Mitsunobu reaction. In short, two processes involving Mitsunobu activity and hydrazine re-oxidation are compatible through the 3,4-dichlorophenyl group (Fig. 3). The catalytic activity of ethyl 2-(4-chlorophenyl)hydrazinecarboxylate (1f) was insufficient under the optimal conditions compared to that of 1a (Fig. 4).
Given the above considerations, ethyl 2-arylhydrazinecarboxylates with strong electron-withdrawing groups on the aromatic ring should be more effective catalysts as these groups should promote the Mitsunobu reaction without significantly suppressing the aerobic oxidation process. For instance, as monitored using NMR spectroscopy, the aerobic oxidation of ethyl 2-(4-cyanophenyl)hydrazinecarboxylate (1j) was completed within 5 hours, which was roughly the same reaction time as that of ethyl 2-phenylhydrazinecarboxylate (1d) (ca. 4 hours).9 On the other hand, higher electrophilicity of 2-(4-cyanophenyl)azocarboxylate (2j) over 3,4-dichlorophenyl derivative 2a is consistent with the higher (3.8 times) reaction rates of 2j (kobs = 3.2 × 10−1 min−1) over 2a (Fig. 3). This suggested that ethyl 2-(4-cyanophenyl)hydrazinecarboxylate (1j) might work as a good catalyst in the catalytic Mitsunobu reaction.
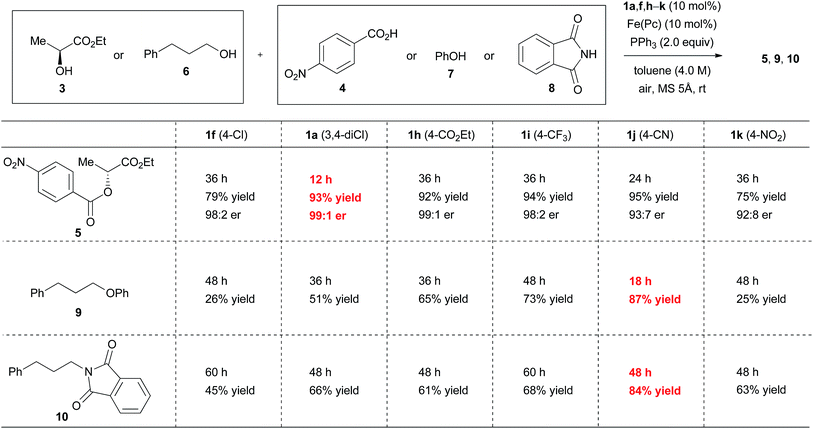
When ethyl 2-(4-cyanophenyl)hydrazinecarboxylate (1j) was used in the reaction between (S)-ethyl lactate (3) and 4-nitrobenzoic acid (4) under optimal conditions, product 5 was obtained in an excellent yield, although with a slightly decreased inversion ratio (Fig. 4). On the other hand, when phenol (7) or phthalimide (8) was used as the reaction partner of 3-phenylpropanol (6), both reactions using 1j provided better results (87% and 84% yields) than the reactions with 1a (51% and 66% yields). Although 2-(4-nitrophenyl)hydrazinecarboxylate (1k) should generate a strongly electrophilic azo compound,20 the results with this catalyst were disappointing. Gradual decomposition of 1k or its azo form was observed in the reaction with triphenylphosphine using 1H NMR analysis, which appears to be the main reason for the poor results.21
The reaction rate of ethyl 2-[4-(ethoxycarbonyl)phenyl]azocarboxylate (2h, kobs = 6.4 × 10−2 min−1) and ethyl 2-[4-(trifluoromethyl)phenyl]azocarboxylate (2i, kobs = 1.2 × 10−1 min−1) with triphenylphosphine was roughly close to that of 2a. Good yields of ester 5 were obtained in the reaction between (S)-ethyl lactate (3) and 4-nitrobenzoic acid (4) using the hydrazine forms 1h and 1i as a catalyst, but reaction times were prolonged (Fig. 4). When phenol (7) or phthalimide (8) were used as a nucleophile in the reaction with 3-phenylpropanol (6), catalysts 1h and 1i did not provide better results than catalyst 1j, though catalyst 1i showed somewhat improved results compared with catalyst 1a. Thus, catalyst 1h showed reactivity similar to that of 1a, and the position of reactivity for catalyst 1i is likely to lie between 1a and 1j. These trends are consistent with the results of the Hammett study. Incidentally, when model experiments of iron-catalyzed aerobic oxidation of 1h and 1i were conducted in dichloromethane, the reactions were completed at 4 h and 6 h, respectively (see the ESI†). The trend of the oxidation process is similar to that of other hydrazide derivatives.9
The above results imply that there is no perfect catalyst for the catalytic Mitsunobu reaction. Instead two catalysts can complement each other. In short, ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a) would be suitable for the reactions of carboxylic acids whereas 2-(4-cyanophenyl)hydrazinecarboxylate (1j) could serve for the reactions of other nucleophiles except for carboxylic acids.
