Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploiting recognition-mediated assembly and reactivity in [2]rotaxane formation†
Annick
Vidonne
,
Tamara
Kosikova
 and
Douglas
Philp
and
Douglas
Philp
 *
*
School of Chemistry and EaStCHEM, University of St Andrews, North Haugh St Andrews, Fife KY16 9ST, UK. E-mail: d.philp@st-andrews.ac.uk; Fax: +44 (0)1334 463808; Tel: +44 (0)1334 467264
First published on 15th January 2016
Abstract
A small molecular reaction network exploits recognition-mediated reactive processes in order to drive the assembly and formation of both a self-replicating linear template (thread) and a [2]rotaxane, in which the linear template is encircled by a diamide macrocycle. Complementary recognition sites, placed at strategic positions on the reactive building blocks, drive these assembly and replication processes. Template-instructed experiments show that the thread is capable of efficient self-replication and that no cross-catalytic relationships exist between the thread and the [2]rotaxane. The rate of [2]rotaxane formation is insensitive to the addition of a preformed template, however, [2]rotaxane formation does show enhanced diastereoselectivity, most likely originating from its recognition-mediated formation through a ternary reactive complex.
Introduction
In recent years, advances in supramolecular synthesis have permitted the construction of a diverse array of molecular machinery1 and nanometre-scale2 objects. In general, the approaches used in these construction processes have featured sophisticated algorithmic self-organisation3 and self-assembly4 processes to control the precise arrangement of structural subunits in space. Despite the attractions of self-assembly processes, other construction methods have also been discussed. In particular, Drexler highlighted5 a radical alternative – the fabrication of replicable networks via atom-by-atom engineering. Whilst this proposal as described6 may be implausible, replication processes remain an attractive alternative to the traditional synthetic approaches. In the last 20 years, numerous examples of synthetic replicating systems capable of templating and catalysing their own synthesis have appeared7 in the chemical literature.Self-replication8 is a subset of autocatalytic reactions.9 In an autocatalytic reaction, a product formed during the reaction acts as a catalyst for its own formation. A system in which self-replication is operating can be defined as an autocatalytic reaction capable of transmitting (and amplifying) structural information. All the synthetic self-replicating systems reported to date utilise precursor subunits that incorporate mutually complementary recognition elements and reactive functionalities. These features have been combined to engineer linear self-complementary platforms capable of autocatalytic self-propagation.
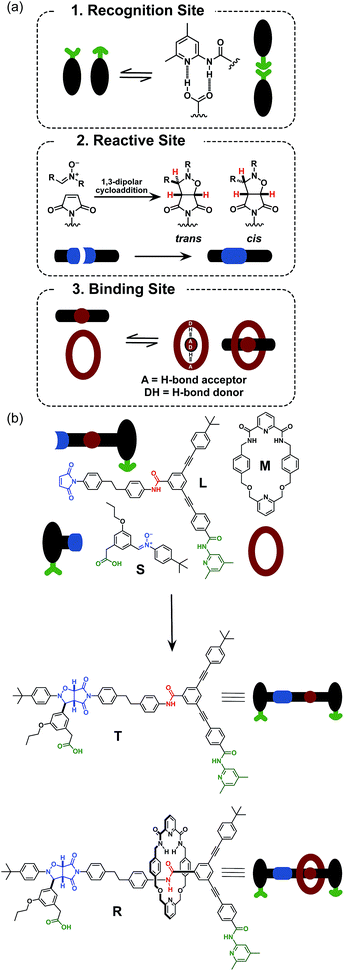
Rotaxanes are archetypal examples of interlocked molecular architectures;10 they consist of a linear molecule, known as the thread, that is encircled by a macrocycle and end-groups (stoppers) large enough to prevent dethreading. Recently, we described11 a kinetics framework for embedding the synthesis of a [2]rotaxane within a self-replicating network. This strategy (Fig. 1) was based on incorporating an efficient replicating template7d,12 as one of the stoppers on the rotaxane. Whilst this approach was successful in assembling and replicating the [2]rotaxane, it suffers from a number of shortcomings.
 | ||
| Fig. 1 Cartoon and chemical structure representations of a kinetic framework utilising orthogonal recognition processes to drive11 the assembly and replication of a [2]rotaxane. Green represents the recognition elements required for self-replication; blue represents the reactive components and red highlights the macrocycle-binding site required for rotaxane assembly. | ||
Firstly, since the stopper is at the periphery of the structure (Fig. 1), the presence or absence of the macrocycle, in principle, has little effect on the replication process. This shortcoming manifests itself as strong crosscatalytic relationships within the reaction network. In fact, in this system, the [2]rotaxane is an equally good catalyst for the formation of the thread as the thread is for its own formation. This crosscatalysis introduces an inherent limitation to the achievable thread![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [2]rotaxane ratio.
[2]rotaxane ratio.
More seriously, there are several unproductive binding modes available to the macrocycle within this design that prevent the optimal replication of the [2]rotaxane. In particular, linear component L* contains the diaryl amide binding site for the macrocycle (Fig. 1, red), the nitrone (Fig. 1, blue) that reacts with maleimide S* to form the replicating framework and the amidopyridine recognition element (Fig. 1, green). In order to ensure optimal replication, macrocycle M11,13 must bind only to the diaryl amide recognition site. In practice, however, there are significant populations of complexes where the macrocycle is bound either to the nitrone or the amidopyridine present in L*. These unproductive binding modes reduce the replication efficiency by around 75%.
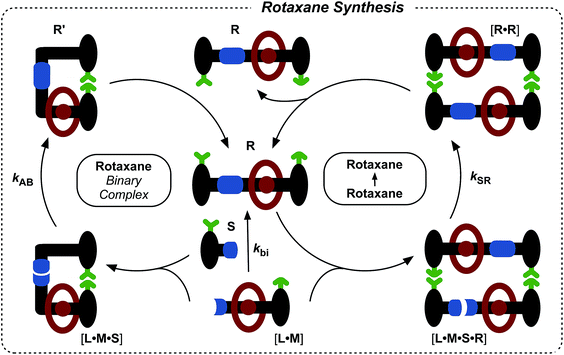
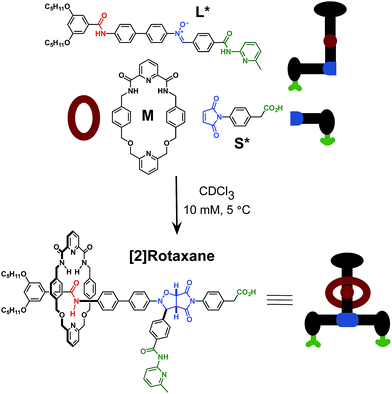
Here, we report an alternative strategy14 for the integration of rotaxane assembly and replication. This approach (Fig. 2) involves the isolation of all of the recognition events required for the assembly and replication from each other at specific loci within the molecular structures of the [2]rotaxane components. In order to achieve this isolation, one of the recognition elements required to drive the replication must be placed on each of the two stoppers (Fig. 2a). This design change permits the use of the same 1,3-dipolar cycloaddition between a nitrone and a maleimide to connect the maleimide L and nitrone stopper S components together. This reaction affords two pairs of diastereoisomeric cycloadducts (Fig. 2a) – labelled7d,15trans and cis, and the reaction is rather insensitive to electronic effects, usually giving a trans/cis ratio between 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3.3
1 and 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. We have described7d,11,12,14,15 the use of this reaction in a number of successful recognition-mediated reaction systems. The same macrocycle and diaryl amide binding motif, used11 in our previous design, can also be utilised in this context. These design changes lead, ultimately, to the identification of the target thread T and [2]rotaxane R (Fig. 2b). Thread T and rotaxane R can be constructed from the corresponding maleimide L, nitrone S and, for the rotaxane only, macrocycle M (Fig. 2b). This new design removes any possibility of unproductive binding from the system. Macrocycle M can associate only with the target diaryl amide (Fig. 2b, red) present in L. The two methyl groups present on the amidopyridine ring in L (Fig. 2b, green) prevent the association of M with this unit. Similarly, the n-propyloxy and tert-butyl groups present in S prevent the association of the macrocycle with the nitrone.
