Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Extremely condensing triplet states of DPEPO-type hosts through constitutional isomerization for high-efficiency deep-blue thermally activated delayed fluorescence diodes†
Jing
Zhang
,
Dongxue
Ding
,
Ying
Wei
* and
Hui
Xu
*
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education & School of Chemistry and Material Science, Heilongjiang University, 74 Xuefu Road, Harbin 150080, P. R. China. E-mail: hxu@hlju.edu.cn; ywei@hlju.edu.cn
First published on 14th January 2016
Abstract
The similarity of thermally activated delayed fluorescence (TADF) dyes and their hosts as pure organic molecules makes hosts predominant in intermolecular interactions and crucial to exciton harvesting and utilization in TADF diodes. DPEPO is the most popular high-energy-gap blue TADF host with steric ortho-substituted diphenylphosphine oxide (DPPO) groups for intermolecular interaction suppression, but suffers from serious efficiency roll-off due to its weak electroactivity. On the contrary, para-substituted DPPO with small steric hindrance is superior in intramolecular electronic coupling. In this work, four constitutional isomers of DPEPO are constructed as diphenylether (DPE) with two diphenylphosphine oxide (DPPO) groups substituted at either the 2 or 4 position, namely 22′DPEPO (viz.DPEPO), 24DPEPO, 24′DPEPO and 44′DPEPO, respectively. On the basis of separation configuration, the steric effect and electroactivity of ortho- and para-substituted DPPOs are successfully integrated in 24′DPEPO, accompanied by remarkably reduced intermolecular interactions due to its unsymmetrical configuration. Compared to its congeners, 24′DPEPO has a rigid structure and locally excited states similar to 22′DPEPO for interaction suppression and improved charge mobility comparable to 44′DPEPO for charge flux balance. Significantly, by virtue of the predominant orientation effect of ortho-DPPO on the T1 location, its T1 state is extremely condensed onto a single phenyl, protected from intermolecular interactions by its remaining five phenyls at its maximum extent. Consequently, 24′DPEPO endowed its DMAC-DPS-based deep-blue devices with state-of-the-art performance, including high color purity with chromaticity coordinates of (0.16, 0.17), external quantum efficiency (EQE) beyond 20% and EQE roll-off as low as 32% at 1000 cd m−2. It is shown that the device performance of 24′DPEPO was far beyond simple integration of those of 22′DPEPO and 44′DPEPO, verifying the significance of host optimization.
1. Introduction
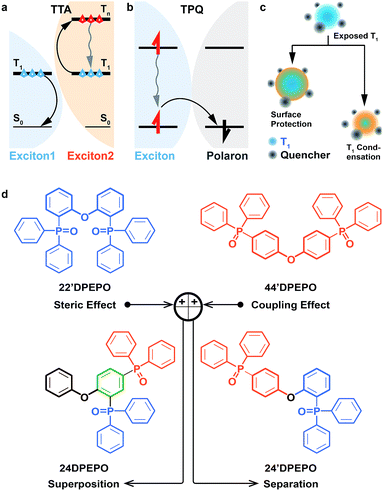
Exciton harvesting and utilization is crucial to realize efficient organic light-emitting diodes (OLEDs).2 After the great success of phosphorescent heavy-metal complexes in harvesting triplet excitons through spin-orbital coupling,3 thermally activated delayed fluorescence (TADF) dyes have emerged in recent years, whose near-zero singlet–triplet splitting can facilitate triplet-to-singlet conversion, by virtue of reverse intersystem crossing (RISC).4 TADF dyes are mostly pure organic compounds featuring donor–acceptor (D–A) structures with high molecular polarity and populated with excited states characteristic of charge transfer (CT).5 On account of the involvement of triplet excitons in the electroluminescence process and the strong intermolecular interactions of TADF dyes, host materials are commonly adopted to dilute emitters and restrain exciton quenching in TADF diodes.6 However, in contrast to their phosphorescent counterparts, as the same pure organic materials, TADF dyes show molecular components and excited characteristics similar to their hosts, especially their comparable triplet lifetimes. It is rational that as it forms the majority of emissive layers (EML), the host is dominant in intermolecular interplay, including host–host and host–dopant interactions.8 In this case, besides the basic functions for host materials, viz. carrier flux balance and energy transfer, their structures and optoelectronic properties would exert great influence on collision-induced exciton quenching effects, such as triplet–triplet annihilation (TTA, Fig. 1a) and triplet-polaron quenching (TPQ, Fig. 1b).9 Therefore, compared to phosphorescent OLEDs, host materials in TADF diodes play a more vital role in exciton harvesting and utilization.8,10The collision between two triplet excitons can render TTA.11 That is, one exciton is annihilated through transferring its energy to the other, making the latter excited to a higher level and then recovering to the first triplet (T1) state through internal conversion (IC) (Fig. 1a). Meanwhile, TPQ occurs during collision between an exciton and polaron, which induces the nonradiative deactivation of the former through charge exchange (Fig. 1b).12 In consequence, three approaches would be effective for quenching suppression: (i) embedding and/or segregating T1-localized moieties of host and dopant molecules to protect triplet excitons from collision quenching with surrounding modification13 and T1 state condensation,14 in spite of only a few reports about T1 condensation (Fig. 1c); (ii) reducing collision probability through shortening exciton lifetime; (iii) enhancing charge flux balance and unifying exciton recombination to decrease polaron concentration. In this case, the excited-state characteristics and carrier-transporting ability of host materials should be a determinant for their electroluminescence (EL) efficiencies.
It is known that the CT-type exciton is labile and highly sensitive to the environment.15 Consequently, although 100% internal quantum efficiencies were already achieved for TADF devices, most of them suffered from serious efficiency roll-off, especially for blue TADF diodes utilizing high-energy excitons. DPEPO is the most popular host for blue TADF diodes.4a,5e,5i,5j,16 Its multi-insulating structure and the steric effect of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups give rise to its high T1 value of ∼3.0 eV for efficient energy transfer to blue TADF dyes, e.g. bis[4-(9,9-dimethyl-9,10-dihydroacridine)phenyl]sulfone (DMAC-DPS) (T1 = 2.90 eV)16a and quenching suppression.17 The external quantum efficiency (EQE) of deep-blue TADF devices with DMAC-DPS as the emitter reached ∼20%, however, this was accompanied by remarkable roll-off of more than 90% at 1000 cd m−2, indicating serious exciton quenching.18 Nevertheless, how to optimize optoelectronic properties of blue TADF host materials is really challenging, since many key issues are still unclear, especially the influences and correlations of their electrical properties and excited-state characteristics and their merits.19 In our previous work, it was shown that these two factors strongly depend on the functional linkage site and molecular sequence.20 In this sense, the isomerization of DPEPO may provide a feasible way to clarify the key determinants of EL performance for deep blue TADF host materials.
O groups give rise to its high T1 value of ∼3.0 eV for efficient energy transfer to blue TADF dyes, e.g. bis[4-(9,9-dimethyl-9,10-dihydroacridine)phenyl]sulfone (DMAC-DPS) (T1 = 2.90 eV)16a and quenching suppression.17 The external quantum efficiency (EQE) of deep-blue TADF devices with DMAC-DPS as the emitter reached ∼20%, however, this was accompanied by remarkable roll-off of more than 90% at 1000 cd m−2, indicating serious exciton quenching.18 Nevertheless, how to optimize optoelectronic properties of blue TADF host materials is really challenging, since many key issues are still unclear, especially the influences and correlations of their electrical properties and excited-state characteristics and their merits.19 In our previous work, it was shown that these two factors strongly depend on the functional linkage site and molecular sequence.20 In this sense, the isomerization of DPEPO may provide a feasible way to clarify the key determinants of EL performance for deep blue TADF host materials.