Scope of substrates using the optimized protocol
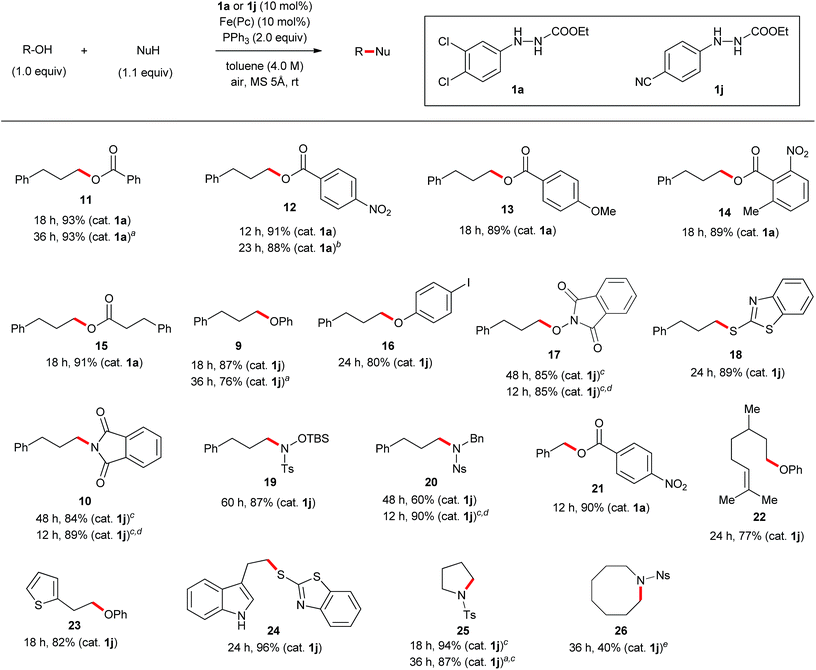
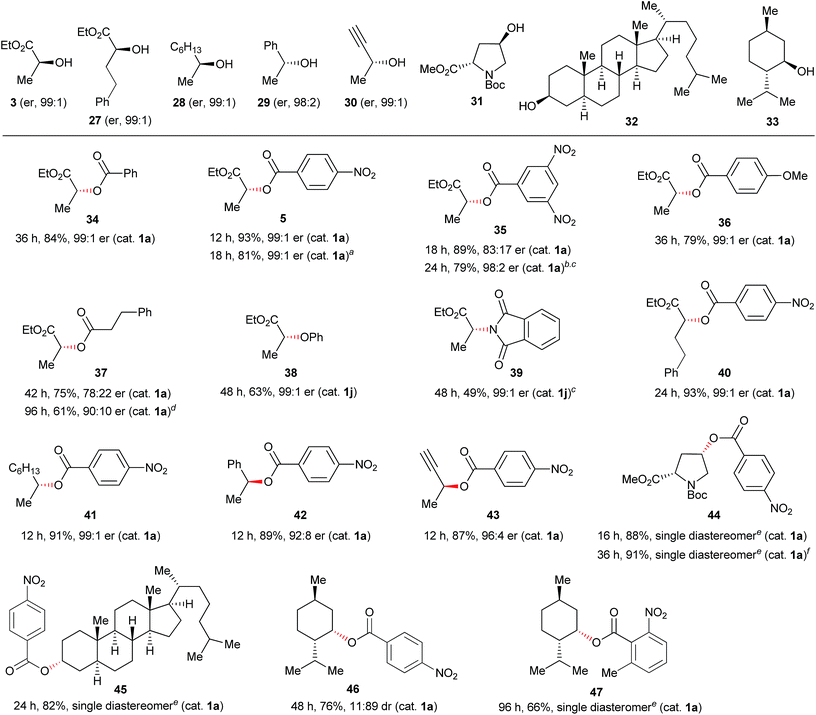
The discovery of new catalyst 1j largely expanded the scope of the catalytic Mitsunobu reaction. Fig. 5 shows the results of catalytic Mitsunobu reactions applying catalyst 1a or 1j to various substrates. Typically, the reactions were performed under the optimal conditions that provided the best result (Table 3, entry 7), but more practical conditions (e.g., Table 3, entry 11) were also applicable to several substrates. Reactions between 3-phenylpropanol and various carboxylic acids with catalyst 1a provided the corresponding esters 11–15 in excellent yields. The reaction of the alcohol with phenols gave the corresponding ethers 9 and 16 in improved yields when catalyst 1j was employed. An iodine atom was intact under the present conditions in the reaction of 4-iodophenol to give 16. N-Hydroxyphthalimide also worked as a good nucleophile to give an O-alkylated product 17 in the presence of catalyst 1j. Similarly, a sulfur nucleophile (2-mercaptobenzothiazole) underwent the Mitsunobu reaction with the alcohol to give the corresponding alkylated sulfide 18 in a good yield. Reactions of the alcohol with representative nitrogen nucleophiles were tested using catalyst 1j and produced alkylated phthalimide 10, and sulfonylamides 19 (ref. 22) and 20 (ref. 23) in good yields. Reactions with phthalimides and the nosylamide needed to be performed in 0.5 M solution due to the solubility issues. In such cases, heating the reaction mixture at 65 °C improved the results in reaction time and product yield. Alcohols sensitive to oxidative conditions were tested with several nucleophiles and were transformed into the corresponding Mitsunobu products 21–24 in good yields. It is noteworthy that a trisubstituted olefin, a thiophene and an indole were intact under the aerobic oxidation conditions. The catalytic Mitsunobu reaction using catalyst 1j was applicable to intramolecular reactions of alkyl sulfonamides having a hydroxyl group to give the corresponding cyclic amines 25 and 26 (ref. 23b) in reasonable yields.Next, various combinations of secondary alcohols and nucleophiles were tested (Fig. 6). Reactions of (S)-ethyl lactate (3) with several aromatic carboxylic acids gave the corresponding esters 34–36 in good yields with almost full inversion of stereochemistry. The reaction of alcohol 3 with 3,5-dinitrobenzoic acid in toluene gave ester 35 in a moderate level of enantioenrichment (er, 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17). The reaction of 3,5-dinitrobenzoic acid, under the previous conditions (in THF at 65 °C) provided 35 in a higher level of enantioenrichment.8 In the reaction of 3 with 3-phenylpropionic acid, the enantioenrichment of ester 37 was not good (er, 78
17). The reaction of 3,5-dinitrobenzoic acid, under the previous conditions (in THF at 65 °C) provided 35 in a higher level of enantioenrichment.8 In the reaction of 3 with 3-phenylpropionic acid, the enantioenrichment of ester 37 was not good (er, 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22), but the reaction at low temperature (0 °C) gave an improved result (er, 90
