Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation†
Oscar
Diaz-Morales
 ,
David
Ferrus-Suspedra
and
Marc T. M.
Koper
*
,
David
Ferrus-Suspedra
and
Marc T. M.
Koper
*
Leiden Institute of Chemistry, Leiden University, PO Box 9502, 2300 RA Leiden, The Netherlands. E-mail: m.koper@lic.leidenuniv.nl
First published on 5th January 2016
Abstract
Nickel oxyhydroxide (NiOOH) is extensively used for energy storage and it is a very promising catalyst for the oxygen evolution reaction (OER). However, the processes occurring on the NiOOH surface during charge accumulation and OER are not well understood. This work presents an in situ Surface Enhanced Raman Spectroscopy (SERS) study of the pH dependent interfacial changes of the NiOOH catalyst under the working conditions used for OER. We demonstrate the important effect of the electrolyte pH on the degree of surface deprotonation of NiOOH, which crucially affects its OER activity. Our results show that the deprotonation of NiOOH produces negatively charged (or proton-deficient) surface species, which are responsible for the enhanced OER activity of NiOOH in highly alkaline pH. Moreover, we provide spectroscopic evidence obtained in an 18O-labeled electrolyte that allows us to assign this surface species to a superoxo-type species (Ni–OO−). Furthermore, we propose a mechanism for the OER on NiOOH which is consistent with the observed pH-sensitivity, and that also explains why NiOOH is not a suitable catalyst for applications in neutral or moderately alkaline pH (in the range 7–11), apart from the lower stability of the catalyst under these conditions.
Introduction
Nickel-based oxides are extensively used for secondary batteries and super capacitors.1–3 These materials are also very promising catalysts for the OER,4–10 which is one of the major bottlenecks for solar energy conversion into storable fuels.11,12 However, the mechanism of nickel charging and its activation towards OER are still a matter of debate. The Bode scheme is one of the accepted mechanisms for the charge/discharge process of the nickel (hydr)oxide, according to which the freshly prepared α-Ni(OH)2 oxidizes to form γ-NiOOH;13 these phases convert into the more crystalline β-Ni(OH)2/β-NiOOH phases upon (electro)chemical ageing. It has been proposed that the formal oxidation state of nickel in the γ-NiOOH lies in the range 3.5–3.67,9,14 which suggests that some nickel sites in this compound have NiO2-like character that may be seen as tetravalent nickel sites; this hypothesis has been supported with X-ray adsorption spectroscopy (XAS), by matching the position of the Ni K-edge of the γ-NiOOH samples with the K-edge of reference compounds in which nickel was thought to be in the NiIV state (BaNiO3 or KNiIO6).15–17 However, the values reported for the oxidation state of nickel in those reference compounds did not consider the possibility of oxygen vacancies, which affect the formal valence of nickel in the compounds and make the conclusions derived from the XAS data uncertain.18,19The OER mechanism on a nickel-based catalyst (nickel-borate) was recently studied by Nocera et al.,20 and they proposed that the formation of the catalytically active species for the OER occurs via an oxidative deprotonation of a nickel oxyhydroxide-like structure; the NiOOH proposed by them is dispersed in a polymeric hydrous network similar to the one suggested by Lyons et al.9,21 The charging mechanism of Ni(OH)2 in KOH and its activation towards OER was also studied by Merrill et al.22 by means of Surface Enhanced Raman Spectroscopy (SERS), who reported the appearance of a broad peak in the 900–1100 cm−1 wavenumber region when α-Ni(OH)2 oxidizes to form γ-NiOOH. This broad feature was attributed to “active oxygen O0” within the NiOOH structure. The spectroelectrochemical evidence for the active oxygen species within the oxyhydroxide network raises the question whether this feature may be related to the deprotonated species reported by Nocera et al. for the OER active form of nickel-borate catalyst, which heralds the onset of oxygen evolution.