In this contribution, four constitutional isomers of DPEPO with the collective name of mDPEPO are constructed as bis(diphenylphosphoryl) diphenylether with two diphenylphosphine oxide (DPPO) groups at either the 2 or 4 position of their diphenylether (DPE) cores, named 22′DPEPO (viz.DPEPO), 24DPEPO, 24′DPEPO and 44′DPEPO, respectively (Fig. 1d). DPPO at the 2-position indicates the strong steric effect and dominant localization effect on the T1 state, while DPPO at the 4-position is superior in enhancing intramolecular electronic coupling. By virtue of the asymmetrical and separated structure for 24′DPEPO, the functions of its two kinds of DPPO groups are successfully integrated. Significantly, its T1 state is extremely condensed to a single phenyl, embedded and protected by its remaining five phenyls at its maximum extent. Meanwhile, compared to 22′DPEPO, the electron mobility of 24′DPEPO is dramatically improved by about 50 times. Consequently, its DMAC-DPS-based deep-blue devices realized state-of-the-art performance, including high color purity with CIE coordinates of (0.16, 0.17), an EQE beyond 20% and EQE roll-off as low as 32% at 1000 cd m−2. The efficiencies of 24′DPEPO-based devices were improved by 20% in comparison to those employing 22′DPEPO as the host; meanwhile, the roll-offs of the former were halved. It is clear that host engineering is a gateway to resolving the key issues of TADF devices in exciton harvesting and utilization.
2. Results and discussions
2.1. Design and synthesis
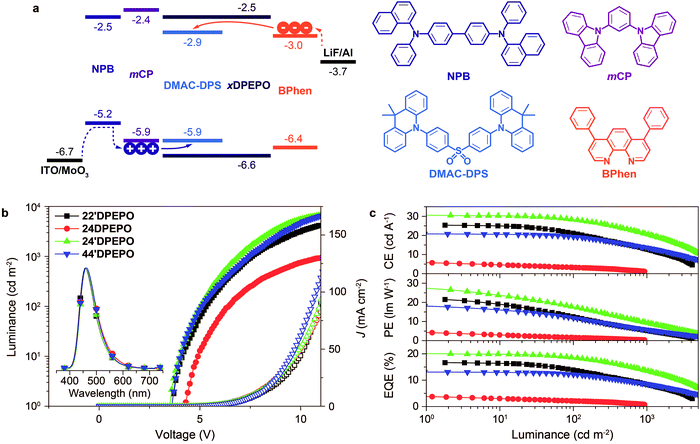
DPPO is an electron-withdrawing group with big steric hindrance. Its dominant function depends on its substitution positions. For instance, DPPOs at ortho-positions of 22′DPEPO are superior in their steric effect; while, DPPOs in 44′DPEPO can enhance intramolecular coupling with an electron-donating ether bridge at the para-position (Fig. 1c). In this case, an intermolecular interaction with 22′DPEPO at the DPPO-substituted side can be thoroughly blocked to effectively suppress TTA. However, electronic coupling in 22′DPEPO is remarkably weaker due to its ortho-substitution configuration.17 In contrast, sufficient electronic coupling in 44′DPEPO can support balanced hole and electron flux in its EMLs, facilitating exciton recombination and reducing TPQ.21 Nonetheless, its DPE chromophore is completely exposed to intermolecular interactions, worsening TTA. Therefore, it is well-reasoned to combine these two kinds of DPPO substitutions in one single molecule for integrating their advantages and thereby simultaneously suppressing TTA and TPQ.More significantly, in our recent works, it was shown that DPPO substitution can regulate T1 location, which should be attributed to the influence of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group on electron cloud distribution.14,20a,22 In this sense, the orientation effect of DPPO on the T1 state would be related to its substitution position. Therefore, with asymmetrical DPPO substitutions, the T1 states of the molecules can be condensed into a designated location by DPPO with a dominant orientation effect. The narrow T1 location can amplify the protective action of surrounding groups and further reduce its probability of involvement in intermolecular interactions. Regarding DPEPO-type analogues, since all of their phenyls are separated by insulating linkages, it can be expected to confine their T1 states into a single phenyl, which is almost the minimum for conjugated compounds with respect to spatial scale.
O group on electron cloud distribution.14,20a,22 In this sense, the orientation effect of DPPO on the T1 state would be related to its substitution position. Therefore, with asymmetrical DPPO substitutions, the T1 states of the molecules can be condensed into a designated location by DPPO with a dominant orientation effect. The narrow T1 location can amplify the protective action of surrounding groups and further reduce its probability of involvement in intermolecular interactions. Regarding DPEPO-type analogues, since all of their phenyls are separated by insulating linkages, it can be expected to confine their T1 states into a single phenyl, which is almost the minimum for conjugated compounds with respect to spatial scale.
With these considerations, two other asymmetrical isomers, namely 24DPEPO and 24′DPEPO, incorporating both ortho- and para-linked DPPO groups were designed. Two DPPOs in 24DPEPO are bonded with the same phenyl, while those in 24′DPEPO are respectively bonded with two different phenyls of DPE. The superposition of two DPPOs in 24DPEPO may render the mutual acceleration or counteraction of their effects. On the contrary, by virtue of the insulating ether bridge, the two separated DPPOs of 24′DPEPO can perform their functions independently. In consequence, it is most likely for 24′DPEPO to integrate the merits of 22′DPEPO and 44′DPEPO. Simultaneously, the multi-insulating linkage establishes the similarity of mDPEPO to the maximum extent, excluding the interference from undesirable intramolecular interplay and conjugation variation, which simplifies the influencing factors of their device performance into electrical and excited-state characteristics.
mDPEPO can be conveniently prepared through Pd-catalyzed phosphorylation with good yields of more than 50%. Their chemical structures were fully characterized on the basis of NMR spectra, mass spectra and elementary analysis. Among mDPEPO, the biggest steric hindrance in 22′DPEPO increases its intramolecular tension, rendering its lowest temperature of decomposition (Td) as 322 °C; while its rigid and monosymmetrical structure results in its highest melting point (Tm) of 280 °C (Fig. S1† and Table 1). On the contrary, owing to negligible steric hindrance in 44′DPEPO, its Td is remarkably improved to 417 °C, however, this is accompanied by a reduced Tm of 190 °C. The rotatable phenyl of DPE in 24DPEPO makes its thermal properties similar to those of 44′DPEPO. Importantly, 24′DPEPO shows a Td equivalent to that of 44′DEPPO and a Tm comparable to that of 22′DPEPO. Therefore, one ortho-substituted DPPO in 24′DPEPO already revealed sufficiently strong molecular rigidity.