1. We have described7d,11,12,14,15 the use of this reaction in a number of successful recognition-mediated reaction systems. The same macrocycle and diaryl amide binding motif, used11 in our previous design, can also be utilised in this context. These design changes lead, ultimately, to the identification of the target thread T and [2]rotaxane R (Fig. 2b). Thread T and rotaxane R can be constructed from the corresponding maleimide L, nitrone S and, for the rotaxane only, macrocycle M (Fig. 2b). This new design removes any possibility of unproductive binding from the system. Macrocycle M can associate only with the target diaryl amide (Fig. 2b, red) present in L. The two methyl groups present on the amidopyridine ring in L (Fig. 2b, green) prevent the association of M with this unit. Similarly, the n-propyloxy and tert-butyl groups present in S prevent the association of the macrocycle with the nitrone.
Results and discussion
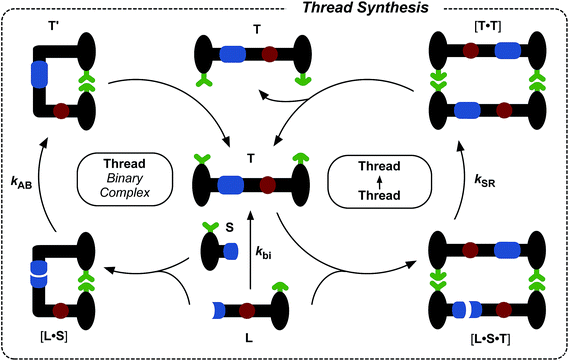
The synthesis of maleimide L, incorporating the maleimide reactive site, the amidopyridine recognition site and the amide-binding site was accomplished14 in five steps starting from methyl 3-bromo-5-iodobenzoate (for details, see ESI†). Compound S, incorporating the nitrone reactive site and the carboxylic acid recognition site, and its seven-step synthesis, as well as the synthesis of macrocycle M, have been reported11,13,14 previously.Initially, we wished to establish that thread T was capable of replication in isolation from the macrocycle. Therefore, we undertook a kinetic analysis of the reactions involving the thread only, shown in Fig. 3. In principle, thread T can be formed through three separate pathways. The first pathway is the uncatalysed bimolecular reaction between maleimide L and nitrone S to form T (Fig. 3, lower centre). Two recognition-mediated pathways can also lead to the formation of thread T. Firstly, the association of maleimide L with nitrone S through their mutually complementary recognition sites results in the formation of the binary complex [L·S]. The reaction between L and S within this complex is pseudounimolecular and affords the closed template T′. This structure is, in principle, in equilibrium with the open thread T (Fig. 3, top left). Secondly, open thread T can assemble L and S to form the ternary complex [L·S·T] within which the reaction between L and S is also pseudounimolecular (Fig. 3, right). The resulting product duplex [T·T] is capable of dissociating to return two molecules of thread to the autocatalytic cycle, thus completing the replication process.
In order to determine which of these recognition-mediated pathways are active in this system, we performed a series of kinetic experiments. A solution of maleimide L and nitrone S, with starting concentrations of the two reagents of 20 mM, was prepared in CDCl3. This concentration was chosen for all experiments reported here as it provides a compromise between a number of competing factors. There is a complex interplay between the various binding equilibria illustrated in Fig. 3 and the rates of the recognition-mediated reactions. It is crucial to balance the Kd (4 mM) for the single point associations, e.g.L + S → [L·S], with the stability of the ternary complex [L·S·T]. The reaction should be performed at a concentration above the Kd for the single point association, but not so high that the binary complex [L·S] is dominant in the system. In general, there is an optimum concentration window7d in which replication can be observed, and we estimated that a concentration of 20 mM would be close to the centre of this window for this system.
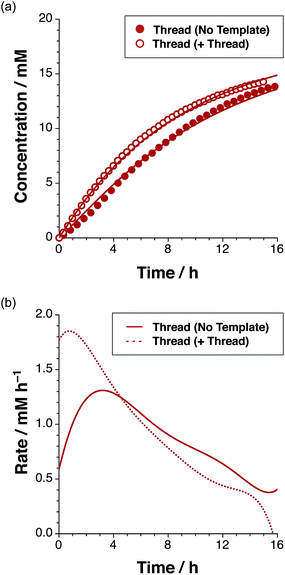
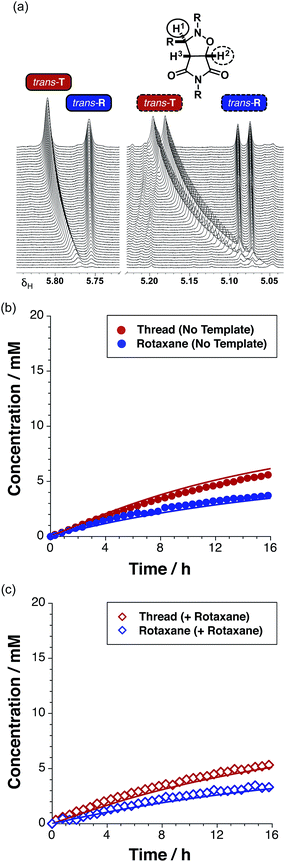
The time course of the reaction (Fig. 4a, filled red circles) at 15 °C was evaluated using 500 MHz 1H NMR spectroscopy. The disappearance of the resonance arising from the maleimide protons present in L at δ 6.85 and the simultaneous appearance of the resonances corresponding to trans-T, initially at δ 5.78, were monitored (for details, see ESI†). The formation of T is very diastereoselective, therefore quantification of the small amount of cis-T, characterised by the resonances around δ 4.76, was challenging. Therefore, the concentration–time profiles presented here show only the data for trans-T.