The oxidative deprotonation process to generate the catalytic species for the OER is not particular for nickel. It has been reported that cobalt, iron and manganese-based catalysts also deprotonate prior to oxygen evolution, in processes that are strongly pH-dependent.23–25 Since the OER activity of NiOOH is also known to be pH-dependent and favored in more alkaline media,9,21 the appearance of the SERS feature attributed to the “active oxygen” should also correlate with the pH, if this species is related to the formation of OER catalytically active sites in the structure of NiOOH. Following this hypothesis, we present here a systematic in situ SERS study of the pH dependence of the catalytic activity of NiOOH towards electrochemical O2 generation. Our electrodes consist of NiOOH electrodeposited on gold in a rigorously Fe-free electrolyte; the importance of removing such impurities was recently demonstrated by the Boettcher group.26 The elimination of the Fe impurities from the electrolyte allows us to conclusively rationalize pH-dependent activity changes to observed spectral changes in the γ-NiOOH catalyst. Based on these results, we will suggest a mechanism for OER reaction on first-row transition-metal oxides that we believe will be useful for guiding future first-principles calculations of novel catalysts.
Experimental section
All glassware was rigorously cleaned before starting experiments by boiling in concentrated H2SO4 to remove metals and organic contaminations, and was subsequently boiled five times in Millipore Milli-Q water (resistivity > 18.2 MΩ cm), which was also used to prepare the solutions for the electrochemical experiments.The chemicals used in this work were of ultra-high purity: Ni(NO3)2·6H2O (Aldrich trace metal basis, 99.999%), HClO4 (Aldrich TraceSelect® for trace analysis, 67–72%) and iron-free NaOH. The purification of commercial NaOH followed the procedure reported by Boettcher's group,26 by shaking a 1 M solution of NaOH (30% solution in H2O, TraceSelect® for trace analysis) with Ni(OH)2 that was precipitated from the 99.999% Ni(NO3)2·6H2O salt. The NaClO4 used as supporting electrolyte was prepared by neutralizing the Fe-free NaOH solution with HClO4, to minimize the amount of iron impurities present in the solution. The pH of the solutions used in all the experiments of this work was adjusted with HClO4, and verified with a pH-meter. All experiments were performed at constant ionic strength, which was kept constant at 0.1 M by adding NaClO4 as supporting electrolyte except for the electrolyte at pH 13 and 14, which did not contain NaClO4; they were NaOH 0.1 M and 1 M, respectively.
In situ Surface Enhanced Raman Spectroscopy (SERS) was performed with a confocal Raman microscope (LabRam HR, Horiba Yobin Yvon) with a 50× objective. The excitation source used was a 30 mW He/Ne laser (633 nm). Backscattered light was filtered with an edge filter at 633 nm, subsequently directed to the spectrograph and to the CCD detector; further details of the setup can be found in ref. 27 and 28. The experiments were made in a two-compartment and three-electrode cell made of glass, with a quartz window at the bottom. A gold spiral was used as counter electrode, Ag/AgCl (sat. KCl) as reference electrode, and nickel electroplated on a roughened gold disk as working electrode; the reference electrode was separated from the working electrode compartment to avoid chloride contamination. The electrochemical experiments were controlled by a μAutolab type III potentiostat/galvanostat (Metrohm-Autolab). Dissolved oxygen in solutions was removed prior to measurements by purging with argon (purity grade 5.0) for at least 30 min, and the argon was kept flowing above the solution during the experiments.