| 22′DPEPO | 24DPEPO | 24′DPEPO | 44′DPEPO | |
|---|---|---|---|---|
| a Absorption peaks. b In CH2Cl2 (10−6 mol L−1). c In polycrystalline powder. d Fluorescence peaks at room temperature. e Estimated according to the absorption edges. f Calculated according to the 0–0 transitions of the phosphorescence spectra. g TDDFT calculated results. h Calculated according to the equation HOMO/LUMO = −(4.78 + onset voltage) eV.1 i Reorganization energy of electron. j For electron. k For hole. l Electron mobility estimated by I–V characteristics of electron-only devices according to field-dependent SCLC model.7 m Data of vacuum-evaporated mDPEPO:DMAC-DPS (10% wt) films with thickness of 100 nm. | ||||
| λ abs (nm) | 227, 274, 284, 300b, 225, 283c | 228, 274, 284, 300b, 225, 283c | 228, 253, 274, 284, 294b, 225, 283c | 228, 250, 273, 283b, 225, 278c |
| λ Em (nm) | 312b/309, 389c | 311b/316, 349, 418, 447c | 310b/315, 412, 433, 461c | 301b/302, 343c |
| S1/T1 (eV) | 3.92e/2.98f, 4.82/3.64g | 3.94e/2.98f, 4.73/3.53g | 3.92e/2.98f, 4.76/3.62g | 3.94e/2.97f, 4.75/3.53g |
| T g/Tm/Td (°C) | —/280/322 | —/203/405 | —/250/417 | —/190/417 |
| HOMO/LUMO (eV) | −6.53/−2.53h, −6.27/−0.76g | −6.51/−2.79h, −6.40/−0.97g | −6.65/−2.63h, −6.34/−0.89g | −6.65/−2.63h, −6.27/−0.93g |
| E R (eV) | 0.2312j/0.4353k | 0.7237j/0.5550k | 0.2884j/1.0176k | 0.1823j/0.3129k |
| μ (cm2 V−1 s−1) | 7.03 × 10−8j/1.40 × 10−9k | 7.72 × 10−9j/1.25 × 10−9k | 4.02 × 10−6j/0.99 × 10−8k | 5.15 × 10−6j/1.20 × 10−7k |
| λ Em (nm) | 470 | 470 | 470 | 470 |
| τ (μs) | 7.2 | 7.4 | 7.5 | 8.6 |
| PLQYm (%) | 85 | 59 | 89 | 77 |
2.2. DFT and TDDFT simulation
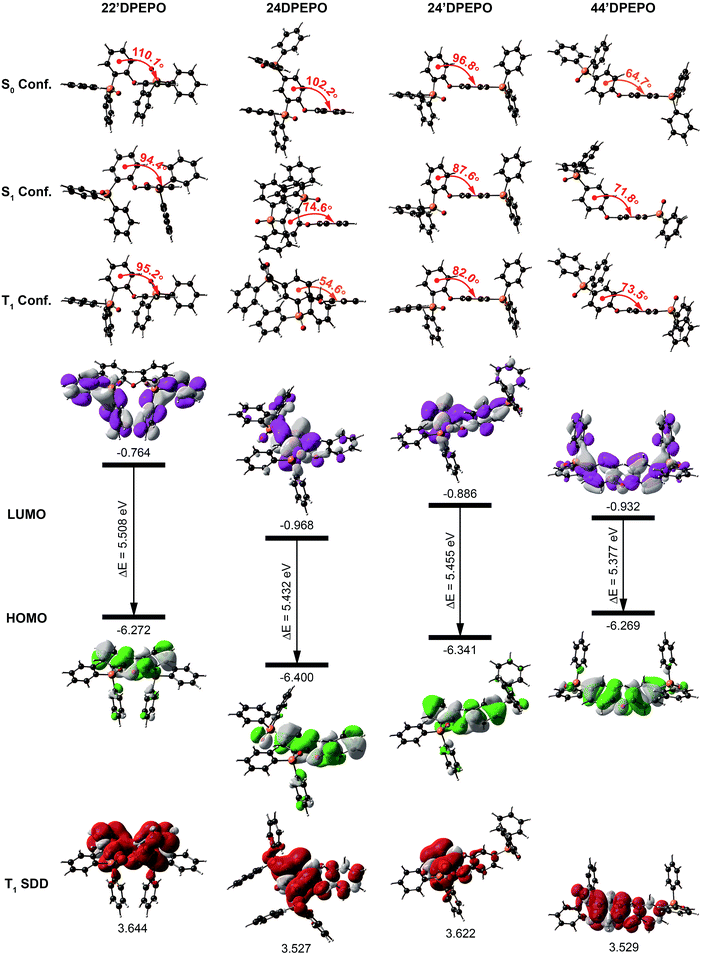
For insight into the nature of the effects of DPPO substitution on electrical and excited-state characteristics of mDPEPO, DFT and TDDFT calculations were performed at the level of B3LYP/6-31+g(d,p), taking into account computational accuracy and cost.The optimized molecular configurations of mDPEPO at ground (S0), the first singlet (S1) and T1 states are shown in Fig. 2. Ascribed to the biggest steric hindrance of its two ortho-substituted DPPOs, the dihedral angle of DPE in 22′DPEPO at the S0 state is the largest among mDPEPO, which is almost preserved at its S1 and T1 states. The dihedral angles of DPE in 44′DPEPO at these states are the smallest, in accord with the smaller steric hindrance of its DPPOs at the para-position. Compared to the ground state, its excited-state configurations are adjusted. Nevertheless, 24DPEPO shows the largest configuration variation at excited states, which is ascribed to the rotational phenyl of its DPE. In this sense, both ortho- and para-DPPO substitution can reduce excited-state structural relaxation. Consequently, the molecular configurations of 24′DPEPO at S0, S1 and T1 states are almost unchanged, accompanied by the dihedral angles of DPE close to those of 22′DPEPO. In comparison to 24DPEPO, the fixed molecular configurations of 24′DPEPO and 22′DPEPO make them superior in reducing relaxation-induced excited-energy loss, which is beneficial to improving luminescent efficiency.
The contours of the highest occupied and the lowest unoccupied molecular orbitals (HOMO and LUMO) for mDPEPO reveal the influence of DPPO substitution position on hole and electron injecting ability, respectively. Although the frontier molecular orbitals (FMO) of 22′DPEPO are respectively localized on its DPE and DPPO groups, its LUMO energy level is the highest among mDPEPO, indicating the weakest electron-withdrawing ability of its DPPOs at the 2-position (Fig. 2, S2† and Table 1). The situations of 44′DPEPO and 24DPEPO are similar, with partially separated HOMO and LUMO. However, the superposition of DPPOs in 24DPEPO renders the simultaneously reduced HOMO and LUMO energy levels. In contrast, 44′DPEPO possesses a HOMO and LUMO that are almost equivalent to those of 22′DPEPO and 24DPEPO, respectively, causing the enhanced intramolecular electronic coupling in 44′DPEPO and its ambipolar characteristics. As expected, through incorporating the para-substituted DPPO, in comparison to 22′DPEPO, the HOMO energy level of 24′DPEPO is preserved, while its LUMO energy level is reduced by 0.12 eV, which is similar to that of 44′DPEPO. Therefore, it is shown that despite its overlapping HOMO and LUMO distributions, the charge injecting ability of 24′DPEPO is mainly determined by its para-linked DPE-DPPO segment.
The calculated S1 and T1 energies of mDPEPO are almost the same as ∼4.7 and ∼3.5 eV, supporting positive energy transfer to DMAC-DPS with the S1 and T1 value of ∼2.9 eV (Fig. 2 and Table 1). The similar excited energy of mDPEPO is attributed to their multi-insulating structures. It is rational that on the basis of monosymmetric configurations and two equivalent DPPOs, the T1 states of 22′DPEPO and 44′DPEPO are uniformly dispersed on their DPE groups. In accord with our previous reports, DPPO substitution has an effect on T1 location regulation.20a The T1 state of 24DPEPO is mainly contributed by the phenyl substituted with two P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Interestingly, 24′DPEPO shows a T1 state that is thoroughly localized on the 2-DPPO substituted phenyl, reflecting the dominant orientation effect of ortho-substituted DPPO on the T1 state. The T1 state of 24′DPEPO is extremely condensed on a single phenyl as one of the smallest conjugated units, which is embedded by its remaining five phenyls. Compared to its isomers, the exposure degree of the T1 state of 24′DPEPO is doubtlessly the smallest, which can support the most effective suppression of collision-induced quenching effects.
O groups. Interestingly, 24′DPEPO shows a T1 state that is thoroughly localized on the 2-DPPO substituted phenyl, reflecting the dominant orientation effect of ortho-substituted DPPO on the T1 state. The T1 state of 24′DPEPO is extremely condensed on a single phenyl as one of the smallest conjugated units, which is embedded by its remaining five phenyls. Compared to its isomers, the exposure degree of the T1 state of 24′DPEPO is doubtlessly the smallest, which can support the most effective suppression of collision-induced quenching effects.