22), but the reaction at low temperature (0 °C) gave an improved result (er, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Other nucleophiles such as phenol and phthalimide were applicable to reactions of chiral secondary alcohol 3 to provide the corresponding Mitsunobu products 38 and 39, though the product yields were somewhat moderate. Reactions of other representative secondary alcohols 27–32 with 4-nitrobenzoic acid (4) readily provided the corresponding inversion products 40–45 in good yields. There was a slight loss of the optical purity of ester 42, which was also observed in the typical Mitsunobu reaction with DEAD.6a However, the case of (−)-menthol (33) was still a limitation in the catalytic Mitsunobu reaction even though a highly acidic carboxylic acid was employed.24 For instance, the reaction of 33 with 4-nitrobenzoic acid gave inversion product 46 as a minor isomer. Fortunately, we found out that inversion product 47 was produced exclusively when the 2-methyl-6-nitrobenzoic acid was used as a nucleophile. These contrasting results could be attributed to the catalytic system. The reaction with a catalytic amount of the azo reagent maintains a low concentration of an intermediary alkoxyphosphonium salt. There would be an equilibrium process between the alkoxyphosphonium intermediate and an acyloxyphosphonium intermediate.25 If a subsequent reaction of the alkoxyphosphonium intermediate with a carboxylic acid to give an inversion product is slow, a retention product would increase via the equilibrium process to give the acyloxyphosphonium intermediate because the concentration of a free carboxylic acid is sufficiently higher than that of the alkoxyphosphonium intermediate in the catalytic system. 2-Methyl-6-nitrobenzoic acid has a sufficient acidity but is sterically hindered. Therefore, conversion of the alkoxyphosphonium intermediate into the corresponding acyloxyphosphonium intermediate would be an unfavourable process due to a steric factor of the carboxylic acid.26
10). Other nucleophiles such as phenol and phthalimide were applicable to reactions of chiral secondary alcohol 3 to provide the corresponding Mitsunobu products 38 and 39, though the product yields were somewhat moderate. Reactions of other representative secondary alcohols 27–32 with 4-nitrobenzoic acid (4) readily provided the corresponding inversion products 40–45 in good yields. There was a slight loss of the optical purity of ester 42, which was also observed in the typical Mitsunobu reaction with DEAD.6a However, the case of (−)-menthol (33) was still a limitation in the catalytic Mitsunobu reaction even though a highly acidic carboxylic acid was employed.24 For instance, the reaction of 33 with 4-nitrobenzoic acid gave inversion product 46 as a minor isomer. Fortunately, we found out that inversion product 47 was produced exclusively when the 2-methyl-6-nitrobenzoic acid was used as a nucleophile. These contrasting results could be attributed to the catalytic system. The reaction with a catalytic amount of the azo reagent maintains a low concentration of an intermediary alkoxyphosphonium salt. There would be an equilibrium process between the alkoxyphosphonium intermediate and an acyloxyphosphonium intermediate.25 If a subsequent reaction of the alkoxyphosphonium intermediate with a carboxylic acid to give an inversion product is slow, a retention product would increase via the equilibrium process to give the acyloxyphosphonium intermediate because the concentration of a free carboxylic acid is sufficiently higher than that of the alkoxyphosphonium intermediate in the catalytic system. 2-Methyl-6-nitrobenzoic acid has a sufficient acidity but is sterically hindered. Therefore, conversion of the alkoxyphosphonium intermediate into the corresponding acyloxyphosphonium intermediate would be an unfavourable process due to a steric factor of the carboxylic acid.26