After 15 hours, the reaction (Fig. 4a) between maleimide L and nitrone S reached 67% overall conversion and the diastereoselectivity was excellent – 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in favour of trans-T. The formation of the major isoxazolidine product, trans-T, displays a rate16 maximum (1.31 mM h−1 at t = 3.2 h), characteristic of a self-replicating system. In order to confirm the ability of trans-T to template its own formation, i.e. to replicate, a further set of experiments were conducted. A solution of maleimide L and nitrone S with starting concentrations of the two reagents of 20 mM, was prepared in CDCl3. Pre-synthesised trans-T (38 mol%) was added to this solution and the time course of this reaction at 15 °C was, once again, evaluated using 500 MHz 1H NMR spectroscopy (Fig. 4a). In this case, an increase in the initial rate of formation of trans-T (71% conversion after 15 h, with 20
1 in favour of trans-T. The formation of the major isoxazolidine product, trans-T, displays a rate16 maximum (1.31 mM h−1 at t = 3.2 h), characteristic of a self-replicating system. In order to confirm the ability of trans-T to template its own formation, i.e. to replicate, a further set of experiments were conducted. A solution of maleimide L and nitrone S with starting concentrations of the two reagents of 20 mM, was prepared in CDCl3. Pre-synthesised trans-T (38 mol%) was added to this solution and the time course of this reaction at 15 °C was, once again, evaluated using 500 MHz 1H NMR spectroscopy (Fig. 4a). In this case, an increase in the initial rate of formation of trans-T (71% conversion after 15 h, with 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 trans
1 trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio), but not of cis-T, was observed and the rate–time profile displays a rate maximum (1.85 mM h−1) at t = 0.75 h (Fig. 4b). This experiment establishes unambiguously that trans-T acts as a template for its own formation, i.e. it is capable of replication.
cis ratio), but not of cis-T, was observed and the rate–time profile displays a rate maximum (1.85 mM h−1) at t = 0.75 h (Fig. 4b). This experiment establishes unambiguously that trans-T acts as a template for its own formation, i.e. it is capable of replication.
By contrast, in the reaction between maleimide L and a recognition-disabled nitrone stopper (for details, see ESI†), which does not possess the carboxylic acid recognition site, the overall conversion after 15 hours was only 37% for trans-T and 16% for cis-T, and the diastereoselectivity was only 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in favour of the trans isoxazolidine. The reliance of the system on molecular recognition was further demonstrated by examining the formation of T in the presence of an inhibitor (for details, see ESI†) – 2.6 equivalents of 4-bromophenylacetic acid. This compound, although unreactive, is still capable of binding to the amidopyridine recognition sites present in L and T, thereby interfering with the recognition processes critical to the operation of the autocatalytic cycle. In this experiment, a decrease in the rate of the reaction, conversion to trans-T reached only 36% after 15 h, was observed and the ratio of trans-T to cis-T was 8.9
1 in favour of the trans isoxazolidine. The reliance of the system on molecular recognition was further demonstrated by examining the formation of T in the presence of an inhibitor (for details, see ESI†) – 2.6 equivalents of 4-bromophenylacetic acid. This compound, although unreactive, is still capable of binding to the amidopyridine recognition sites present in L and T, thereby interfering with the recognition processes critical to the operation of the autocatalytic cycle. In this experiment, a decrease in the rate of the reaction, conversion to trans-T reached only 36% after 15 h, was observed and the ratio of trans-T to cis-T was 8.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after 15 h. The conversion to trans-T observed in the inhibitor experiment is nearly identical to the conversion observed in the kinetic experiment where the thread formation can proceed only through the bimolecular pathway (37% after 15 h). This decrease in conversion was accompanied by the disappearance of the sigmoidal concentration–time profile for trans-T. Taken together, these kinetic experiments clearly demonstrate that the formation of trans-T is the result of a recognition-mediated replication process.
1 after 15 h. The conversion to trans-T observed in the inhibitor experiment is nearly identical to the conversion observed in the kinetic experiment where the thread formation can proceed only through the bimolecular pathway (37% after 15 h). This decrease in conversion was accompanied by the disappearance of the sigmoidal concentration–time profile for trans-T. Taken together, these kinetic experiments clearly demonstrate that the formation of trans-T is the result of a recognition-mediated replication process.
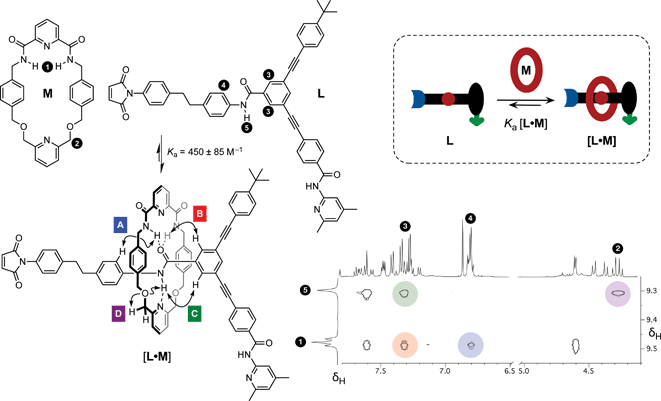
Having established the replicating ability of the core thread structure, we next sought to establish the viability of the linear component L as a suitable host for macrocycle M. The association of the macrocycle to the amide binding site is a key feature of the rotaxane. The position of the equilibrium between the linear component L and macrocycle M (Fig. 5, cartoon) and the corresponding [L·M] complex is crucial to the success of the formation of the [2]rotaxane. The ability of maleimide L to thread through the cavity of macrocycle M and to associate with the diaryl amide was assessed by performing a binding experiment. A solution containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of macrocycle M and maleimide L (25 mM each) in CDCl3 was prepared and analysed at 0 °C using 1H NMR spectroscopy. The 499.9 MHz 1H NMR spectrum of this mixture (for details, see ESI†) exhibits three different sets of resonances, corresponding to (i) free M, (ii) uncomplexed L, and (iii) the complex [L·M]. This observation indicates that the bound and unbound species equilibrate slowly with one another on the 1H NMR chemical shift timescale. This phenomenon allowed the determination of the association constant using17 the single-point method and a value for Ka was estimated to be 450 ± 85 M−1 in CDCl3 at 0 °C. The chemical shift changes observed are entirely consistent with pseudorotaxane formation. The resonance arising from the macrocycle NH protons (Fig. 5, protons marked 1) is shifted downfield (+1.37 ppm) as a result of the hydrogen bonding between these protons and the maleimide amide carbonyl group. The upfield shifts for the resonances of the macrocycle phenylene protons are characteristic of protons residing in the shielding zone of an aromatic ring. The downfield resonance arising from the NH proton associated with the amide in L, which is the binding target of macrocycle M, (Fig. 5, proton marked 5, δ 9.29) suggests that this proton is hydrogen bonded to the macrocycle pyridine nitrogen atom. Additionally, a complex pattern resulting from the resonances of the macrocycle methylene groups was observed; the end-to-end asymmetry of L renders the protons located on opposite faces of the macrocycle diastereotopic. A 1H–1H ROESY NMR experiment (Fig. 5) gave detailed structural information about the [L·M] complex and the ROE cross peaks observed prove unambiguously that pseudorotaxane [L·M] has the expected geometry.