The nature of electronic transitions for the excited states of mDPEPO was further evaluated with natural transition orbitals (NTO) of the first singlet and triplet excitations (Fig. 3 and S4†).23 In the cases of NTOs about S0 → S1 states for mDPEPO, “holes” are thoroughly localized on their DPE. However, the distributions of “particles” are various, such that for 22′DPEPO and 24′DPEPO, “particles” are also dispersed on their DPE, featuring locally excited (LE) state transition; while, for 24DPEPO, “holes” are concentrated to one phenyl of DPE, and for 44′DPEPO, “holes” are partially dispersed on DPPO groups, characteristic of hybridized local and charge transfer excited states. Nevertheless, the biggest oscillator strength (f) suggests the LE transition of DPE as the major part for 44′DPEPO, while CT transition between two phenyls of DPE is dominant for 24DPEPO with the smallest f. NTOs of S0 → T1 states for mDPEPO reveal an LE character with overlapped “hole” and “particle” locations. In accord with the DFT results, triplet states of 22′DPEPO and 44′DPEPO are dispersed on their DPE, while only a single phenyl of 24DPEPO and 24′DPEPO is involved in their triplet transitions. Regarding CT character of excitons on TADF dyes, LE-dominant excited states of hosts can provide uniform and apparently neutral matrices to restrain Coulomb interaction induced CT exciton dissociation.
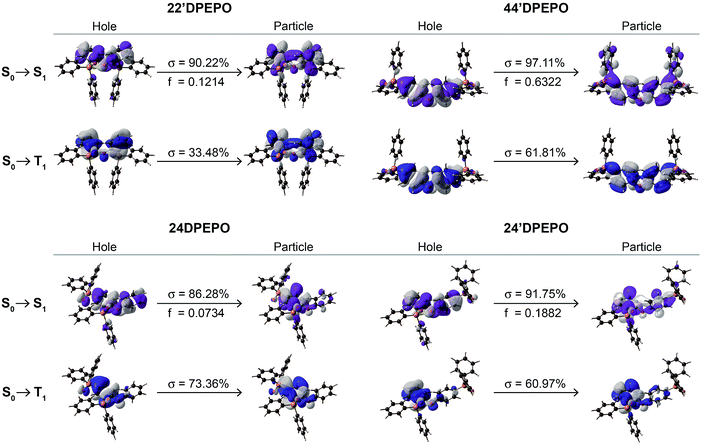 | ||
| Fig. 3 Natural transition orbitals (NTO) of the S1 and T1 states for mDPEPO. σ and f refer to the associated weight and oscillator strength, respectively. | ||
It is shown that 24′DPEPO successfully integrates the merits of ortho- and para-DPPO substitutions, including excited structural stability, singlet characteristics comparable to 22′DPEPO and FMO energy levels close to 44′DPEPO. In addition to significantly reinforced T1 state protection through an extremely condensed T1 location on the minimum unit phenyl, 24′DPEPO shows superiority in quenching suppression and charge balance. In contrast, 24DPEPO exhibits some shortages of serious excited structural relaxation and a CT-dominant S1 characteristic.
2.3. Optical properties
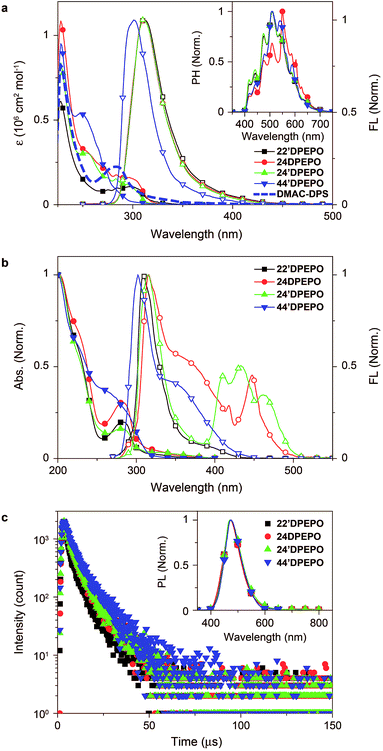
The absorption spectra of mDPEPO in dilute solutions (10−6 M in CH2Cl2) consist of three bands at around 230, 270 and 300 nm, corresponding to π → π* transitions of DPPO and DPE and the n → π* transition of DPE, respectively (Fig. 4a). The spectra of 22′DPEPO, 24DPEPO and 24′DPEPO are almost identical, but different to that of 44′DPEPO. As indicated by the DFT results, the smallest dihedral angle of DPE in 44′DPEPO facilitates π–π interactions between the two phenyls of DPE and decreases p–π conjugation between the O atom and phenyls, accordingly enhancing π → π* transition and weakening n → π* transition. Nevertheless, the optical energy gaps of mDPEPO estimated by absorption edges are almost identical to 3.9 eV, consistent with TDDFT results (Table 1).In the same way, the fluorescence (FL) spectra of 22′DPEPO, 24DPEPO and 24′DPEPO in dilute solutions (10−6 M in CH2Cl2) are exactly the same in range and profile. Meanwhile, the FL spectrum of 44′DPEPO reveals a blue shift of ∼10 nm. Nonetheless, there are large-range spectral overlaps between FL emissions of mDPEPO and absorption of DMAC-DPS from 275 to 350 nm, facilitating Förster resonance energy transfer (FRET). The low-temperature time-resolved phosphorescence (PH) spectra of mDPEPO are identical, with the same peaks and profiles, which is in accord with their similar T1 transition characteristics as shown by TDDFT simulation (inset of Fig. 4a). Estimated with 0 → 0 transitions, the T1 value of mDPEPO is equivalent to 2.98 eV, supporting the efficient triplet energy transfer to DMAC-DPS. The similar excited energy of these DPEPO-type isomers fully verifies the effectiveness of multi-insulating linkages in excited energy preservation.
The optical properties of mDPEPO in the solid state were further investigated to exclude solvent effects (Fig. 4b). The main absorption bands are preserved in the solid-state spectra, reflecting the limited intermolecular interactions. However, in contrast to the unchanged fluorescence emission of 22′DPEPO in the solid state, 44′DPEPO shows an additional broad aggregation-induced emission band in the long-wavelength range from 325 to 450 nm, in accord with the weak steric effect of its para-DPPOs. The flexible DPE in 24DPEPO also facilitates aggregation, rendering a similar broad band at around 340 nm. As expected, by virtue of the strong steric effect of ortho-substituted DPPO in 24′DPEPO with a separation configuration, the aggregation in the solid state is successfully suppressed, making one of its solid-state emission bands at 315 nm almost overlapped with its emission in solution. Significantly, solid-state emissions of 24′DPEPO and 24DPEPO contain additional distinct multi-peak bands coincident with the blue parts of their phosphorescence, whose lifetimes (τ) are also comparable to ∼7 μs (Fig. S3†); meanwhile, for 22′DPEPO and 44′DPEPO, their solid-state phosphorescence is fully quenched through nonradiative transitions due to their exposed T1 states. In dilute solutions, the small volumes of solvent molecules make collision with every part of solute molecules facile, resulting in serious solvent quenching effects on T1 states, which can be excluded in the solid state. Recently, room-temperature phosphorescence from crystalline pure organic molecules was realized on the basis of D–A systems with overlapping S1 and T1 locations.24 It is rational to attribute the visible room-temperature solid-state phosphorescence of 24′DPEPO and 24DPEPO to their extremely condensed T1 state, effectively protected from interaction-induced triplet quenching, which validates T1 condensation as a feasible and effective strategy to achieving and enhancing room-temperature phosphorescence from pure organic systems.
The host–dopant energy transfer was further investigated through steady-state and transient photoluminescence (PL) spectra of vacuum-evaporated DMAC-DPS-doped mDPEPO films (Fig. 4c). The emission spectra are almost identical, with the maxima at 470 nm, corresponding to the pure DMAC-DPS-originated emission, which indicates the same positive energy transfer from mDPEPO consistent with their equivalent S1 and T1 energy. As mentioned above, the τ of T1 states for mDPEPO and emissions of their DMAC-DPS-doped films is of the same order of magnitude, making mDPEPO-involved interactions dominant in triplet quenching processes. The τ of the 44′DPEPO-based film is 8.6 μs, about 1 μs longer than those of the other isomer based films, revealing the effect of 44′DPEPO with a partial CT-featured S1 state on stabilizing DMAC-DPS excitons.8 In spite of the CT-dominant S1 state, the τ of the 24DPEPO-based film is much shorter due to its structural relaxation-induced exciton quenching, which is further manifested by its lowest PL quantum yield (PLQY) of only 59% (Table 1). Furthermore, it is known that a longer lifetime would worsen collision-induced exciton quenching. Therefore, in comparison to the PLQY of 77% for the 44′DPEPO-based film, 22′DPEPO and 24′DPEPO endow their films with PLQYs as high as 85 and 89%, respectively.
