 Open Access Article
Open Access ArticleTargeted anion transporter delivery by coiled-coil driven membrane fusion†
Nestor Lopez
Mora
a,
Azadeh
Bahreman
a,
Hennie
Valkenier‡
 b,
Hongyu
Li
b,
Hongyu
Li
 c,
Thomas H.
Sharp
d,
David N.
Sheppard
c,
Thomas H.
Sharp
d,
David N.
Sheppard
 c,
Anthony P.
Davis
*b and
Alexander
Kros
c,
Anthony P.
Davis
*b and
Alexander
Kros
 *a
*a
aLeiden Institute of Chemistry, Leiden University, 2300 RA Leiden, The Netherlands. E-mail: a.kros@chem.leidenuniv.nl
bSchool of Chemistry, University of Bristol Cantock's Close, Bristol BS8 1TS, UK. E-mail: Anthony.Davis@bristol.ac.uk
cSchool of Physiology, Pharmacology and Neuroscience, University of Bristol Biomedical Sciences Building, University Walk, Bristol BS8 1TD, UK
dDepartment of Molecular Cell Biology, Section Electron Microscopy, Leiden University Medical Center, 2300 RC Leiden, The Netherlands
First published on 7th January 2016
Abstract
Synthetic anion transporters (anionophores) have potential as biomedical research tools and therapeutics. However, the efficient and specific delivery of these highly lipophilic molecules to a target cell membrane is non-trivial. Here, we investigate the delivery of a powerful anionophore to artificial and cell membranes using a coiled-coil-based delivery system inspired by SNARE membrane fusion proteins. Incorporation of complementary lipopeptides into the lipid membranes of liposomes and cell-sized giant unilamellar vesicles (GUVs) facilitated the delivery of a powerful anionophore into GUVs, where its anion transport activity was monitored in real time by fluorescence microscopy. Similar results were achieved using live cells engineered to express a halide-sensitive fluorophore. We conclude that coiled-coil driven membrane fusion is a highly efficient system to deliver anionophores to target cell membranes.
Introduction
There is urgent interest in Drug Delivery Systems (DDSs), such as cell penetrating peptides or liposomes, which are non-invasive and cause no damage to cellular membranes.1 Liposomes of less than 1 μm in diameter have been used as models for studying biological and biophysical membrane properties, as well as DDSs, due to their biocompatibility and low toxicity. Delivery of molecules into the cytoplasm of cells can be achieved by functionalizing liposomes with positively charged lipids, polymers, antibodies or cell penetrating peptides.2,3 Whilst the encapsulation of water-soluble drugs into liposomes is one of the most used tools for drug delivery, the incorporation of lipophilic drugs into the phospholipid bilayer of liposomes has been less exploited.4,5 Alternative approaches to delivering drugs with low water-solubility include the use of solubilizing agents or vehicles, such as micelle forming amphiphiles,6 cyclodextrins7 or cucurbiturils,8 although these are not targeted and tend to have limited stability.One class of lipophilic compounds that could benefit from novel DDSs are transmembrane anion transporters (anionophores).9 These molecules have potential as tools for biomedical research, and might also replace the function of anion channels which are defective or deficient in genetic diseases.10–13 There is particular interest in bypassing the cystic fibrosis transmembrane conductance regulator (CFTR) whose dysfunction causes cystic fibrosis.14–16 Anionophores require sufficient lipophilicity to partition exclusively into the membrane and to carry anions such as chloride across the apolar membrane interior.17 They therefore tend to be water-insoluble, with low intrinsic deliverabilities using conventional DDS.18,19
Inspired by the specific molecular recognition of native SNARE proteins,20,21 we have developed a DDS employing a synthetic model system to induce targeted membrane fusion.22,23 Our membrane fusion system consists of the use of two complementary peptide amphiphiles located in different membranes.24 The formation of a dimeric coiled-coil by these peptides brings the two opposing membranes into close proximity, thereby inducing efficient membrane fusion.25 In previous work, we successfully used this coiled-coil motif to modify surfaces of cancer cells and one-day-old zebra fish embryos in vivo.26,27
Herein, we report the use of this DDS as a highly specific recognition system for delivering a lipophilic anion transporter to both giant unilamellar vesicles (GUVs) and the plasma membrane of cells. The pair of complementary lipopeptides employed in this study is presented in Scheme 1. This synthetic model system is constructed from two complementary amphiphilic coiled-coil peptides K4 [(KIAALKE)4] (1) and E4 [(EIAALEK)4] (2) coupled to a cholesterol anchor through a flexible polyethylene glycol linker. The heterodimeric coiled coil acts as a molecular zipper by the binding of two α-helical peptide strands, while the cholesterol anchor allows the insertion of the peptides into the lipid membrane of vesicles or cells.