1 ratio of macrocycle M and maleimide L (25 mM each) in CDCl3 was prepared and analysed at 0 °C using 1H NMR spectroscopy. The 499.9 MHz 1H NMR spectrum of this mixture (for details, see ESI†) exhibits three different sets of resonances, corresponding to (i) free M, (ii) uncomplexed L, and (iii) the complex [L·M]. This observation indicates that the bound and unbound species equilibrate slowly with one another on the 1H NMR chemical shift timescale. This phenomenon allowed the determination of the association constant using17 the single-point method and a value for Ka was estimated to be 450 ± 85 M−1 in CDCl3 at 0 °C. The chemical shift changes observed are entirely consistent with pseudorotaxane formation. The resonance arising from the macrocycle NH protons (Fig. 5, protons marked 1) is shifted downfield (+1.37 ppm) as a result of the hydrogen bonding between these protons and the maleimide amide carbonyl group. The upfield shifts for the resonances of the macrocycle phenylene protons are characteristic of protons residing in the shielding zone of an aromatic ring. The downfield resonance arising from the NH proton associated with the amide in L, which is the binding target of macrocycle M, (Fig. 5, proton marked 5, δ 9.29) suggests that this proton is hydrogen bonded to the macrocycle pyridine nitrogen atom. Additionally, a complex pattern resulting from the resonances of the macrocycle methylene groups was observed; the end-to-end asymmetry of L renders the protons located on opposite faces of the macrocycle diastereotopic. A 1H–1H ROESY NMR experiment (Fig. 5) gave detailed structural information about the [L·M] complex and the ROE cross peaks observed prove unambiguously that pseudorotaxane [L·M] has the expected geometry.
Having established that the formation of the desired pseudorotaxane complex is possible, we wished to examine the formation of the rotaxane from the individual building blocks (Fig. 6). As with the formation of T, there are three main pathways that can afford rotaxane R within this kinetic framework. The bimolecular reaction between pseudorotaxane [L·M] (Fig. 6, centre) and stoppering reagent S affords the [2]rotaxane R. Stoppering reagent S and maleimide L bear complementary recognition sites on their stoppers, hence S and [L·M] can bind reversibly to form a reactive ternary complex [S·L·M], which results in the formation of closed rotaxane R′ (Fig. 6, left) through a recognition-mediated reaction. The closed form R′ is in equilibrium with the open rotaxane template R (Fig. 6, top left), which can also associate with the pseudorotaxane and stopper in a catalytic quaternary complex [L·M·S·R] (Fig. 6, right). Bond formation occurs between S and [L·M] to give the product duplex [R·R], which then dissociates to return two molecules of R to the start of the autocatalytic cycle (Fig. 6, right).
In order to ensure that rotaxane R can be formed using our three building blocks, its synthesis was attempted on a preparative scale. A mixture of maleimide L and macrocycle M was pre-equilibrated in chloroform at ambient temperature in order to allow the formation of the pseudorotaxane. The addition of nitrone S to this solution afforded, after six days at ambient temperature, rotaxane R in 46% yield, as a 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of its trans- and cis-diastereoisomers, and thread T in 45% yield, as a 35
1 mixture of its trans- and cis-diastereoisomers, and thread T in 45% yield, as a 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of its trans- and cis-diastereoisomers after purification using column chromatography. These products were characterised using a combination of one- and two-dimensional NMR experiments (see ESI†).
1 mixture of its trans- and cis-diastereoisomers after purification using column chromatography. These products were characterised using a combination of one- and two-dimensional NMR experiments (see ESI†).
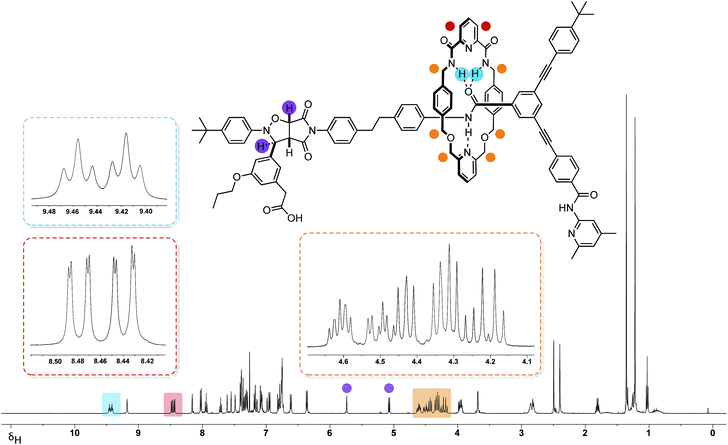
The 499.9 MHz 1H NMR spectrum of trans-R (Fig. 7) reveals a number of features consistent with the mechanical interlocking of the thread and macrocycle components. The resonances arising from the macrocycle NH protons are shifted significantly downfield (δ 9.46 and 9.42, Δδ −1.58 and −1.54) and appear (Fig. 7, blue) as two separate triplets. The observation of two resonances for these protons is consistent with the reduction in symmetry of the macrocycle from CS to C1 on the formation of the cycloadduct. This effect also manifests itself in the observation of two magnetically inequivalent pyridine ring protons (Fig. 7, red). The magnitudes of these chemical shift differences are surprising given the somewhat remote relative locations of the macrocycle and cycloadduct, but may reflect the fact that, in solution, the closed structure R′ (Fig. 6, top left) predominates. The end-to-end asymmetry that arises from the threading of the linear component through the macrocycle is maintained in the rotaxane and is responsible for the complex pattern observed (Fig. 7, orange) for the resonances arising from the CH2 groups present in the macrocycle.
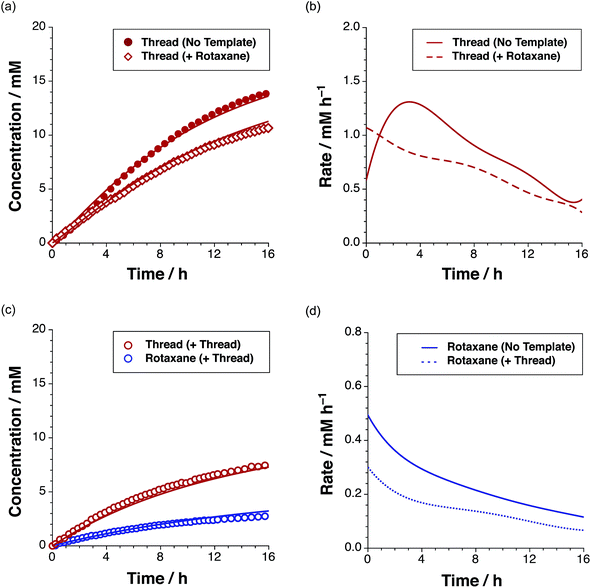
Having established that the rotaxane synthesis is viable, we next undertook a kinetic analysis aimed at assessing the relative efficiencies of the different reaction pathways available for the formation of the rotaxane. To this end, we first explored the formation of R in the absence of any preformed template. An equimolar solution of maleimide L and macrocycle M in CDCl3 was pre-equilibrated, before the addition of nitrone S to the mixture. The starting concentration of the three reagents was 19 mM. The time course of the reaction was evaluated using 500.1 MHz 1H NMR spectroscopy. The appearance (Fig. 8a) of the resonance that arises from trans-T (initially at δ 5.77), and of a resonance that arises from trans-R (δ 5.76), was monitored. Interestingly, while the resonance that arises from trans-T experiences a downfield shift from δ 5.77 to δ 5.81, the corresponding resonance arising from trans-R does not exhibit any chemical shift change. A similar pattern of shift changes is also observed for the resonance that arises from trans-T initially at δ 5.06, which experiences a downfield shift to δ 5.19 over the course of the reaction. However, the resonance that arises from trans-R (δ 5.08), once again, does not exhibit any chemical shift change. The spectroscopic behaviour exhibited by trans-T is typical18 for reactions mediated by autocatalytic processes, whereas, the spectroscopic behaviour exhibited by trans-R is more characteristic of a reaction that is either bimolecular or mediated19 by a binary reactive complex.