In consequence, the optical properties of 24′DPEPO and 22′DPEPO are identical, originating from their similar excited-state characteristics as TDDFT simulated.
2.4. Electrical properties
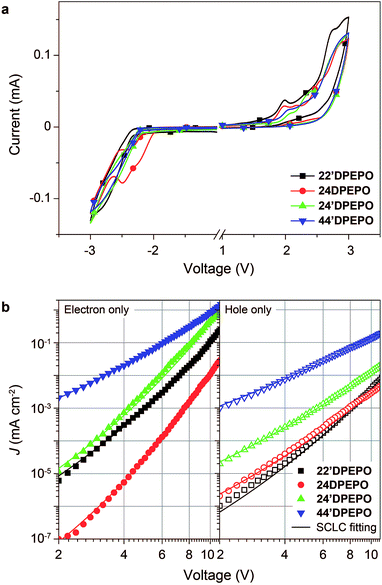
The influence of DPPO substitution position on the HOMO and LUMO energy levels is experimentally investigated with cyclic voltammetry (CV) analysis (Fig. 5a). The oxidation voltammograms of mDPEPO consist of two irreversible peaks characteristic of DPE and DPPO, respectively. Estimated with onset voltages of the anodic peaks, the HOMO energy level of 24′DPEPO is equivalent to that of 44′DPEPO at −6.65 eV, which is 0.1 eV lower than those of 22′DPEPO and 24DPEPO (Table 1). On the other hand, the reduction voltammograms of 22′DPEPO, 24′DPEPO and 44′DPEPO only contain single irreversible cathodic peaks, while 24′DPEPO shows two irreversible reduction peaks attributed to DPE and DPPO, respectively. It is shown that the reduction curves of 24′DPEPO and 44′DPEPO are almost overlapped with the same onset voltages of −2.15 V, corresponding to the LUMO of −2.63 eV. The superposition of two DPPOs in 24DPEPO further reduces its LUMO to −2.79 eV, which is in accord with the DFT results. It is clear that in comparison to 22′DPEPO, the stronger electron-withdrawing effect of para-DPPO substitution endows 24′DPEPO and 44′DPEPO with the deeper LUMOs. Significantly, the identical FMO energy levels of 24′DPEPO and 44′DPEPO verify the dominant effect of para-DPPO in FMO energy level regulation.The nominal single-carrier transporting devices with single-layer configurations of ITO|MoO3 (6 nm)|mDPEPO (100 nm)|MoO3 (6 nm)|Al for hole only and ITO|LiF (1 nm)|mDPEPO (100 nm)|LiF (1 nm)|Al for electron only, where MoO3 and LiF respectively served as hole- and electron-injecting layers, were fabricated to evaluate the intrinsic carrier transporting ability of mDPEPO (Fig. 5b). All of these materials show electron predominant characteristics with electron-only current density (J) remarkably larger than hole-only J. According to field-dependent space charge limited current model, the hole mobility (μh) of 44′DPEPO is estimated to be 1.20 × 10−7 cm2 V−1 s−1, which is 10 fold that of 24′DPEPO and 100 fold those of 22′DPEPO and 24DPEPO (Table 1). On account of the DPE-localized HOMOs for mDPEPO and the electron-transporting character of DPPO, the hole transporting abilities of mDPEPO should be in direct proportion to the exposure degree of their DPE as the main hole transporting channel. Although the superposition of the two DPPOs renders the deepest LUMO that of 24DPEPO, its electron mobility (μe) is the lowest at 7.72 × 10−9 cm2 V−1 s−1, which is only one tenth of that of 22′DPEPO. In contrast, the μe of 24′DPEPO at 4.02 × 10−6 cm2 V−1 s−1 is dramatically improved by 3 orders of magnitude, which is almost equivalent to that of 44′DPEPO. According to the DFT results, both DPE and DPPO are involved in unoccupied molecular orbitals and incorporated into electron transportation (Fig. S2†). In this case, the various μe of mDPEPO actually corresponds to their different intermolecular interplay. Compared to the over-concentration of DPPO with big steric hindrance in 24DPEPO and 22′DPEPO, by virtue of the relatively weaker steric effect of para-substituted DPPO and separation configurations, the intermolecular interplay of 24′DPEPO and 44′DPEPO is more effective to facilitate charge hopping between adjacent molecules. Furthermore, it is shown that the J of 24′DPEPO-based devices is intermediate between those of 22′DPEPO and 44′DPEPO-based devices, revealing the carrier transporting ability of 24′DPEPO as a combined result of steric and electronic coupling effects of its ortho- and para-substituted DPPOs. Nevertheless, on the contrary to 24DPEPO with superposition configuration, the separation configuration of 24′DPEPO causes the superiority of its para-DPPO in electrical performance enhancement.
In general, through an asymmetrical separation configuration incorporating both ortho- and para-DPPO substitutions, 24′DPEPO successfully integrates the complementary advantages of 22′DPEPO and 44′DPEPO in favorable optical characteristics and improved electrical performance, respectively, revealing its great potential in exciton harvesting and utilization.
2.5. Device performance of deep-blue TADF diodes
The well-controlled and differentiated optoelectronic properties of constitutional isomers mDPEPO establish the basis to selectively investigate the determinants of EL performance of blue TADF host materials. The devices were fabricated with a configuration of ITO|MoO3 (6 nm)|NPB (70 nm)|mCP (5 nm)|mDPEPO:DMAC-DPS (20 nm, 10% wt)|mDPEPO (5 nm)|Bphen (30 nm)|LiF (1 nm)|Al, in which MoO3 and LiF served as the hole and electron-injecting layer, NPB and Bphen are 4,4′-bis[N-(1-naphthyl)-N-phenylamino]biphenyl and 4,7-diphenyl-1,10-phenanthroline as hole and electron transporting layers and mCP (N,N′-dicarbazole-3,5-benzene) and mDPEPO were used as exciton-blocking layers, respectively (Fig. 6a). The doping concentration was optimized as 10% wt (Fig. S5†). In contrast to the large energy barriers of ∼0.5 eV between FMOs of mDPEPO and mCP and Bphen, the HOMO and LUMO energy levels of DMAC-DPS perfectly match with the corresponding FMO energy levels of mCP and Bphen, making the charge capture and exciton recombination on DMAC-DPS dominant in exciton harvesting. At high operation voltages, high-energy carriers can surmount the barriers to recombine on mDPEPO, making host-to-dopant energy transfer considerable. In this case, exciton quenching by host–host and host–dopant interactions can directly influence the device performance.All of the devices showed deep blue emissions peaking at 460 nm at 1000 cd m−2 (inset in Fig. 6b). In comparison to the PL spectra, the hypochromatic shift of EL emissions should be attributed to the optical microcavity effect of nanometer-scaled devices. 22′DPEPO and 24′DPEPO-based devices achieved the highest color purity with Commission Internationale Ed I'eclairage (CIE) coordinates of (0.16, 0.17), owing to their rigid structures and LE-featured excited states (Table 2). In contrast, the relatively flexible structures and partially CT-type excited states of 24DPEPO and 44′DPEPO rendered broadened EL spectra with CIE coordinates of (0.16, 0.20) and (0.16, 0.18), respectively. The remarkably increased intensity of the long-wavelength part in the EL emission from 24DPEPO-based devices was actually due to the decreased intensity of the short-wavelength part, which is readily quenched through nonradiative transitions during host–dopant interactions.