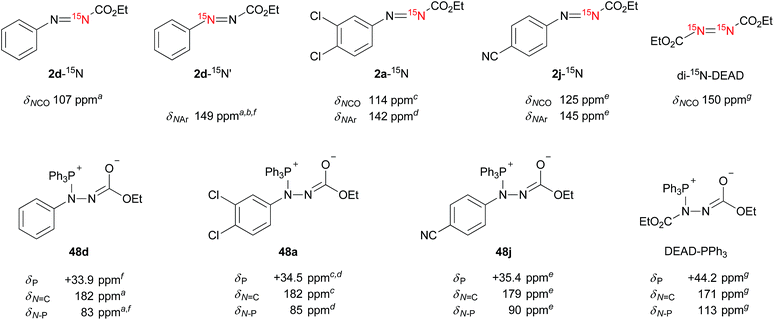
Mechanistic studies of the reaction using NMR spectroscopic methodologies
Does the reaction of the ethyl 2-arylazocarboxylates with triphenylphosphine form Morrison–Brunn–Huisgen betaine intermediates27 like in the typical Mitsunobu reaction? Precedent mechanistic studies indicate the formation of betaine intermediates from azo reagents and phosphines. To obtain insights into the intermediates in the present reaction, we monitored the reactions of ethyl 2-arylazocarboxylates with triphenylphosphine using multinuclear (1H, 13C, 31P, 15N) 1D and 2D NMR spectroscopy. To assist an unambiguous structure elucidation and assignment of NMR parameters, two kinds of 15N-labeled ethyl 2-phenylazocarboxylates 2d-15N and 2d-15N′, 15N-labeled potent Mitsunobu reagents 2a-15N, 2j-15N and doubly 15N-labeled DEAD (di-15N-DEAD) were prepared and used in the study, along with some unlabeled analogues (Fig. 7).The addition of triphenylphosphine (10 equiv.) into the solution of azo compounds in CDCl3 resulted in the appearance of low-field resonances in the 31P NMR spectra (2d: +33.9 ppm, 2a: +34.5 ppm, 2j: +35.4 ppm) that are supportive of the formation of betaine intermediates 48. In light of the electron density of a nitrogen atom, these chemical shifts are roughly consistent with that of di-15N-DEAD (+44.2 ppm) and DEAD (+44.8 ppm).27
Although it is predicted that Michael-type addition of triphenylphosphine to ethyl 2-arylazocarboxylates (an attack to N2) takes place to form betaines,28 the formation of other intermediary structures should be considered. Unlike for the symmetric DEAD,29 the issue of the regiochemistry of the triphenylphosphine attack to ethyl 2-arylazocarboxylates is raised as a consequence of their non-symmetric nature and the potential electrophilicity of the azo benzene derivatives toward triphenylphosphine.30,31
15N NMR spectroscopy was sought as a probe for the in situ investigation of the regiochemistry. The formation of adducts formed between the triphenylphosphine and azo reagents was monitored using 1H, 13C, 31P, 1H–1H COSY, 1H–13C HSQC, 1H–13C HMBC, 1H–31P HMBC, 1H–15N HMBC experiments, as well as HRMS. The results are summarized in Fig. 7 (and Tables S3 and S4 in the ESI†). In ethyl 2-arylazocarboxylates (e.g.2a, d, j), the NCO and N–Ar nitrogen atoms resonate in the regions of 107–125 ppm and 142–149 ppm, respectively. Upon the addition of triphenylphosphine, a large downfield shift of NCO to around 180 ppm, and a significant upfield shift of NAr to approximately 83–90 ppm is observed for the betaine intermediates. The nitrogen atoms resonating in di-15N-DEAD at 150 ppm appear after the addition of triphenylphosphine at 113 ppm and 171 ppm.
Since, to the best of our knowledge, this is the first 15N NMR study of the intermediates formed in the Mitsunobu reaction, no direct comparison with the literature data is possible. Nevertheless, the downfield 15N resonances, which are common for all phosphine intermediates from Fig. 7, suggest carbonimidate structural fragments as they are consistent with the 15N NMR data of dimethyl cyclohexylcarbonimidate (50 in Scheme 1, δN 181 ppm).32 Although this is reminiscent of a five-membered oxadiazophosphole ring structure (e.g., O,N-phosphorane 52 in Scheme 1), the 31P NMR chemical shift of such an intermediate should possess a negative value.27c Perhaps, the O,N-phosphorane is formed as a transient intermediate,27c but formation of the betaine intermediate having a carbonimidate anion appears to be predominant in the reaction mixture.