A concentration–time profile for the formation of both trans-T and trans-R (Fig. 8b) was constructed using deconvolution of these resonances, using tetrakis(trimethylsilyl) silane as an internal standard. The formation of the cis cycloadducts for the thread and rotaxane could not be monitored across the entire time course of the reaction as these resonances overlapped with others in the spectrum. Reaction of the equimolar solution of L, S and M (15 °C, CDCl3) resulted in 27% and 18% conversion to trans-T (maximum rate 0.47 mM h−1 at t = 1.25 h) and trans-R (maximum rate 0.49 mM h−1 at t = 0 h) cycloadducts after 15 hours, respectively. The ratio of trans-T (Fig. 8b, filled red circles) to trans-R (Fig. 8b, filled blue circles) was 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The sigmoidal rate profile (indicative of a self-replicating system) could not be unambiguously identified for either of the trans cycloadducts. In order to determine whether self-replication is operating within the rotaxane system, an experiment in which the reaction components were instructed with the preformed rotaxane trans-R was conducted. The addition of 38 mol% pre-synthesised rotaxane trans-R at the beginning of the reaction between M, L and S at 15 °C did not result in an increase in the conversion of either trans-T or trans-R (Fig 8c, empty red and blue diamonds, respectively) – reaching only 26% and 17% conversion, respectively, values mirroring closely those observed in the uninstructed kinetic experiment, giving a T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R ratio (1.5
R ratio (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) that is identical to that determined in the absence of the added template. This result suggests that rotaxane trans-R is not capable of templating and accelerating its own formation via the catalytic quaternary [L·M·S·R] complex pathway, nor is it a suitable template for the formation of trans-T.
1) that is identical to that determined in the absence of the added template. This result suggests that rotaxane trans-R is not capable of templating and accelerating its own formation via the catalytic quaternary [L·M·S·R] complex pathway, nor is it a suitable template for the formation of trans-T.
Additionally, we wanted to assess the role of recognition in the formation of the rotaxane through a reactive ternary complex [L·M·S]. To this end, we performed a kinetic experiment examining the reaction of M, L and S in the presence of 3.5 equivalents of 4-bromophenylacetic acid as an inhibitor (for details, see ESI†). The reaction resulted in a decrease in the conversion for both trans-T (18%) and trans-R (13%) after 15 hours, resulting in T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R of 1.4
R of 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The decrease in the conversion of both the thread and rotaxane in this control experiment clearly demonstrates that formation of both the thread and rotaxane is enhanced by the recognition processes within the system, and, in particular, suggests that the rotaxane could be formed via the [L·M·S] reactive complex.
1. The decrease in the conversion of both the thread and rotaxane in this control experiment clearly demonstrates that formation of both the thread and rotaxane is enhanced by the recognition processes within the system, and, in particular, suggests that the rotaxane could be formed via the [L·M·S] reactive complex.
In addition to the three thread (Fig. 3) and three rotaxane (Fig. 6) forming pathways described thus far, two crosscatalytic cycles potentially exist in this network – R can direct the formation of T and vice versa. In this experimental system, only trans-T was shown to be capable of catalysing its own formation via the template-mediated autocatalytic pathway. The results of the kinetic experiment examining rotaxane formation in the presence of the preformed rotaxane template (Fig. 8c) suggest that the rotaxane cannot cross-catalyse the formation of the thread. In order to confirm this result, we examined the reaction between maleimide L and nitrone S in the presence of 35 mol% pre-synthesised rotaxane R (Fig. 9a, empty red diamonds). The addition of R resulted in significantly lower conversion to trans-T, which reached only 52% after 15 h. This result suggests that the rotaxane template is acting as a non-specific inhibitor in the thread forming reaction. The recognition sites present in R act to sequester reagents into unproductive complexes in a manner similar to the competitive inhibitor experiments performed using 4-bromophenylacetic acid. The rate profile for the formation of the thread (Fig. 9b, dashed red line) changed noticeably, exhibiting a decrease in the maximum rate (1.07 mM h−1 at t = 0 h) relative to that observed in the uninstructed experiment (Fig. 9b, full red line). In order to probe the second cross-catalytic pathway, 35 mol% pre-synthesised thread trans-T was added to maleimide L, macrocycle M and nitrone S at the beginning of the reaction (Fig. 9c). Conversion to trans-T (Fig. 9c, empty red circles) increased to 37% after 15 h, yet again confirming the ability of the thread to template its own formation (maximum rate 0.58 mM h−1 at t = 0.55 h). The conversion to trans-R (Fig. 9c, empty blue circles), on the other hand, only reached 13% after 15 h and max. rate 0.30 mM h−1 at t = 0 h (Fig. 9d, blue dashed line).
The decrease in the formation of the rotaxane suggests that the thread template is not a suitable platform for the production of rotaxane in a recognition-mediated fashion. Taken together, these two experiments revealed that the rotaxane and thread are unable to catalyse the formation of each other.
In order to build a better understanding of the kinetic pathways active in the system and the parameters governing these pathways, we performed a kinetic simulation and fitting of all of the thread and rotaxane kinetic data acquired. Firstly, we constructed a model for the bimolecular formation of the thread in the absence of recognition elements (for details, see ESI†). Thus, we were able to extract the bimolecular rate constant (kbi) for the formation of trans-T and cis-T. Using the value of kbi determined for the trans diastereoisomer, we next fitted the three recognition-enabled thread kinetic experiments simultaneously (−T, +T, +R, data in Fig. 4a and 9a), which allowed us to determine the rate constant20 for the template-directed unimolecular reaction (kSR), the thread duplex dimerisation constant21 (Kduplex) and the corresponding effective molarity22 (Table 1).
| Thread | Rotaxane | |
|---|---|---|
| trans | trans | |
| k bi/10−4 M−1 s−1 | 7.73 | 5.80 |
| k AB/10−4 s−1 | 0.15 | 0.092 |
| k SR/10−4 s−1 | 3.62 | — |
| EMAB/mM | 20 | 16 |
| EMSR/mM | 470 | — |
The rate constant for the cross-catalytic formation of the thread on the rotaxane template was not fitted, as we determined through our kinetic analyses that this pathway does not operate in this system. The fitting procedure was repeated23 for the rotaxane kinetic data. In this instance however, only kAB for the recognition-mediated formation of the rotaxane through the reactive complex [L·M·S] was fitted, as the kinetic analyses revealed that the rotaxane is not capable of establishing either an auto- or cross-catalytic reaction pathway. An overview of the kinetic parameters determined through this fitting procedure is presented in Table 1 and the best fits of the appropriate models to the experimental data are shown as solid lines in Fig. 4, 8 and 9. In the case of the thread, trans-T, it is clear that the major pathway exploited in the formation of this product is the autocatalytic template-mediated pathway. The value of EMSR – 470 mM – is considerably lower than that observed7d,11,12,15 (2 → 200 M) in other replicating systems studied in our laboratory. This observation probably reflects the increased flexibility of trans-T when compared to the other autocatalytic templates we have reported. The fitting of the kinetic data also reveals that the pathway involving the binary reactive complex [L·S] (EMAB = 20 mM) has a minimal role in the formation of trans-T.