The working electrode used in this work was a gold disk back-contacted with a gold wire and it was not mounted in any material to allow annealing during the cleaning procedure; the electrochemical measurements were performed with the disk in meniscus configuration. Prior to each measurement, the disk was mechanically polished to mirror finish using aqueous diamond pastes (Buehler Limited) with different grain sizes to 0.25 μm, rinsed with Milli-Q water and ultrasonicated during 5 min to remove all residuals of mechanical polishing; next the gold electrode was annealed with a butane flame and electrochemically roughened by 25 oxidation–reduction cycles (ORC) in a 0.1 M solution of KCl. The ORC were performed between −0.30 and 1.20 V vs. SCE, during which the potential was held for 30 seconds at the negative limit and for 1.3 seconds at the positive limit; this method has been reported to give a brownish surface that is SERS active.29 The roughened gold electrode was thoroughly rinsed with water to measure a cyclic voltammetry in the potential range 0–1.75 V vs. RHE in 0.1 M HClO4 at 0.05 V s−1. The real surface area of the electrode was measured from the charge of the reduction peak of the gold oxide, assuming 390 μC cm−2 for the charge for one monolayer of gold oxide.30 The surface area obtained from this measurement was used to calculate the current density in the cyclic voltammetry reported in the work. The capacitance-corrected plots of catalytic activity were obtained from the cyclic voltammetry curves by averaging the current of the backward and forward scans.31,32
Nickel was plated on the roughened gold electrode by galvanostatic electrodeposition from a 5 × 10−3 M Ni(NO3)2·6H2O solution, using 0.1 M NaClO4 as supporting electrolyte. The deposition was carried out by applying a cathodic current (10 μA) for a given time, in order to obtain ca. five monolayers of coverage; the time for nickel plating was calculated according to the real surface area of the working electrode in order to deposit 5 × 726 μC cm−2, the latter value corresponding to the charge needed to deposit one monolayer of closely packed metallic nickel from a NiII solution, taking the atomic radius of Ni as 0.124 nm and its density as 8.908 g cm−3.33
All potentials in this work are reported versus the reversible hydrogen electrode (RHE) in the working pH, unless otherwise stated. The potentials were converted into the RHE scale according to the eqn (1).
| ERHE = EAg/AgCl(sat. KCl) + E0Ag/AgCl(sat. KCl) + 0.059ΔpH | (1) |
The Raman experiments in H218O (98% isotopic purity, GMP standard, purchased from CMR) were performed at pH 13 (NaOH 0.1 M). The electrolyte for these experiments was used without further purification, and the electrochemical cell had a smaller internal volume (ca. 1 mL); schematic details of this cell can be found in ref. 28.
Results and discussion
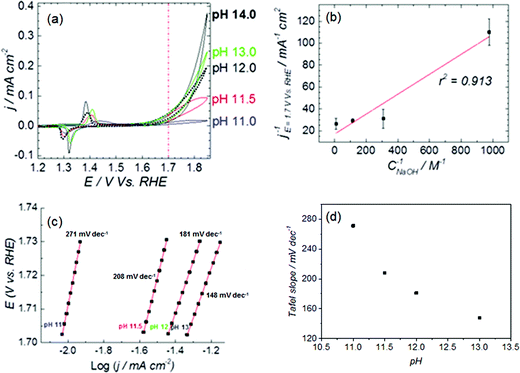
The cyclic voltammetry (CV) in Fig. 1a shows the Ni(OH)2/NiOOH (NiII/NiIII) redox transition in the potential region 1.3–1.5 V vs. RHE. The potential at which the redox transition occurs does not show significant pH dependence (see Fig. S1a and b in the ESI†). However, the OER activity (expressed as current density) does depend on the pH of the electrolyte, as confirmed in the capacitance-corrected plot of OER activity as function of the applied potential in Fig. S2 in the ESI.†Fig. 1a show that the OER activity at pH 11.0 is negligible and increases with the electrolyte pH, with a tendency to saturate at the highest pH values (see Fig. S3 in the ESI†).The potential of the NiII/NiIII transition and the OER current of NiOOH in 1 M NaOH (see Fig. 1a) compares well with the results reported by Boettcher's group,26 and confirms that the hydroxide solution was free of iron traces. We can therefore assert that our NiOOH catalyst is not contaminated with Fe during the electrochemical experiments, and the pH effect is not an artifact caused by impurities in the electrolyte. The elimination of Fe impurities in the electrolyte is important because the presence of Fe in the electrolyte shifts the OER onset potential to lower values due to the formation of NiFe mixed oxyhydroxide (Ni1−xFexOOH), which has a higher catalytic activity for oxygen evolution than NiOOH itself;26 the formation of Ni1−xFexOOH also affects the position of the NiII/NiIII redox transition, and produces an apparent pH dependence of the redox pair (compare Fig. S1c and d to Fig. S1a and b†). Fig. S3a and b in the ESI† shows polarization curves of Ni(OH)2 deposited on Au, obtained in purified (Fe-free) and non-purified electrolytes, respectively. The cyclic voltammetry in the Fe-containing electrolytes (Fig. S3b in the ESI†) differs from the one obtained in Fe-free electrolyte: the NiII/NiIII redox peaks of NiOOH shift with pH and the OER activity increases ca. 20-fold from pH 11 to pH 13 whereas the enhancement is about 10-fold for the purified electrolyte. In general, the activity measured in the Fe-containing electrolyte is ca. 10-fold higher than in the Fe-free electrolyte.