| Host | Operation voltagea (V) | Maximum efficiencyb | Efficiency roll-offc (%) | Emission peak (nm) | CIE (x, y) | ||
|---|---|---|---|---|---|---|---|
| CE | PE | EQE | |||||
| a Operating voltages for onset, 100 and 1000 cd m−2. b The maximum efficiencies of CE (cd A−1), PE (lm W−1) and EQE (%). c At 100 and 1000 cd m−2. | |||||||
| 22′DPEPO | 3.7, 5.4, 7.6 | 25.3, 21.5, 16.7 | 18, 50 | 44, 75 | 19, 50 | 460 | 0.16, 0.17 |
| 24DPEPO | 4.3, 6.5, — | 5.7, 4.2, 3.7 | 44, — | 63, — | 44, — | 460 | 0.16, 0.20 |
| 24′DPEPO | 3.5, 5.1, 7.0 | 30.6, 27.5, 20.1 | 8, 33 | 37, 66 | 8, 32 | 460 | 0.16, 0.17 |
| 44′DPEPO | 3.6, 5.1, 7.5 | 20.8, 18.1, 13.1 | 7, 30 | 35, 53 | 7, 30 | 460 | 0.16, 0.18 |
I–V characteristics of these light-emitting devices showed a trend consistent with the charge mobility of their host materials (Fig. 6b). 44′DPEPO endowed its devices with the highest J, while the J of 24′DPEPO-based devices was secondary. Along with increasing operation voltage, the effect of 44′DPEPO and 24′DPEPO on enhancing J became more prominent. However, the difference between the J of these devices was remarkably smaller than that between the J of the single-carrier transporting devices, especially at low driving voltages, which indicated the significant contribution of DMAC-DPS to carrier injection and transportation. Nevertheless, 44′DPEPO and 24′DPEPO endowed their devices with a maximum luminance of ∼6500 cd m−2, which was more than 2000 cd m−2 higher than that of 22′DPEPO-based devices; meanwhile, when using 24DPEPO as a host, the maximum luminance was less than 1000 cd m−2. The higher luminance of 44′DPEPO and 24′DPEPO-based devices corresponded to higher exciton concentrations owing to larger and balanced charge flux in their EMLs. Furthermore, the driving voltages of 44′DPEPO-based devices were 3.6, 5.1 and 7.5 V for onset, 100 and 1000 cd m−2, respectively, which were lower than those of 22′DPEPO-based analogues (Table 2). 24′DPEPO further reduced the driving voltages of its devices to 3.5, 5.1 and 7.0 V, which were about 1 V lower than those of 24DPEPO-based devices. In comparison to 44′DPEPO with stronger electroactivity, the lower driving voltages achieved by 24′DPEPO actually reflected the higher exciton harvesting efficiency of its devices.25
On the basis of the four-layer device structure, 22′DPEPO-based devices realized maximum efficiencies of 25.3 cd A−1 for current efficiency (CE), 21.5 lm W−1 for power efficiency (PE) and 16.7% for EQE, which were about 20% higher than those of 44′DPEPO-based devices (Fig. 6c and Table 2). The superiority of 22′DPEPO in the maximum efficiencies should be attributed to the effectively suppressed collision-induced quenching through reducing host–dopant interactions by virtue of the strong steric effect of its ortho-DPPOs. Significantly, in addition to the advantages of ortho-substituted DPPO, the unsymmetrical separation structure of 24′DPEPO further weakens intermolecular interactions, improving the maximum efficiencies to 30.6 cd A−1, 27.5 lm W−1 and 20.1%, which are among the best results for deep-blue TADF devices reported so far.4a,5d,5e,5i,5j,16a Therefore, compared to 22′DPEPO, the asymmetric configuration of 24′DPEPO gives rise to the remarkable efficiency increase by 20%. In contrast, the maximum efficiencies of 24DPEPO-based devices were the lowest among these devices, suffering from serious exciton quenching through structural relaxation-induced nonradiative transitions.
Despite larger maximum efficiencies, 22′DPEPO-based devices showed serious efficiency reduction with roll-offs as large as 18 and 50% at 100 and 1000 cd m−2, respectively. With the best electrical performance among mDPEPO, 44′DPEPO facilitated charge flux balance in the EML of its devices, enhanced exciton recombination and reduced redundant charge concentration, thereby decreasing exciton-polaron collision probability for TPQ suppression. Consequently, on the contrary to 22′DPEPO, 44′DPEPO supported its devices with remarkably reduced roll-offs as low as 7 and 30% at 100 and 1000 cd m−2, respectively. Significantly, 24′DPEPO-based devices also realized low roll-offs of 8 and 32% at 100 and 1000 cd m−2, respectively, comparable to those of 44′DPEPO-based analogues. The efficiency roll-offs of 24′DPEPO and 44′DPEPO-based devices are among the lowest values reported so far for deep-blue TADF diodes.4a,5d,5e,5i,5j,16a If proportional to their charge mobility, TPQ suppression in these devices can be roughly evaluated in the order 44′DPEPO > 24′DPEPO > 22′DPEPO > 24DPEPO. On the other hand, compared to the exposed T1 states of 22′DPEPO and 44′DPEPO, the extremely condensed T1 state of 24′DPEPO can more effectively restrain collision-induced quenching, which offsets the slight inferiority of 24′DPEPO to 44′DPEPO in charge mobility. It would be rational that to some extent the enhanced efficiency stability at high luminance would reflect improved device durability, since device aging can be accelerated at high luminance to generate charge traps and exciton quenching sites, making both luminance and efficiency decrease.5e,26 In this case, 24′DPEPO with reduced efficiency roll-off would be superior to 22′DPEPO in device stability.
The state-of-the-art performance of 24′DPEPO-based devices clearly shows that for high-energy-gap blue TADF host materials, the strong steric effect is beneficial to suppress TTA for high efficiencies; while, under high driving voltages, the improved charge mobility can facilitate flux balance in EML to suppress TPQ. Significantly, with an effectively protected T1 state with an extremely condensed location, the EL performance of asymmetric 24′DPEPO was far beyond simple integration of those of 22′DPEPO and 44′DPEPO, verifying the great importance of suppressing host-involved intermolecular interactions. The sky-blue devices of another TADF dye 1,2-bis(carbazol-9-yl)-4,5-dicyanobenzene (2CzPN) employing 24′DPEPO also showed higher efficiencies and reduced roll-off in contrast to 22′DPEPO, displaying the universality of the host design strategy for 24′DPEPO (Fig. S6†).
3. Experimental section
3.1. Materials and instruments
All the reagents and solvents used for the synthesis were purchased from Aldrich and Acros and used without further purification. DPEPO and DPESPO bromides were prepared according to our previous report.20d1H NMR spectra were recorded using a Varian Mercury plus 400NB spectrometer relative to tetramethylsilane (TMS) as an internal standard. Molecular masses were determined using a FINNIGAN LCQ Electro-Spraying Ionization-Mass Spectrometer (ESI-MS), or a MALDI-TOF-MS. Elemental analyses were performed on a Vario EL III elemental analyzer. Absorption and photoluminescence (PL) emission spectra of the target compounds were measured using a SHIMADZU UV-3150 spectrophotometer and a SHIMADZU RF-5301PC spectrophotometer, respectively. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed on Shimadzu DSC-60A and DTG-60A thermal analyzers under a nitrogen atmosphere at a heating rate of 10 °C min−1. Cyclic voltammetric (CV) studies were conducted using an Eco Chemie B. V. AUTOLAB potentiostat in a typical three-electrode cell with a platinum sheet working electrode, a platinum wire counter electrode, and a silver/silver nitrate (Ag/Ag+) reference electrode. All electrochemical experiments were carried out under a nitrogen atmosphere at room temperature in dichloromethane. Phosphorescence spectra were measured in dichloromethane using an Edinburgh FPLS 920 fluorescence spectrophotometer at 77 K cooled by liquid nitrogen with a delay of 300 μs using the Time-Correlated Single Photon Counting (TCSPC) method with a microsecond pulsed xenon light source for 10 μs to 10 s lifetime measurement, and a synchronization photomultiplier for signal collection and the Multi-Channel Scaling Mode of a PCS900 fast counter PC plug-in card for data processing. The absolute PLQY of the films was measured with an integrating sphere.