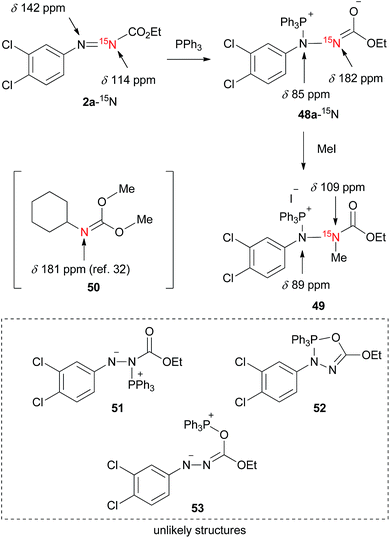 | ||
| Scheme 1 A trapping experiment of a betaine with iodomethane and the chemical shifts of the 15N NMR analysis. | ||
To further support the structure of the intermediate we carried out a trapping experiment in which betaine 48a, formed in situ from 2a-15N and triphenylphosphine in CDCl3, was treated in an NMR tube with iodomethane. 15N NMR chemical shifts of the starting compounds and products are shown in Scheme 1. The reaction of 2a-15N with triphenylphosphine followed by treatment with iodomethane readily afforded a methylated product holding a phosphine, as confirmed using 1H–31P HMBC. A correlation between the N–CH3 proton resonance with that of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl in the 1H–13C HMBC spectrum, along with the absence of N–CH3 correlations with aromatic carbons, strongly suggested the formation of 15N-methylated phosphonium salt 49. An upfield 15N NMR shift from 182 ppm (in 48a-15N) to 109 ppm upon methylation additionally supports the structure of 49. By repeating the trapping experiment with 15N-unlabeled 2a in a preparative way, the corresponding phosphonium salt decomposed during chromatographic purification on silica gel into ethyl 2-(3,4-dichlorophenyl)-1-methylhydrazine-1-carboxylate (see the ESI†). Although the intermediates generated from the dialkyl azodicarboxylates and triphenylphosphine are generally presented in a form of a resonance structure with a negatively charged nitrogen atom and a C
O carbonyl in the 1H–13C HMBC spectrum, along with the absence of N–CH3 correlations with aromatic carbons, strongly suggested the formation of 15N-methylated phosphonium salt 49. An upfield 15N NMR shift from 182 ppm (in 48a-15N) to 109 ppm upon methylation additionally supports the structure of 49. By repeating the trapping experiment with 15N-unlabeled 2a in a preparative way, the corresponding phosphonium salt decomposed during chromatographic purification on silica gel into ethyl 2-(3,4-dichlorophenyl)-1-methylhydrazine-1-carboxylate (see the ESI†). Although the intermediates generated from the dialkyl azodicarboxylates and triphenylphosphine are generally presented in a form of a resonance structure with a negatively charged nitrogen atom and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond, our NMR data suggest that the alternative with the sp2 hybridized nitrogen atom more accurately represents the true structure of the betaine (Fig. 7). This is also in agreement with oxygen being more electronegative than nitrogen.
O double bond, our NMR data suggest that the alternative with the sp2 hybridized nitrogen atom more accurately represents the true structure of the betaine (Fig. 7). This is also in agreement with oxygen being more electronegative than nitrogen.
Overall, the NMR experimental results support the formation of P–N betaines such as 48 in the Mitsunobu reaction using our reagents and indicate that other structures such as regioisomer 51 and P–O betaine 53 are unlikely. Formation of O,N-phosphorane 52 could not be ruled out but was not detected in our NMR analysis.
By treating butan-1-ol (10 equiv.) with triphenylphosphine (10 equiv.) and azo reagent 2a (1 equiv.) in solvents like THF-d8, CD3CN, CDCl3, or toluene-d8, a 31P NMR resonance corresponding to di-n-butoxytriphenylphosphorane (54) appeared in the spectra between −56.0 ppm and −55.2 ppm (Table 4), which is consistent with the data for DEAD (−55.0 ppm in THF-d8).27a On the other hand, unlike for THF-d8, CD3CN and CDCl3, the resonance of betaine 48a in toluene-d8 could not be detected. This suggests that an equilibrium toward betaine 48a from 2a is unfavorable but the reactivity of 48a toward an alcohol is sufficiently high in toluene. Thus, the fate of the betaine generated from the ethyl 2-arylazocarboxylates and triphenylphosphine appears to be very similar to that from the typical Mitsunobu reaction using DEAD.