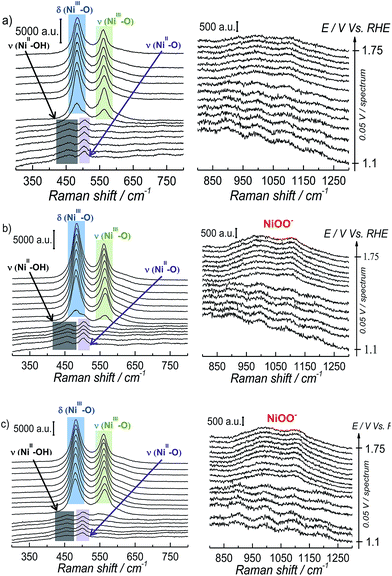
The interfacial structural changes during the electrochemical oxidation of Ni(OH)2 and subsequent OER were studied by means of in situ SERS at different pH, keeping the ionic strength of the electrolyte constant; Fig. 2 shows the results obtained from these experiments.
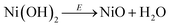
The SERS spectra acquired at potentials below ca. 1.4 V vs. RHE show two weak peaks at 457 cm−1 and 504 cm−1 (see left panel of Fig. 2a–c), which can be assigned to the A1g stretching modes of Ni–OH and Ni–O, respectively, in the Ni(OH)2.35–37 The stretching mode of the dehydrated form of nickel hydroxide (Ni–O peak at ca. 504 cm−1) has been attributed to a potential-assisted dehydration process of the nickel hydroxide to NiO-like structures, as expressed in eqn (2).37
 | (2) |
The Ni(OH)2/NiOOH redox transition occurs at potentials higher than ca. 1.35 V vs. RHE (see Fig. 1a), and the SERS spectra in the left panel of Fig. 2a–c show the appearance of two well-defined peaks at ca. 482 cm−1 and 562 cm−1 that can be assigned to the eg bending vibration and the A1g stretching vibration modes, respectively, of Ni–O in NiO(OH).37 The Raman peaks of Ni(OH)2 are weak in comparison with the intensity observed for the peaks assigned to NiO(OH), as previously reported by Bell's group; this has been attributed to the low Raman scattering cross-section of Ni(OH)2, in contrast to the stronger bands observed for NiO(OH) due to a resonance enhancing effect.38 At higher potentials, we observe the peak attributed to “active oxygen” in the oxyhydroxide structure in the 800–1150 cm−1 wavenumber region. The spectra in the right-hand panel of Fig. 2a–c show that the intensity of this peak increases as the pH of the electrolyte becomes more alkaline (spectra taken at pH 11.5 and 14 are shown in Fig. S4 in the ESI†).
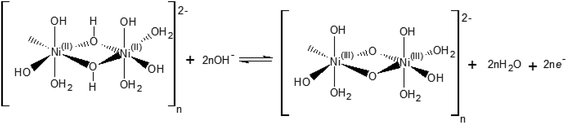
Our electrochemical results indicate that the oxidation of Ni(OH)2 occurs via a hydroxide-mediated deprotonation process that can be described by Scheme 1.
Scheme 1 shows the NiII/NiIII oxidation process of a polymeric hydrous nickel(II) hydroxide ([(NiII)2(OH)6(H2O)3]n2−), which is the actual state of the hydroxide on the electrode surface as reported by Lyons et al.9,21 This redox process is a OH−/e− – coupled reaction which should exhibit no pH dependence on the RHE scale.
The [(NiIII)2O2(OH)4(H2O)3]n2− may be further deprotonated if the pH of the electrolyte is higher than pKa of the proton attached to the NiO(OH) species, leading to formation of a NiO− species, as shown in Scheme 2.