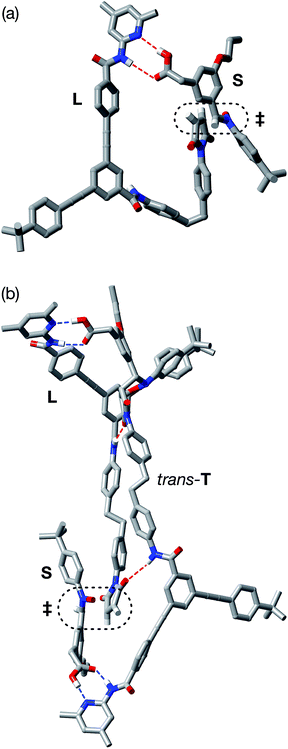
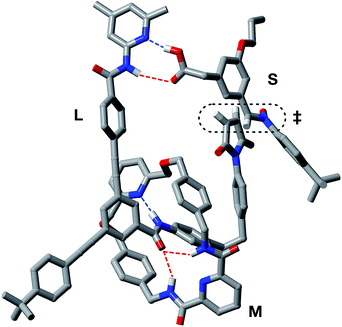
In order to assess the plausibility of these two recognition-mediated pathways for the formation of trans-T, we performed a series of calculations. We located the transition states for the formation of trans-T from the binary complex [L·S] and the ternary complex [L·S·T] using the RM1 (ref. 24) method. Although the binary complex [L·S] can access (Fig. 10a) a plausible transition state (rC–O = 2.03 Å; rC–C = 2.13 Å; νi = 543 cm−1), this structure exhibits significant extensions (rN⋯HO = 2.40 Å; rNH⋯O = 2.48 Å) of both the hydrogen bonding distances in the key amidopyridine·carboxylic acid recognition element away from their optimum values. This disruption of the hydrogen bonds limits the efficiency of the [L·S] complex as a vehicle for the formation of trans-T. By contrast, the ternary complex [L·S·T] can access (Fig. 10b) a transition state in which, in addition to interactions formed between the amidopyridines and carboxylic acids (average rN⋯HO = 1.57 Å; rNH⋯O = 1.71 Å), there are a number of additional stabilising and polarising25 interactions between the amide NH protons and the carbonyl oxygen atoms of the maleimide and isoxazolidine rings. The structure of the transition state associated with the forming isoxazolidine ring is as expected (rC–O = 2.03 Å; rC–C = 2.14 Å; νi = 571 cm−1).
By contrast, rotaxane trans-R barely benefits from recognition-mediated assistance at all in its formation – the value of EMAB is the same as the reaction concentration. It is likely that the bimolecular pathway and the pathway mediated by the [L·M·S] complex are equally important in the formation of trans-R. This hypothesis is supported by the fact that, although trans-R is not formed at a rate significantly higher than the bimolecular cycloaddition, the diastereoselectivity of the reaction forming trans-R is significantly better. However, attempts to fit the observed kinetic profiles involving rotaxane formation using a model that excludes the ternary complex pathway afforded catastrophically poor fits.
In order to assess potential issues with the two recognition-mediated pathways for the formation of trans-R, we, once again, performed a series of calculations. We located the transition state for the formation of trans-R from the ternary complex [L·M·S] using the RM1 (ref. 24) method. Although the ternary complex [L·M·S] can access (Fig. 11) a plausible transition state (rC–O = 2.02 Å; rC–C = 2.14 Å; νi = 556 cm−1), this structure, in common with the structure discussed above in the context of the [L·S] complex, also has a significant distortion of the key amidopyridine·carboxylic acid recognition motif. However, in this case, the hydrogen bond between the pyridine ring and the acid proton is intact (rN⋯HO = 1.61 Å), but the hydrogen bond between the amide proton and the carbonyl oxygen is lengthened significantly (rNH⋯O = 2.37 Å). This distortion of the critical recognition element probably limits the efficiency of this complex as a vehicle for the formation of trans-R. It proved impossible to locate a plausible transition state structure for the formation of trans-R from the quaternary complex [L·M·S·R]. The location of a macrocycle at each of the two amide binding sites cannot be accommodated within a structure similar to that calculated for [L·M·S·R]. The presence of the macrocycle also prevents the formation of the additional stabilising and polarising hydrogen bonds between the amide NH protons and the carbonyl oxygen atoms of the maleimide and isoxazolidine rings with [L·M·S·R] that appear to be critical to the successful operation of the [L·S·T] ternary complex. This observation may also help to explain the absence of any crosscatalysis between trans-T and trans-R.
Conclusions
In conclusion, we have described the full characterisation of a reaction network that integrates recognition-mediated reaction processes with the assembly of a [2]rotaxane. The initial goals of this research were to address two critical shortcomings of our previous, successful design of a replicating [2]rotaxane. The issues associated with the unproductive binding modes have been addressed completely in the new design reported here. In order to remove unwanted crosscatalytic relationships between the [2]rotaxane and the corresponding thread, the macrocyclic component was relocated into a central location in the design of the [2]rotaxane (Fig. 2). We envisaged that, by placing the macrocyclic component M in this location, discrimination between the catalytic cycles that constructed the [2]rotaxane, trans-R, and those associated with the corresponding thread, trans-T, would be enhanced. However, the outcome of this design change was only partly successful. While thread T can template its own formation and does not participate in any crosscatalytic pathways, the formation of the rotaxane did not proceed through an autocatalytic cycle, but rather through a relatively inefficient ternary complex reaction channel. Kinetic fitting and semi-empirical electronic structure calculations, traced these issues to the unwanted distortion of one of the key recognition elements in the system. Additionally, the presence of a flexible spacer at the heart of the molecular design compounded these issues by allowing the unwanted folding of the rotaxane and thread structures, thereby hampering their ability to act as templates. This structural flexibility can be removed by a careful structural redesign of the components of this system, in particular maleimide L, and this work is currently underway in our laboratory.Acknowledgements
We thank EaStCHEM (Graduate Studentship to AV), EPSRC (Graduate Studentship to TK, Grant EP/K503162/1) and the University of St Andrews for financial support. The research data supporting this publication can be accessed at: http://dx.doi.org/10.17630/2df6cf7e-7cd4-4e5a-b972-93170dc00773.Notes and references
- (a) E. R. Kay and D. A. Leigh, Angew. Chem., Int. Ed., 2015, 54, 2 CrossRef PubMed; (b) K. Zhu, C. A. O'Keefe, V. N. Vukotic, R. W. Schurko and S. J. Loeb, Nat. Chem., 2015, 7, 514 CrossRef CAS PubMed; (c) G. de Bo, S. Kuschel, D. A. Leigh, B. Lewandowski, M. Papmeyer and J. W. Ward, J. Am. Chem. Soc., 2014, 136, 5811 CrossRef CAS PubMed; (d) A. Carlone, S. M. Goldup, N. Lebrasseur, D. A. Leigh and A. Wilson, J. Am. Chem. Soc., 2012, 134, 8321 CrossRef CAS PubMed; (e) E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72 CrossRef CAS PubMed; (f) J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163 CrossRef PubMed; (g) C. A. Schalley, K. Beizai and F. Vögtle, Acc. Chem. Res., 2001, 34, 465 CrossRef CAS PubMed; (h) L. Raehm, J.-M. Kern and J.-P. Sauvage, Chem.–Eur. J., 1999, 5, 3310 CrossRef CAS; (i) M. Gómez-López, J. A. Preece and J. F. Stoddart, Nanotechnology, 1999, 7, 183 CrossRef; (j) V. Balzani, M. Gomez-Lopez and J. F. Stoddart, Acc. Chem. Res., 1998, 31, 405 CrossRef CAS.