3.2. Density functional theory (DFT) and time-dependent DFT (TDDFT) calculations
DFT computations were carried out with different parameters for structure optimizations and vibration analyses. The ground states and triplet states of molecules in vacuum were optimized by the restricted and unrestricted formalism of Beck's three-parameter hybrid exchange functional27 and Lee, Yang and Parr’s correlation functional28 (B3LYP)/6-31+G(d,p), respectively. The fully optimized stationary points were further characterized by harmonic vibrational frequency analysis to ensure that real local minima had been found without imaginary vibrational frequency. The total energies were also corrected by zero-point energy both for the ground state and triplet state. Natural transition orbital (NTO) analysis was performed on the basis of optimized ground-state geometries at the level of (B3LYP)/6-31+G(d,p).23 The contours were visualized with Gaussview 5.0. All computations were performed using the Gaussian 09 package.293.3. Device fabrication and testing
Before loading into a deposition chamber, the ITO substrate was cleaned with detergents and deionized water, dried in an oven at 120 °C for 4 h, and treated with oxygen plasma for 3 min. Devices were fabricated by evaporating the organic layers at a rate of 0.1–0.2 nm s−1 onto the ITO substrate sequentially at a pressure below 4 × 10−4 Pa. Onto the electron-transporting layer, a layer of LiF with 1 nm thickness was deposited at a rate of 0.1 nm s−1 to improve electron injection. Finally, a 100 nm-thick layer of Al was deposited at a rate of 0.6 nm s−1 as the cathode. The emission area of the devices was 0.09 cm2 as determined by the overlap area of the anode and the cathode. After fabrication, the devices were immediately transferred to a glove box for encapsulation with glass cover slips using epoxy glue. The EL spectra and CIE coordinates were measured using a PR650 spectra colorimeter. The current–density–voltage and brightness–voltage curves of the devices were measured using a Keithley 4200 source meter and a calibrated silicon photodiode. All the measurements were carried out at room temperature under ambient conditions. For each structure, five devices were fabricated to confirm the performance repeatability. To make conclusions reliable, the data reported herein were the closest to the average results.4. Conclusions
A series of DPEPO-type host materials named mDPEPO as constitutional isomers with two DPPO groups substituted on a DPE core at either the ortho- or para-position were designed and prepared. The steric effect of ortho-substituted DPPO and the electron coupling effect of para-substituted DPPO were successfully integrated through the separation configuration of 24′DPEPO, giving rise to its harmonious excited-state characteristics and electrical performance inherited from 22′DPEPO and 44′DPEPO, respectively. The asymmetrical configuration and the dominant orientation effect of ortho-substituted DPPO on the T1 location extremely condense the T1 state of 24′DPEPO onto a single phenyl, completely protected from intermolecular interactions. Consequently, 24′DPEPO endowed its DMAC-DPS-based deep blue TADF diodes with state-of-the-art performance featuring high EQE beyond 20% and low roll-off at 32% at 1000 cd m−2, which was dramatically improved in comparison to those of 22′DPEPO and 44′DPEPO and successfully demonstrated its potential. This showed that besides common energy level optimization, more delicate and purposeful modulation on the optoelectronic properties of host materials is crucial for developing high-performance TADF diodes.Acknowledgements
JZ and DD contributed equally to this work. HX thanks Prof. Runfeng Chen (Nanjing University of Posts and Telecommunication) for his assistance in the TDDFT simulation. This project was financially supported by NSFC (61176020 and 51373050), the New Century Excellent Talents Supporting Program of the Ministry of Education (China) (NCET-12-0706), the Program for Innovative Research Team in University (MOE) (IRT-1237), the Science and Technology Bureau of Heilongjiang Province (ZD201402 and JC2015002), the Education Bureau of Heilongjiang Province (2014CJHB005), the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (141012) and Harbin Science and Technology Bureau (2015RAYXJ008).Notes and references
- D. M. de Leeuw, M. M. J. Simenon, A. R. Brown and R. E. F. Einerhand, Synth. Met., 1997, 87, 53 CrossRef CAS.
- (a) C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS; (b) J. Kido, M. Kimura and K. Nagai, Science, 1995, 267, 1332 CrossRef CAS PubMed; (c) M. A. Baldo, M. E. Thompson and S. R. Forrest, Nature, 2000, 403, 750 CrossRef CAS PubMed; (d) C. D. Muller, A. Falcou, N. Reckefuss, M. Rojahn, V. Wiederhirn, P. Rudati, H. Frohne, O. Nuyken, H. Becker and K. Meerholz, Nature, 2003, 421, 829 CrossRef PubMed; (e) Y. R. Sun, N. C. Giebink, H. Kanno, B. W. Ma, M. E. Thompson and S. R. Forrest, Nature, 2006, 440, 908 CrossRef CAS PubMed; (f) S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lussem and K. Leo, Nature, 2009, 459, 234 CrossRef CAS PubMed; (g) F. B. Dias, K. N. Bourdakos, V. Jankus, K. C. Moss, K. T. Kamtekar, V. Bhalla, J. Santos, M. R. Bryce and A. P. Monkman, Adv. Mater., 2013, 25, 3707 CrossRef CAS PubMed; (h) G. Schwartz, S. Reineke, T. C. Rosenow, K. Walzer and K. Leo, Adv. Funct. Mater., 2009, 19, 1319 CrossRef CAS; (i) Q. Wang and D. Ma, Chem. Soc. Rev., 2010, 39, 2387 RSC.
- (a) M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS; (b) H. Wu, L. Ying, W. Yang and Y. Cao, Chem. Soc. Rev., 2009, 38, 3391 RSC; (c) L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Gong and J. Kido, Adv. Mater., 2011, 23, 926 CrossRef CAS PubMed; (d) K. S. Yook and J. Y. Lee, Adv. Mater., 2012, 24, 3169 CrossRef CAS PubMed; (e) Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943 RSC; (f) H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259 RSC; (g) H. Xu, Q. Sun, Z. An, Y. Wei and X. Liu, Coord. Chem. Rev., 2015, 293–294, 228 CrossRef CAS.
- (a) H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234 CrossRef CAS PubMed; (b) H. Nakanotani, T. Higuchi, T. Furukawa, K. Masui, K. Morimoto, M. Numata, H. Tanaka, Y. Sagara, T. Yasuda and C. Adachi, Nat. Commun., 2014, 5, 4016 CAS; (c) V. Jankus, P. Data, D. Graves, C. McGuinness, J. Santos, M. R. Bryce, F. B. Dias and A. P. Monkman, Adv. Funct. Mater., 2014, 24, 6178 CrossRef CAS; (d) Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 26, 7931 CrossRef CAS PubMed.