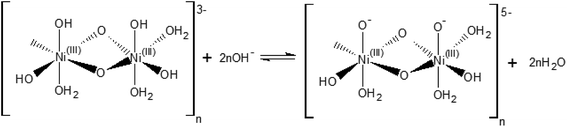 | ||
| Scheme 2 Hydroxide-mediated deprotonation process of the surface of NiO(OH) towards negatively charged species. | ||
The reaction presented in Scheme 2 is somewhat similar to the activation process towards oxygen evolution proposed by Nocera et al.20 for nickel-borate catalyst, which we have recently shown to be essentially identical to the NiOOH catalyst.39 The main difference between the deprotonation step depicted in Scheme 2 and the activation process of nickel-borate proposed by Nocera et al. is that Scheme 2 proposes a chemical deprotonation of the nickel oxyhydroxide towards a negatively-charged (or proton-deficient) surface species instead of a concerted proton – electron transfer step towards oxyl radicals; the generation of negatively charged surface species as shown in Scheme 2 allows to explain the strong pH dependency of the OER (see Fig. 1a and b).
We mention that we assume the oxidation state of nickel in NiO(OH) to be trivalent; this assumption is based on the XPS data indicating that the oxidation state of nickel in the oxyhydroxide is most likely trivalent.40–43
In a time-resolved spectroscopic study of photocatalytic water oxidation on Co3O4, Frei et al.24 also report a vibrational peak at ca. 1013 cm−1 when the photo-induced OER experiment was performed in H216O. This peak shifts to lower frequencies (by ca. 47 cm−1) when the experiment is performed in H218O. Based on the position of the peak in the spectrum and its shift in frequency due to the isotopic labeling, they assigned the vibrational peak to superoxo intermediates in the cobalt catalyst (CoOO). Moreover, the time-dependence of the peak intensity during the photocatalytic reaction led them to propose that the water oxidation occurs via decomposition of this superoxo species.
Since the frequency of the Raman peak attributed to the “active oxygen” species is very close to the position reported for the infrared peak assigned to the superoxo species on the cobalt-based catalyst,24 we performed a similar isotopic labeling experiment for the electrocatalytic oxygen evolution on NiOOH to confirm the nature of this species; Fig. 3 shows a comparison between the SER spectrum of the “active oxygen” in NiOOH, measured at pH 13 in H216O and H218O (at 1.7 V vs. RHE), showing a clear shift of the Raman peak to lower frequencies in the labeled media. The peaks attributed to the “active oxygen” shift ca. 64 cm−1 to lower frequencies in H218O (see Table S1 in the ESI†). This value is close to the shift observed by Frei et al.24 for the superoxo species in cobalt oxide. The position of the “active oxygen” peak in the spectrum and its shift in H218O therefore renders further credence to the assertion that the “active oxygen” peak corresponds to a superoxo (O–O) vibration (SERS of NiOOH at pH 13 in H218O in the potential range of 1.45–1.75 V vs. RHE are shown in Fig. D5 in the ESI†). The nature of the shallow minimum in both spectra in Fig. 3 is unknown but suggests the existence of two spectroscopically discernible O–O species on the surface.
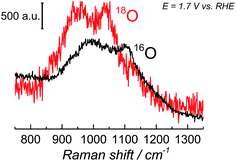 | ||
| Fig. 3 SER spectra of NiOOH in the wavenumber region 800–1350 cm−1. The spectra were obtained at 1.7 V vs. RHE in Na16OH 0.1 M. The electrolyte was prepared with H216O and H218O. | ||
The superoxidic nature of the species in the SER spectra can be further confirmed by comparing the above SERS results to the existing DFT calculations of NiO2 complexes; the calculations show that NiO2 has vibrational modes in the wavenumber region 900–1150 cm−1,44 when the O2 in NiO2 is of peroxidic or superoxidic character, i.e. the 900–1150 cm−1 region corresponds to O–O stretching modes.