Thermal stability of the developed Mitsunobu reagents
When typical azo reagents such as DEAD are used, sufficient care is often required from the viewpoint of their thermal instability. Ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a), ethyl 2-(4-cyanophenyl)hydrazinecarboxylate (1j) and their azo forms 2a and 2j are stable crystalline solids under ambient conditions, and no decomposition of these compounds was observed after two months. Incidentally, when di(2-methoxyethyl) azodicarboxylate (DMEAD), that is a crystalline solid, was exposed to ambient conditions for two months, a partial but clear decomposition was observed using 1H NMR analysis. It is known, from differential scanning calorimetry (DSC), that DEAD, diisopropyl azodicarboxylate (DIAD) and di(2-methoxyethyl) azodicarboxylate (DMEAD) show a large exothermic peak at 210–250 °C, indicating exponential decomposition of these compounds.33We investigated the thermal properties of ethyl 2-(3,4-dichlorophenyl)azocarboxylate (2a) and ethyl 2-(4-cyanophenyl)azocarboxylate (2j) using thermogravimetry-differential thermal analysis (TG-DTA). Interestingly, it indicated the absence of exothermic peaks, whereas endothermic peaks were observed at 191.3 °C (3,4-dichlorophenyl derivative 2a, mp: 52.1 °C) and 225.7 °C (4-cyanophenyl derivative 2j, mp: 55.4 °C) with a loss of weight of the samples. These peaks likely show boiling points of the azo compounds that are accompanied by some evaporation. A possibility of endothermic decomposition is unlikely because decomposition of azo compounds is generally exothermic. To eliminate the possibility of the endothermic decomposition, we representatively tested by heating 2a in the solution-phase. A solution of 2a in benzene-d6 was kept for 10 min at 200 °C in an autoclave and then analyzed using 1H NMR spectroscopy, which indicated no decomposition (see the ESI†). Similarly, TG-DTA of ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a, mp: 114.0 °C) and ethyl 2-(4-cyanophenyl)hydrazinecarboxylate (1j, mp: 138.1 °C) showed endothermic peaks with a loss of weight of the samples at 250.3 °C and 267.4 °C, though partial decomposition seems to occur around this temperature in the case of 1j. Thus, we did not observe clear exponential decomposition of our azo and hydrazine compounds under the ambient pressure unlike in typical Mitsunobu reagents, though we did not test the thermal stability of these compounds at higher temperatures in a pressured vessel.34 Overall, the experimental results support that our Mitsunobu catalysts can be safely stored and used without special precautions.
Conclusions
Ethyl 2-arylazocarboxylates can operate in the Mitsunobu reaction like typical Mitsunobu reagents such as diethyl azodicarboxylate (DEAD). The former, however, are recyclable using aerobic re-oxidation of the resultant ethyl 2-arylhydrazinecarboxylate with cheap and nontoxic iron phthalocyanine. This outstanding ability enables catalytic Mitsunobu reactions by using these reagents as organocatalysts. Our systematic study reveals that Mitsunobu activity of azo forms of these catalysts is compatible with an oxidation process of hydrazine forms. Two effective catalysts have been identified. Ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (1a) is suitable for catalytic Mitsunobu reactions with carboxylic acids, working best for the inversion of stereochemistry of secondary alcohols. Ethyl 2-(4-cyanophenyl)hydrazinecarboxylate (1j) provides excellent results in reactions with nucleophiles other than carboxylic acids, serving for the transformation of the hydroxyl groups of alcohols to other functional groups. Thus, the catalytic Mitsunobu reaction has been complemented by two potent reagents and strict optimization of the reaction conditions. The present catalytic protocol is comparable to the original Mitsunobu reaction in both, reactivity and scope. It is also noteworthy that these reagents are stable solids, and their thermal behavior is different from the typical Mitsunobu reagents. Our study has illustrated that serious limitations of the Mitsunobu reaction are avoidable using new reagents and improved procedures. We expect that the improved method will promote the use of the Mitsunobu reaction in practical synthesis.Acknowledgements
Authors are thankful to Dr Jun Kamitani (Industrial Research Institute of Ishikawa) for measuring the TG-DTA and for helpful discussion. D.H. and T.T. are thankful to Prof. Shigeyoshi Kanoh, Katsuhiro Maeda and Tomoyuki Ikai (Kanazawa University) for their kind support. This work was supported by MEXT/JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (Grant no. 25460011) and Grant-in-Aid for JSPS Fellows (Grant no. 14J02441). Financial support from the Ministry of Education, Science and Sport, Republic of Slovenia, the Slovenian Research Agency (Grant P1-0230) is acknowledged. Dedicated with deep respect to Professor Miha Tišler on the occasion of his 90th birthday.Notes and references
- (a) L. Kürti and B. Czakó, Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, Burlington, MA, 2005 Search PubMed; (b) Z. Wang, Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc., Hoboken, NJ, 2009, vol. 1–3 Search PubMed; (c) J. J. Li, Name Reactions: A Collection of Detailed Mechanism and Synthetic Applications, Springer, Cham, Heidelberg, New York, Dordrecht, London, 5th edn, 2014 Search PubMed.
- Reviews: (a) O. Mitsunobu, Synthesis, 1981, 1–28 CrossRef CAS; (b) D. L. Hughes, Org. React., 1992, 42, 335–656 CAS; (c) T. Y. S. But and P. H. Toy, Chem. Asian J., 2007, 2, 1340–1355 CrossRef CAS PubMed; (d) K. C. Kumara Swamy, N. N. Bhuvan Kumar, E. Balaraman and K. V. P. Pavan Kumar, Chem. Rev., 2009, 109, 2551–2651 CrossRef PubMed; (e) S. Fletcher, Org. Chem. Front., 2015, 2, 739–752 RSC.