- (a) J.-C. Chambron and J.-P. Sauvage, New J. Chem., 2013, 37, 49 RSC; (b) K. Tiefenbacher, D. Ajami and J. Rebek Jr., Angew. Chem., Int. Ed., 2011, 50, 12003 CrossRef CAS PubMed; (c) K. S. Chichak, S. J. Cantrill, A. R. Pease, S.-H. Chiu, G. W. V. Cave, J. L. Atwood and J. F. Stoddart, Science, 2004, 304, 1308 CrossRef CAS PubMed; (d) H. Yan, S. H. Park, G. Finkelstein, J. H. Reif and T. H. LaBean, Science, 2003, 301, 1882 CrossRef CAS PubMed; (e) F. H. Beijer, H. Kooijman, A. L. Spek, R. P. Sijbesma and E. W. Meijer, Angew. Chem., Int. Ed., 1998, 37, 75 CrossRef CAS; (f) C. Mao, W. Sun and N. C. Seeman, Nature, 1997, 386, 137 CrossRef CAS PubMed; (g) C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607 CrossRef CAS PubMed; (h) A. Harada, J. Li and M. Kamachi, Nature, 1992, 356, 325 CrossRef CAS.
- (a) M. Lista, E. Orentas, J. Areephong, P. Charbonnaz, A. Wilson, Y. Zhao, A. Bolag, G. Sforazzini, R. Turdean, H. Hayashi, Y. Domoto, A. Sobczuk, N. Sakai and S. Matile, Org. Biomol. Chem., 2013, 11, 1754 RSC; (b) A. G. Salles, S. Zarra, R. Turner and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 19143 CrossRef CAS PubMed; (c) A. Caballero, F. Zapata, N. G. White, P. J. Costa, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2012, 51, 1876 CrossRef CAS PubMed; (d) B. H. Northrop, Y.-R. Zheng, K.-W. Chi and P. J. Stang, Acc. Chem. Res., 2009, 42, 1554 CrossRef CAS PubMed; (e) J. D. Halley and D. A. Winkler, Complexity, 2008, 14, 10 CrossRef; (f) E. Karsenti, Nat. Rev. Mol. Cell Biol., 2008, 9, 255 CrossRef CAS PubMed; (g) I. R. Epstein, J. A. Pojman and O. Steinbock, Chaos, 2006, 16, 037101 CrossRef PubMed; (h) F. J. Nédélec, T. Surrey, A. C. Maggs and S. Leibler, Nature, 1997, 389, 305 CrossRef PubMed.
- (a) L. Favereau, A. Cnossen, J. B. Kelber, J. Q. Gong, R. M. Oetterli, J. Cremers, L. M. Herz and H. L. Anderson, J. Am. Chem. Soc., 2015, 137, 14256 CrossRef CAS PubMed; (b) P. J. Stang, J. Org. Chem., 2009, 74, 2 CrossRef CAS PubMed; (c) S. R. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972 CrossRef CAS PubMed; (d) G. M. Whitesides and M. Boncheva, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4769 CrossRef CAS PubMed; (e) G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed; (f) L. M. Greig and D. Philp, Chem. Soc. Rev., 2001, 30, 287 RSC; (g) A. Klug, Philos. Trans. R. Soc., B, 1999, 354, 531 CrossRef CAS PubMed; (h) D. Philp and J. F. Stoddart, Angew. Chem., Int. Ed. Engl., 1996, 35, 1154 CrossRef; (i) G. M. Whitesides, J. P. Mathias and C. T. Seto, Science, 1991, 254, 1312 CAS; (j) D. J. Kushner, Bacteriol. Rev., 1969, 33, 302 CAS; (k) S. Anderson, H. L. Anderson and J. K. M. Sanders, J. Chem. Soc., Perkin Trans. 1, 1995, 2256 Search PubMed.
- E. K. Drexler, Engines of Creation: The Coming Era of Nanotechnology, Anchor Press, Doubleday, 1986 Search PubMed.
- (a) R. E. Smalley, Sci. Am., 2001, 285, 76 CrossRef CAS PubMed; (b) G. M. Whitesides, Sci. Am., 2001, 285, 78 CrossRef CAS PubMed.
- (a) T. A. Plöger and G. von Kiedrowski, Org. Biomol. Chem., 2014, 12, 6908 RSC; (b) M. P. Robertson and G. F. Joyce, Chem. Biol., 2014, 21, 238 CrossRef CAS PubMed; (c) J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J.-P. Peyralans and S. Otto, Science, 2010, 327, 1502 CrossRef CAS PubMed; (d) E. Kassianidis and D. Philp, Angew. Chem., Int. Ed., 2006, 45, 6344 CrossRef PubMed; (e) N. Paul and G. F. Joyce, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12733 CrossRef CAS PubMed; (f) V. C. Allen, D. Philp and N. Spencer, Org. Lett., 2001, 3, 777 CrossRef CAS PubMed; (g) B. Wang and I. O. Sutherland, Chem. Commun., 1997, 1495 RSC; (h) D. H. Lee, J. R. Granja, J. A. Martinez, K. Severin and M. R. Ghadri, Nature, 1996, 382, 525 CrossRef CAS PubMed; (i) A. Terfort and G. von Kiedrowski, Angew. Chem., Int. Ed. Engl., 1992, 31, 654 CrossRef; (j) V. Rotello, J.-I. Hong and J. Rebek Jr., J. Am. Chem. Soc., 1991, 113, 9422 CrossRef CAS; (k) T. Tjivikua, P. Ballester and J. Rebek Jr., J. Am. Chem. Soc., 1990, 112, 1249 CrossRef CAS; (l) W. S. Zielinski and L. E. Orgel, Nature, 1987, 327, 346 CrossRef CAS PubMed; (m) G. von Kiedrowski, Angew. Chem., Int. Ed. Engl., 1986, 25, 932 CrossRef.
- (a) V. Patzke and G. von Kiedrowski, ARKIVOC, 2007, 5, 293 Search PubMed; (b) Z. Dadon, N. Wagner and G. Ashkenasy, Angew. Chem., Int. Ed., 2008, 47, 6128 CrossRef CAS PubMed; (c) A. Vidonne and D. Philp, Eur. J. Org. Chem., 2009, 593 CrossRef CAS; (d) P. L. Luisi, The Emergence of Life, Cambridge University Press, 2006 Search PubMed; (e) N. Paul and G. F. Joyce, Curr. Opin. Chem. Biol., 2004, 8, 634 CrossRef CAS PubMed; (f) A. Robertson, A. J. Sinclair and D. Philp, Chem. Soc. Rev., 2000, 29, 141 RSC; (g) L. E. Orgel, Nature, 1992, 358, 203 CrossRef CAS PubMed.