- (a) J.-Y. Hu, Y.-J. Pu, F. Satoh, S. Kawata, H. Katagiri, H. Sasabe and J. Kido, Adv. Funct. Mater., 2014, 24, 2064 CrossRef CAS; (b) W. Li, D. Liu, F. Shen, D. Ma, Z. Wang, T. Feng, Y. Xu, B. Yang and Y. Ma, Adv. Funct. Mater., 2012, 22, 2797 CrossRef CAS; (c) C. Mayr, S. Y. Lee, T. D. Schmidt, T. Yasuda, C. Adachi and W. Brütting, Adv. Funct. Mater., 2014, 24, 5232 CrossRef CAS; (d) V. Jankus, C. J. Chiang, F. Dias and A. P. Monkman, Adv. Mater., 2013, 25, 1455 CrossRef CAS PubMed; (e) M. Kim, S. K. Jeon, S.-H. Hwang and J. Y. Lee, Adv. Mater., 2015, 27, 2515 CrossRef CAS PubMed; (f) X.-L. Chen, R. Yu, Q.-K. Zhang, L.-J. Zhou, X.-Y. Wu, Q. Zhang and C.-Z. Lu, Chem. Mater., 2013, 25, 3910 CrossRef CAS; (g) T. Hofbeck, U. Monkowius and H. Yersin, J. Am. Chem. Soc., 2015, 137, 399 CrossRef CAS PubMed; (h) M. J. Leitl, V. A. Krylova, P. I. Djurovich, M. E. Thompson and H. Yersin, J. Am. Chem. Soc., 2014, 136, 16032 CrossRef CAS PubMed; (i) Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706 CrossRef CAS PubMed; (j) S. Hirata, Y. Sakai, K. Masui, H. Tanaka, S. Y. Lee, H. Nomura, N. Nakamura, M. Yasumatsu, H. Nakanotani, Q. Zhang, K. Shizu, H. Miyazaki and C. Adachi, Nat. Mater., 2015, 14, 330 CrossRef CAS PubMed; (k) D. Zhang, L. Duan, D. Zhang, J. Qiao, G. Dong, L. Wang and Y. Qiu, Org. Electron., 2013, 14, 260 CrossRef CAS; (l) H. Wang, L. Meng, X. Shen, X. Wei, X. Zheng, X. Lv, Y. Yi, Y. Wang and P. Wang, Adv. Mater., 2015, 27, 4041 CrossRef CAS PubMed; (m) W. Y. Hung, G. C. Fang, Y. C. Chang, T. Y. Kuo, P. T. Chou, S. W. Lin and K. T. Wong, ACS Appl. Mater. Interfaces, 2013, 5, 6826 CrossRef CAS PubMed; (n) X.-K. Liu, Z. Chen, C.-J. Zheng, C.-L. Liu, C.-S. Lee, F. Li, X.-M. Ou and X.-H. Zhang, Adv. Mater., 2015, 27, 2378 CrossRef CAS PubMed; (o) D. G. Cuttell, S.-M. Kuang, P. E. Fanwick, D. R. McMillin and R. A. Walton, J. Am. Chem. Soc., 2002, 124, 6 CrossRef CAS PubMed.
- (a) B. S. Kim and J. Y. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 8396 CrossRef CAS PubMed; (b) B. S. Kim and J. Y. Lee, Adv. Funct. Mater., 2014, 24, 3970–3977 CrossRef CAS; (c) Y. J. Cho, K. S. Yook and J. Y. Lee, Adv. Mater., 2014, 26, 4050 CrossRef CAS PubMed; (d) L.-S. Cui, Y.-M. Xie, Y.-K. Wang, C. Zhong, Y.-L. Deng, X.-Y. Liu, Z.-Q. Jiang and L.-S. Liao, Adv. Mater., 2015, 27, 4213 CrossRef CAS PubMed; (e) D. Zhang, L. Duan, C. Li, Y. Li, H. Li, D. Zhang and Y. Qiu, Adv. Mater., 2014, 26, 5050 CrossRef CAS PubMed.
- Z. B. Wang, M. G. Helander, M. T. Greiner, J. Qiu and Z. H. Lu, J. Appl. Phys., 2010, 107, 034506 CrossRef.
- G. Méhes, K. Goushi, W. J. Potscavage Jr and C. Adachi, Org. Electron., 2014, 15, 2027 CrossRef.
- (a) S. Reineke, K. Walzer and K. Leo, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125328 CrossRef; (b) J. Kalinowski, W. Stampor, J. Mecedilzdotyk, M. Cocchi, D. Virgili, V. Fattori and P. Di Marco, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 235321 CrossRef; (c) S. Reineke and M. A. Baldo, Phys. Status Solidi A, 2012, 209, 2341 CrossRef CAS; (d) M. A. Baldo, C. Adachi and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 10967 CrossRef CAS.
- H. Zamani Siboni and H. Aziz, Org. Electron., 2013, 14, 2510 CrossRef CAS.
- S. K. Lower and M. A. El-Sayed, Chem. Rev., 1966, 66, 199 CrossRef CAS.
- D. Hertel and K. Meerholz, J. Phys. Chem. B, 2007, 111, 12075 CrossRef CAS PubMed.
- (a) B. Zhang, G. Tan, C.-S. Lam, B. Yao, C.-L. Ho, L. Liu, Z. Xie, W.-Y. Wong, J. Ding and L. Wang, Adv. Mater., 2012, 24, 1873 CrossRef CAS PubMed; (b) C. Han, Z. Zhang, H. Xu, J. Li, G. Xie, R. Chen, Y. Zhao and W. Huang, Angew. Chem., Int. Ed., 2012, 51, 10104 CrossRef CAS PubMed.
- C. Han, L. Zhu, F. Zhao, Z. Zhang, J. Wang, Z. Deng, H. Xu, J. Li, D. Ma and P. Yan, Chem. Commun., 2014, 50, 2670 RSC.
- W. Huang, B. Mi and Z. Gao, Organic Electronics, Science Press, Beijing, 2011 Search PubMed.
- (a) Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326 CrossRef CAS; (b) D. R. Lee, M. Kim, S. K. Jeon, S.-H. Hwang, C. W. Lee and J. Y. Lee, Adv. Mater., 2015, 27, 5861 CrossRef CAS PubMed; (c) M. Numata, T. Yasuda and C. Adachi, Chem. Commun., 2015, 51, 9443 RSC.
- C. Han, Y. Zhao, H. Xu, J. Chen, Z. Deng, D. Ma, Q. Li and P. Yan, Chem.–Eur. J., 2011, 17, 5800 CrossRef CAS PubMed.
- W. Song, I. H. Lee, S.-H. Hwang and J. Y. Lee, Org. Electron., 2015, 23, 138 CrossRef CAS.
- W. Song, I. Lee and J. Y. Lee, Adv. Mater., 2015, 27, 4358–4363 CrossRef CAS PubMed.
- (a) C. Han, L. Zhu, J. Li, F. Zhao, Z. Zhang, H. Xu, Z. Deng, D. Ma and P. Yan, Adv. Mater., 2014, 26, 7070 CrossRef CAS PubMed; (b) D. Yu, F. Zhao, C. Han, H. Xu, J. Li, Z. Zhang, Z. Deng, D. Ma and P. Yan, Adv. Mater., 2012, 24, 509 CrossRef CAS PubMed; (c) Y. Tao, J. Xiao, C. Zheng, Z. Zhang, M. Yan, R. Chen, X. Zhou, H. Li, Z. An, Z. Wang, H. Xu and W. Huang, Angew. Chem., Int. Ed., 2013, 52, 10491 CrossRef CAS PubMed; (d) C. Han, Z. Zhang, H. Xu, S. Yue, J. Li, P. Yan, Z. Deng, Y. Zhao, P. Yan and S. Liu, J. Am. Chem. Soc., 2012, 134, 19179 CrossRef CAS PubMed.
- (a) N. C. Giebink and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235215 CrossRef; (b) D. Song, S. Zhao, Y. Luo and H. Aziz, Appl. Phys. Lett., 2010, 97, 243304 CrossRef.
- C. Han, L. Zhu, J. Li, F. Zhao, H. Xu, D. Ma and P. Yan, Chem.–Eur. J., 2014, 20, 16350 CrossRef CAS PubMed.
- R. L. Martin, J. Chem. Phys., 2003, 118, 4775 CrossRef CAS.
- (a) Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685 CrossRef CAS PubMed; (b) S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386 CrossRef CAS.
- Z. Zhang, Z. Zhang, D. Ding, Y. Wei, H. Xu, J. Jia, Y. Zhao, K. Pan and W. Huang, J. Phys. Chem. C, 2014, 118, 20559 CAS.
- Y. Noguchi, H.-J. Kim, R. Ishino, K. Goushi, C. Adachi, Y. Nakayama and H. Ishii, Org. Electron., 2015, 17, 184 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D. 1, Gaussian, Inc., Wallingford CT, USA, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc04848f |
| This journal is © The Royal Society of Chemistry 2016 |