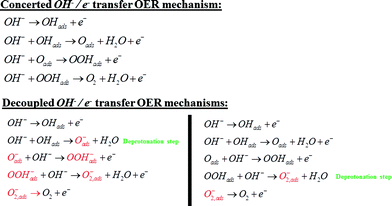
The dependence of the activity and the corresponding Raman bands on pH suggests that the species is formed upon deprotonation of the γ-NiOOH phase, which has been shown to be the more OER active phase of NiOOH.26 Moreover, in the light of the abovementioned results reported for the photocatalytic OER on cobalt oxide,24 the comparison to DFT calculations, the shift of the “active oxygen” Raman peak due to isotopic labeling and its pH-dependence, we conclude that this species is of superoxo nature and acts as precursor for oxygen evolution. As a consequence, we propose two possible mechanisms for the electrocatalytic OER on NiOOH that are similar to the one reported by Nocera et al.20 for the OER on nickel borate, and by Frei et al.24 for the light-assisted water oxidation on Co3O4. Our mechanisms incorporate a deprotonation step of the polymeric hydrous nickel oxyhydroxide towards formation of either oxide (NiO−) or superoxo species (NiOO−), as shown in Scheme 3. These mechanisms propose O2 formation via decomposition of the negatively charged surface species (O2−), which differs from the “classical” concerted OH−/e− transfer mechanism that only considers uncharged adsorbates (or adsorbates with all equal charge).
The surface character of the pH-dependent active precursor (NiO− or NiOO−) species is suggested by the Langmuir-type dependence of the OER activity on the NaOH concentration: a plot of 1/j versus 1/CNaOH gives a reasonably straight line (see Fig. 1b). The deviation from linearity in the plot of Fig. 1b can be partially attributed to the strong pH dependence of the OER Tafel slope, as can be shown in Fig. 1c and d. The Tafel slope for OER on nickel oxyhydroxide varies from ca. 271 mV dec−1 at pH 11 to ca. 148 mV dec−1 at pH 13. Lyons et al.9 reported a similar trend in the values of the Tafel slope in experiments performed at higher concentrations of NaOH (0.1–5 M).
The decoupled OH−/e− transfer mechanisms proposed in Scheme 3 consider two possible deprotonation pathways: OHads deprotonation towards negatively charged surface oxide (Oads−), or OOHads deprotonation towards negatively charged surface superoxide (O2,ads−); in both pathways the O2 formation occurs via decomposition of the negatively charged surface superoxide. The pH-dependence of the NiOOH activity towards OER may in principle be ascribed to both pathways: deprotonation giving rise to the negatively charged surface oxide (Oads−) and subsequent formation of the O–O bond, or OOHads deprotonation giving rise to the negatively charged surface superoxide. Ultimately, the (surface) pKa of the corresponding acid–base equilibrium needs to be determined in order to assess which of the two explains the observed pH dependence. The importance of proton loss for generating localized reactive intermediates was also emphasized by Bediako et al., who speculated that changes in ligand field strength upon deprotonation could localize the unpaired spin density to favor further reactivity.20
The relevance of negatively charged species, either of “O−” or of “OO−” character, on the surface of the catalyst during the OER has been suggested for many types of (transition-metal) oxide catalysts.9,20,21,23,25,39,45,46 However, theoretical descriptions of the OER mechanism employing density functional theory calculations have not yet incorporated this important pH effect in the reaction kinetics.47 We believe that the data reported here and the associated mechanism suggested in Scheme 3 provide another clear experimental example of the importance of negatively charged (surface) intermediates in generating pH dependent electrocatalytic activities, in agreement with a general model reported previously.48 Further understanding of the role of the oxo (MO−) and superoxo (MOO−) intermediates in the OER kinetics would require detailed DFT calculations that consider the pKa and the relative stabilities of these intermediates. Future computational approaches towards modeling OER should account for this important acid–base surface chemistry.