- (a) O. Mitsunobu, M. Yamada and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1967, 40, 935–939 CrossRef CAS; (b) O. Mitsunobu and M. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380–2382 CrossRef CAS.
- Examples: (a) M. Girardin, S. J. Dolman, S. Lauzon, S. G. Ouellet, G. Hughes, P. Fernandez, G. Zhou and P. D. O'Shea, Org. Process Res. Dev., 2011, 15, 1073–1080 CrossRef CAS; (b) G. Schmidt, S. Reber, M. H. Bolli and S. Abele, Org. Process Res. Dev., 2012, 16, 595–604 CrossRef CAS.
- For reviews on the modification of Mitsunobu reactions: (a) R. Dembinski, Eur. J. Org. Chem., 2004, 2763–2772 CrossRef CAS; (b) S. Dandapani and D. P. Curran, Chem.–Eur. J., 2004, 10, 3130–3138 CrossRef CAS PubMed. See also ref. 2c–e. For a recent example of modification, see: (c) M. Figlus, N. Wellaway, A. W. J. Cooper, S. L. Sollis and R. C. Hartley, ACS Comb. Sci., 2011, 13, 280–285 CrossRef CAS PubMed; (d) P. K. Maity, A. Rolfe, T. B. Samarakoon, S. Faisal, R. D. Kurtz, T. R. Long, A. Schätz, D. L. Flynn, R. N. Grass, W. J. Stark, O. Reiser and P. R. Hanson, Org. Lett., 2011, 13, 8–10 CrossRef CAS PubMed.
- (a) T. Y. B. But and P. H. Toy, J. Am. Chem. Soc., 2006, 128, 9636–9637 CrossRef CAS PubMed; (b) T. Y. S. But, J. Lu and P. H. Toy, Synlett, 2010, 1115–1117 CAS.
- For Mitsunobu and related reactions without azo reagents: (a) C. M. Vanos and T. H. Lambert, Angew. Chem., Int. Ed., 2011, 50, 12222–12226 CrossRef CAS PubMed; (b) E. D. Nacsa and T. H. Lambert, Org. Lett., 2013, 15, 38–41 CrossRef CAS PubMed; (c) X. Tang, C. Chapman, M. Whiting and R. Denton, Chem. Commun., 2014, 50, 7340–7343 RSC.
- D. Hirose, T. Taniguchi and H. Ishibashi, Angew. Chem., Int. Ed., 2013, 52, 4613–4617 CrossRef CAS PubMed.
- T. Hashimoto, D. Hirose and T. Taniguchi, Adv. Synth. Catal., 2015, 357, 3346–3352 CrossRef CAS.
- Examples of catalytic Mitsunobu reactions using a catalytic amount of phosphine reagents: (a) C. J. O'Brien, PCT Int. Appl. WO2010/118042A2, 2010; (b) J. A. Buonomo and C. C. Aldrich, Angew. Chem., Int. Ed., 2015, 54, 13041–13044 CrossRef CAS PubMed. Although Aldrich reported “a fully catalytic system” of the Mitsunobu reaction by combining with our catalytic system in this publication, only limited scope of substrates (combination of two benzyl alcohols and 4-nitrobenzoic acid) has been shown. An original concept of phosphine catalysts: (c) C. J. O'Brien, J. L. Tellez, Z. S. Nixon, L. J. Kang, A. L. Carter, S. R. Kunkel, K. C. Przeworski and G. A. Chass, Angew. Chem., Int. Ed., 2009, 48, 6836–6839 CrossRef PubMed.
- See the Supporting Information of ref. 8.
- For the effect of solvents in the Mitsunobu reaction: D. Camp, P. J. Harvey and I. D. Jenkins, Tetrahedron, 2015, 71, 3932–3938 CrossRef CAS.
- K. Watanabe, N. Yamagiwa and Y. Torisawa, Org. Process Res. Dev., 2007, 11, 251–258 CrossRef CAS.
- For the side reaction to give the retention product, see: T. Taniguchi, D. Hirose and H. Ishibashi, ACS Catal., 2011, 1, 1469–1474 CrossRef CAS.
- A. Ogawa and D. P. Curran, J. Org. Chem., 1997, 62, 450–451 CrossRef CAS PubMed.
- For molecular sieves as desiccants, see: D. Bradley, G. Williams and M. Lawton, J. Org. Chem., 2010, 75, 8351–8354 CrossRef PubMed.
- (a) D. Camp and I. D. Jenkins, Aust. J. Chem., 1992, 45, 47–55 CrossRef CAS; (b) T. Tsunoda, Y. Yamamiya and S. Itô, Tetrahedron Lett., 1993, 34, 1639–1642 CrossRef CAS.
- D. Crich, H. Dyker and R. J. Harris, J. Org. Chem., 1989, 54, 257–259 CrossRef CAS.