- (a) G. N. Lewis, Proc. Am. Acad. Arts Sci., 1905, 40, 719 CrossRef; (b) R. Breslow, Tetrahedron Lett., 1959, 1, 22 CrossRef; (c) D. G. Blackmond, Angew. Chem., Int. Ed., 2009, 48, 386 CrossRef CAS PubMed; (d) R. Plasson, A. Brandenburg, L. Jullien and H. Bersini, J. Phys. Chem. A, 2011, 115, 8073 CrossRef CAS PubMed; (e) A. J. Bissette and S. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800 CrossRef CAS PubMed; (f) M. H. Todd, Chem. Soc. Rev., 2002, 31, 211 RSC; (g) K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1 CrossRef CAS; (h) S. B. Tsogoeva, J. Syst. Chem., 2010, 1, 8 CrossRef CAS.
- (a) D. B. Amabilino and J. F. Stoddart, Chem. Rev., 1995, 95, 2725 CrossRef CAS; (b) Molecular Catenanes, Rotaxanes and Knots, ed. J.-P. Sauvage and C. Dietrich-Buchecker, Wiley-VCH, Weinheim, 1999 Search PubMed; (c) T. J. Hubin and D. H. Busch, Coord. Chem. Rev., 2000, 200–202, 5 CrossRef CAS; (d) J. S. Siegel, Science, 2004, 304, 1256 CrossRef CAS PubMed; (e) C. A. Schalley, T. Weilandt, J. Brüggemann and F. Vögtle, Top. Curr. Chem., 2004, 248, 141 CrossRef; (f) F. Aricó, J. D. Badjic, S. J. Cantrill, A. H. Flood, K. C.-F. Leung, Y. Liu and J. F. Stoddart, Top. Curr. Chem., 2005, 249, 203 Search PubMed; (g) S. J. Loeb, Chem. Soc. Rev., 2007, 36, 226 RSC; (h) M. S. Vickers and P. D. Beer, Chem. Soc. Rev., 2007, 36, 211 RSC; (i) J. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530 RSC; (j) B. M. Rambo, H.-Y. Gong, M. Oh and J. L. Sessler, Acc. Chem. Res., 2012, 45, 1390 CrossRef CAS PubMed; (k) J.-F. Ayme, J. E. Beves, C. J. Campbell and D. A. Leigh, Chem. Soc. Rev., 2013, 42, 1700 RSC; (l) R. J. Bordoli and S. M. Goldup, J. Am. Chem. Soc., 2014, 136, 4817 CrossRef CAS PubMed; (m) M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935 CrossRef CAS PubMed; (n) J. F. Stoddart, Chem. Soc. Rev., 2009, 38, 1802 RSC; (o) V. Balzani, A. Credi and M. Venturi, Molecular Devices and Machines, Wiley-VCH, Weinheim, 2008 Search PubMed; (p) J.-P. Sauvage, J.-P. Collin, S. Durot, J. Frey, V. Heitz, A. Sour and C. Tock, C. R. Chim., 2010, 13, 315 CrossRef CAS; (q) L. D. Movsisyan, D. V. Kondratuk, M. Franz, A. L. Thompson, R. R. Tykwinski and H. L. Anderson, Org. Lett., 2012, 14, 3424 CrossRef CAS PubMed; (r) N. Ponnuswamy, F. B. Cougnon, J. M. Clough, G. D. Pantoş and J. K. M. Sanders, Science, 2012, 338, 783 CrossRef CAS PubMed.
- T. Kosikova, N. I. Hassan, D. B. Cordes, A. M. Z. Slawin and D. Philp, J. Am. Chem. Soc., 2015, 137, 16074 CrossRef CAS PubMed.
- J. W. Sadownik and D. Philp, Angew. Chem., Int. Ed., 2008, 47, 9965 CrossRef CAS PubMed.
- T.-H. Ho, C.-C. Lai, Y.-H. Liu, S.-M. Peng and S.-H. Chiu, Chem.–Eur. J., 2014, 20, 4563 CrossRef CAS PubMed.
- A. Vidonne and D. Philp, Tetrahedron, 2008, 64, 8464 CrossRef CAS.
- E. Kassianidis and D. Philp, Chem. Commun., 2006, 4072 RSC.
- The rate vs. time (d[X]/dt) profiles for the thread (X = T) and rotaxane (X = R) kinetic experiments were determined by computing the first derivative of a seventh-order polynomial fitted to the respective concentration vs. time data.
- Analytical Methods in Supramolecular Chemistry, ed. C. A. Schalley, Wiley-VCH, Weinheim, 2007 Search PubMed.
- A. Dieckmann, S. Beniken, C. Lorenz, N. L. Doltsinis and G. von Kiedrowski, J. Syst. Chem., 2010, 1, 10 CrossRef CAS.
- (a) R. J. Pearson, E. Kassianidis, A. M. Z. Slawin and D. Philp, Org. Biomol. Chem., 2004, 2, 3434 RSC; (b) R. J. Pearson, E. Kassianidis and D. Philp, Tetrahedron Lett., 2004, 45, 4777 CrossRef CAS.
- The current experimental system did not allow us to unambiguously establish whether thread formation proceeds through the reactive binary complex in addition to the autocatalytic self-replicating pathway, and hence both kAB and kSR were enabled throughout the fitting procedure. (EMAB was confined to 20 mM).
- Throughout the fitting of thread and rotaxane data, the formation of [R·R] or [R·T] duplexes was not enabled as the kinetic analyses suggested that these complexes are not formed within the network. Instead, the Ka value for the formation of these complexes was confined to the value of the individual binding event, 250 M−1 (recognition between single –CH2COOH and 4,6-dimethylamidopyridine).
- (a) M. I. Page and W. P. Jencks, Proc. Natl. Acad. Sci. U. S. A., 1971, 68, 1678 CrossRef CAS PubMed; (b) M. I. Page, Chem. Soc. Rev., 1973, 2, 295 RSC; (c) A. J. Kirby, Adv. Phys. Org. Chem., 1980, 17, 183 CrossRef CAS.
- The value for kbi for the formation of trans rotaxane used throughout the rotaxane fitting was estimated from the extracted kbi for the formation of thread and the ratio of thread
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rotaxane formed in the kinetic experiment examining rotaxane formation in the presence of an inhibitor (for details, see ESI†) (where kbi rotaxane is 0.75 × kbi thread M−1 s−1).
rotaxane formed in the kinetic experiment examining rotaxane formation in the presence of an inhibitor (for details, see ESI†) (where kbi rotaxane is 0.75 × kbi thread M−1 s−1). - G. B. Rocha, R. O. Freire, A. M. Simas and J. J. P. Stewart, J. Comput. Chem., 2006, 27, 1101 CrossRef CAS PubMed.
- R. M. Cowie, S. M. Turega and D. Philp, Org. Lett., 2006, 8, 5179 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of kinetic experiments, computational methods and characterisation data for all compounds are provided. See DOI: 10.1039/c5sc04805b |
| This journal is © The Royal Society of Chemistry 2016 |