Conclusions
In this paper, we have provided spectro-electrochemical evidence for the active species that is responsible for the pH dependent OER activity of rigorously Fe-free NiOOH in alkaline electrolytes. We identify this species as a deprotonated γ-NiOOH surface phase in which stable (i.e. Raman observable) O–O bonds are formed. Based on our observations and other literature data on pH dependent OER kinetics, we propose a mechanism for the OER on NiOOH which is consistent with the observed pH-sensitivity; it involves the formation of a superoxo-type intermediate (NiOO−) that acts as preferential oxygen precursor at pH > 11. The proposed OER mechanism also considers the possibility of O2 formation via decomposition of OOH intermediates formed from the negatively charged surface oxide (NiO−). However, theoretical calculations to determine the (surface) pKa of the corresponding acid–base equilibrium need to be performed to ultimately assess the relative importance of the NiOO− and NiO− intermediates in the OER mechanism. The pH dependence presented in this work rationalizes the unsuitability of NiOOH as electrocatalyst for applications in neutral or moderately alkaline pH (in the range 7–11), apart from the lower stability of the catalyst under these conditions.Acknowledgements
This work was also supported by the Netherlands Organization for Scientific Research (NWO) and in part by the BioSolar Cells open innovation consortium, supported by the Dutch Ministry of Economic Affairs, Agriculture and Innovation.References
- H. Chen, T. N. Cong, W. Yang, C. Tan, Y. Li and Y. Ding, Prog. Nat. Sci., 2009, 19, 291 CrossRef CAS.
- F. Luan, G. Wang, Y. Ling, X. Lu, H. Wang, Y. Tong, X.-X. Liu and Y. Li, Nanoscale, 2013, 5, 7984 RSC.
- Q. Yang, Z. Lu, J. Liu, X. Lei, Z. Chang, L. Luo and X. Sun, Prog. Nat. Sci., 2013, 23, 351 CrossRef.
- D. A. Corrigan and R. M. Bendert, J. Electrochem. Soc., 1989, 136, 723 CrossRef CAS.
- R. L. Doyle, I. J. Godwin, M. P. Brandon and M. E. G. Lyons, Phys. Chem. Chem. Phys., 2013, 15, 13737 RSC.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452 CrossRef CAS PubMed.
- Z. Lu, W. Xu, W. Zhu, Q. Yang, X. Lei, J. Liu, Y. Li, X. Sun and X. Duan, Chem. Commun., 2014, 50, 6479 RSC.
- M. E. G. Lyons and M. P. Brandon, Int. J. Electrochem. Sci., 2008, 3, 1386 CAS.
- M. E. G. Lyons, A. Cakara, P. O'Brien, I. Godwin and R. L. Doyle, Int. J. Electrochem. Sci., 2012, 7, 11768 CAS.
- Y. Matsumoto and E. Sato, Mater. Chem. Phys., 1986, 14, 397 CrossRef CAS.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS PubMed.
- K. S. Joya, Y. F. Joya, K. Ocakoglu and R. van de Krol, Angew. Chem., Int. Ed., 2013, 52, 10426 CrossRef CAS PubMed.
- H. Bode, K. Dehmelt and J. Witte, Electrochim. Acta, 1966, 11, 1079 CrossRef CAS.
- P. Oliva, J. Leonardi, J. F. Laurent, C. Delmas, J. J. Braconnier, M. Figlarz, F. Fievet and A. D. Guibert, J. Power Sources, 1982, 8, 229 CrossRef CAS.
- Y. Hu, I. T. Bae, Y. Mo, D. A. Scherson and M. R. Antonio, Can. J. Chem., 1997, 75, 1721 CrossRef CAS.
- A. N. Mansour, C. A. Melendres, M. Pankuch and R. A. Brizzolara, J. Electrochem. Soc., 1994, 141, L69 CrossRef CAS.
- W. E. O'Grady, K. I. Pandya, K. E. Swider and D. A. Corrigan, J. Electrochem. Soc., 1996, 143, 1613 CrossRef.
- R. Gottschall, R. Schöllhorn, M. Muhler, N. Jansen, D. Walcher and P. Gütlich, Inorg. Chem., 1998, 37, 1513 CrossRef CAS.
- X. Qian, H. Sambe, D. E. Ramaker, K. I. Pandya and W. E. O'Grady, J. Phys. Chem. B, 1997, 101, 9441 CrossRef CAS.
- D. K. Bediako, Y. Surendranath and D. G. Nocera, J. Am. Chem. Soc., 2013, 135, 3662 CrossRef CAS PubMed.