- (a) L. P. Hammett, J. Am. Chem. Soc., 1937, 59, 96–103 CrossRef CAS; (b) C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- Since the reaction of ethyl 2-(4-nitrophenyl)azocarboxylate (2k) with triphenylphosphine and water is very fast and completed within 1 minute (k > 1 min−1), we could not monitor the reaction by the same method measuring adsorption of 2k.
- The poor result of 1k is a reason why we have overlooked a potent electron-deficient catalyst 1j. It misled us into believing that aerobic oxidation of ethyl 2-arylhydrazinecarboxylates having strong electron-withdrawing groups was slow in the previous study.
- K. Kitahara, T. Toma, J. Shimokawa and T. Fukuyama, Org. Lett., 2008, 10, 2259–2261 CrossRef CAS PubMed.
- For amination reactions using 2-nitrobenzenesulfonyl (Ns)-strategies: (a) T. Fukuyama, C.-K. Jow and M. Cheung, Tetrahedron Lett., 1995, 36, 6373–6374 CrossRef CAS; (b) T. Kan and T. Fukuyama, Chem. Commun., 2004, 353–359 RSC.
- (a) S. F. Martin and J. A. Dodge, Tetrahedron Lett., 1991, 32, 3017–3020 CrossRef CAS; (b) J. A. Dodge, J. I. Trujillo and M. Presnell, J. Org. Chem., 1994, 59, 234–236 CrossRef CAS.
- (a) D. Camp and I. D. Jenkins, J. Org. Chem., 1989, 54, 3045–3049 CrossRef CAS; (b) D. Camp and I. D. Jenkins, J. Org. Chem., 1989, 54, 3049–3054 CrossRef CAS; (c) P. J. Harvey, M. von Itzstein and I. D. Jenkins, Tetrahedron, 1997, 53, 3933–3942 CrossRef CAS; (d) C. Ahn, R. Correia and P. DeShong, J. Org. Chem., 2002, 67, 1751–1753 CrossRef CAS PubMed; (e) J. McNulty, A. Capretta, V. Laritchev, J. Dyck and A. J. Robertson, Angew. Chem., Int. Ed., 2003, 42, 4051–4054 CrossRef CAS PubMed.
- J. McNulty, A. Capretta, V. Laritchev, J. Dyck and A. J. Robertson, J. Org. Chem., 2003, 68, 1597–1600 CrossRef CAS PubMed.
- (a) E. Grochowski, B. D. Hilton, R. J. Kupper and C. J. Michejda, J. Am. Chem. Soc., 1982, 104, 6876–6877 CrossRef CAS; (b) D. L. Hughes, R. A. Reamer, J. J. Bergan and E. J. J. Grabowski, J. Am. Chem. Soc., 1988, 110, 6487–6491 CrossRef CAS; (c) D. Camp, M. von Itzstein and I. D. Jenkins, Tetrahedron, 2015, 71, 4946–4948 CrossRef CAS.
- Recent examples of Michael addition reactions to 2-arylazocarboxylates: R. Lasch and M. R. Heinrich, J. Org. Chem., 2015, 80, 10412–10420 CrossRef CAS PubMed and references therein.
- An example of nonsymmetrical Mitsunobu reagents: D. P. Furkert, B. Breitenbach, L. Juen, I. Sroka, M. Pantin and M. A. Brimble, Eur. J. Org. Chem., 2014, 7806–7809 CrossRef CAS.
- (a) M. Yamamura, N. Kano and T. Kawashima, J. Am. Chem. Soc., 2005, 127, 11954–11955 CrossRef CAS PubMed; (b) N. Iranpoor, H. Firouzabadi, D. Khalili and S. Motevalli, J. Org. Chem., 2008, 73, 4882–4887 CrossRef CAS PubMed.
- A possibility of an N1 attack of triphenylphosphine to 2-phenylazocarboxamides in the presence of acids has been raised: J. Košmrlj, M. Kočevar and S. Polanc, Synlett, 2009, 2217–2235 Search PubMed.
- M. Gazvoda, K. Höferl-Prantz, R. Barth, W. Felzmann, A. Pevec and J. Košmrlj, Org. Lett., 2015, 17, 512–515 CrossRef CAS PubMed.
- (a) K. Hagiya, N. Muramoto, T. Misaki and T. Sugimura, Tetrahedron, 2009, 65, 6109–6114 CrossRef CAS; (b) A. Berger and K. D. Wehrstedt, J. Loss Prev. Process Ind., 2010, 23, 734–739 CrossRef CAS . In our TG-DTA and DSC experiments of DMEAD, an exothermic peak was observed at 210–220 °C (see the ESI†).
- In our tentative DSC analysis of four compounds in a sealed pan under ambient pressure, no exothermic peak was observed below 300 °C similarly to TG-DTA (see the ESI†).
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and copies of analytical data. See DOI: 10.1039/c6sc00308g |
| This journal is © The Royal Society of Chemistry 2016 |