- M. E. G. Lyons, R. L. Doyle and M. P. Brandon, Phys. Chem. Chem. Phys., 2011, 13, 21530 RSC.
- M. Merrill, M. Worsley, A. Wittstock, J. Biener and M. Stadermann, J. Electroanal. Chem., 2014, 717–718, 177 CrossRef CAS.
- Y. Surendranath, M. W. Kanan and D. G. Nocera, J. Am. Chem. Soc., 2010, 132, 16501 CrossRef CAS PubMed.
- M. Zhang, M. de Respinis and H. Frei, Nat. Chem., 2014, 6, 362 CrossRef CAS PubMed.
- T. Takashima, K. Hashimoto and R. Nakamura, J. Am. Chem. Soc., 2012, 134, 1519 CrossRef CAS PubMed.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744 CrossRef CAS PubMed.
- S. C. S. Lai, S. E. F. Kleyn, V. Rosca and M. T. M. Koper, J. Phys. Chem. C, 2008, 112, 19080 CAS.
- O. Diaz-Morales, F. Calle-Vallejo, C. de Munck and M. T. M. Koper, Chem. Sci., 2013, 4, 2334 RSC.
- P. Gao, D. Gosztola, L. W. H. Leung and M. J. Weaver, J. Electroanal. Chem., 1987, 233, 211 CrossRef CAS.
- S. Trasatti and O. A. Petrii, J. Electroanal. Chem., 1992, 327, 353 CrossRef CAS.
- A. Grimaud, K. J. May, C. E. Carlton, Y. L. Lee, M. Risch, W. T. Hong, J. Zhou and Y. Shao-Horn, Nat. Commun., 2013, 4, 2439 Search PubMed.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383 CrossRef CAS PubMed.
- J. House, Inorganic Chemistry, Academic Press, Waltham, 2nd edn, 2013 Search PubMed.
- R. West, Handbook of Chemistry and Physics, CRC Press, Cleveland, 53rd edn, 1972 Search PubMed.
- P. Hermet, L. Gourrier, J. L. Bantignies, D. Ravot, T. Michel, S. Deabate, P. Boulet and F. Henn, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 235211 CrossRef.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- Y. L. Lo and B. J. Hwang, Langmuir, 1998, 14, 944 CrossRef CAS.
- B. S. Yeo and A. T. Bell, J. Phys. Chem. C, 2012, 116, 8394 CAS.
- B. J. Trześniewski, O. Diaz-Morales, D. A. Vermaas, A. Longo, W. Bras, M. T. M. Koper and W. A. Smith, J. Am. Chem. Soc., 2015, 137, 15112 CrossRef PubMed.
- T. Dickinson, A. F. Povey and P. M. A. Sherwood, J. Chem. Soc., Faraday Trans. 1, 1977, 73, 327 RSC.
- I. G. Casella, M. R. Guascito and M. G. Sannazzaro, J. Electroanal. Chem., 1999, 462, 202 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, L. W. M. Lau, A. Gerson and R. S. C. Smart, Surf. Interface Anal., 2009, 41, 324 CrossRef CAS.
- A. P. Grosvenor, M. C. Biesinger, R. S. C. Smart and N. S. McIntyre, Surf. Sci., 2006, 600, 1771 CrossRef CAS.
- E. L. Uzunova, H. Mikosch and G. S. Nikolov, J. Chem. Phys., 2008, 128, 094307 CrossRef PubMed.
- A. I. Nguyen, M. S. Ziegler, P. Oña-Burgos, M. Sturzbecher-Hohne, W. Kim, D. E. Bellone and T. D. Tilley, J. Am. Chem. Soc., 2015, 137, 12865 CrossRef CAS PubMed.
- H. Willems, A. G. C. Kobussen, J. H. W. de Wit and G. H. J. Broers, J. Electroanal. Chem., 1984, 170, 227 CrossRef CAS.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Norskov, J. Electroanal. Chem., 2007, 607, 83 CrossRef CAS.
- M. T. M. Koper, Chem. Sci., 2013, 4, 2710 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc04486c |
| This journal is © The Royal Society of Chemistry 2016 |