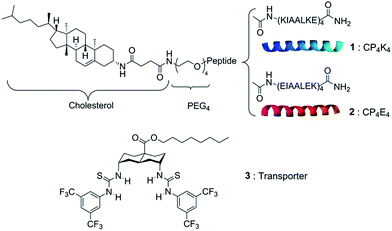 | ||
| Scheme 1 Chemical structures of the synthetic membrane fusion model: lipopeptide CP4K4 (1), lipopeptide CP4E4 (2) and the anionophore bis-(thioureido)decalin (3). | ||
The anionophore bis-(thioureido)decalin (3) has remarkable ability to transport anions across lipid bilayers, promoting rapid chloride–nitrate exchange even when operating as single molecules.28 Recently, we evaluated the activity of 3 in individual GUVs by its direct incorporation into the lipid mixture prior to GUV formation. The average initial rate of chloride transport per molecule was determined by analyzing the quenching of the halide-sensitive fluorophore lucigenin encapsulated in the GUVs.29 Transporter 3 showed exceptional chloride/nitrate exchange activity (820 ± 260 Cl−/s) when incorporated a priori into the lipid membrane of liposomes or GUVs at different concentrations. However, the high lipophilicity of 3 limits its deliverability. Not only is it poorly delivered when added in methanol, but the use of simple vesicles as delivery vehicles is also ineffective (Fig. S1 in the ESI†).
The poor deliverability of 3 and similar anionophores is a critical barrier to future applications. When rates of anion transport were studied in cells, the best performance was obtained by an anionophore with excellent deliverability, but modest intrinsic activity (two orders of magnitude lower than 3 in liposomes).19 Thus, solving the deliverability problem of 3 has great potential for applications in biophysics and perhaps therapeutics. Here we present a facile method to deliver the highly lipophilic transporter 3 pre-incorporated in liposomes by simple incubation with GUVs and cells.
Fig. 1 outlines the protocol for delivering transporter 3 to the lipid membrane of cell-sized GUVs using our synthetic membrane fusion system. The experimental design comprises three main components: (i) GUVs functionalized with lipopeptide 1 as the target membrane and biophysical cell model; (ii) the halide sensitive fluorophore lucigenin encapsulated in the GUVs as a sensing dye and (iii) the DDS which uses liposomes decorated with lipopeptide 2, and with transporter 3 incorporated a priori into the lipid membrane. Following targeting and incorporation of anionophore 3 into the lipid membrane of GUVs by membrane fusion, the quenching of lucigenin fluorescence by chloride is used to measure the chloride transport activity.
Results and discussion
Liposomes were formed from the lipid mixture 1-palmitoyl-2-oleoylphosphatidylcholine (POPC) and cholesterol in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio by sonication method in the presence of 10 mol% transporter 3 and 1 mol% lipopeptide CP4E4 (see ESI for experimental details†). The hybrid lipid film was hydrated in an aqueous solution of 225 mM NaNO3, 10 mM Tris and 200 mM glucose (pH = 7, adjusted with H2SO4) and sonicated for 4 minutes at 50–55 °C. Liposome formation was confirmed by dynamic light scattering measurements (Fig. S2 and S3 in the ESI†) and the peptide functionalized liposomes were characterized by cryo-transmission electron microscopy (cryoTEM) in the absence and presence of transporter (Fig. S4 in the ESI†). Electron microscopy showed that the lipid membrane of the CP4E4 functionalized liposomes was not altered by the incorporation of 3.
3 molar ratio by sonication method in the presence of 10 mol% transporter 3 and 1 mol% lipopeptide CP4E4 (see ESI for experimental details†). The hybrid lipid film was hydrated in an aqueous solution of 225 mM NaNO3, 10 mM Tris and 200 mM glucose (pH = 7, adjusted with H2SO4) and sonicated for 4 minutes at 50–55 °C. Liposome formation was confirmed by dynamic light scattering measurements (Fig. S2 and S3 in the ESI†) and the peptide functionalized liposomes were characterized by cryo-transmission electron microscopy (cryoTEM) in the absence and presence of transporter (Fig. S4 in the ESI†). Electron microscopy showed that the lipid membrane of the CP4E4 functionalized liposomes was not altered by the incorporation of 3.
In parallel, GUVs were grown by lipid film hydration (POPC and cholesterol, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio) on chemically crosslinked dextran (polyethylene glycol) hydrogel (DexPEG) substrates.30 The hydration of the lipid film was performed with a solution of 225 mM NaNO3, 10 mM Tris, 200 mM sucrose and 0.8 mM lucigenin at room temperature. The DexPEG hydrogel allows both the efficient encapsulation of the lucigenin fluorophore and the growth of GUVs under the high ionic strength conditions which are required to perform chloride/nitrate exchange.
3 molar ratio) on chemically crosslinked dextran (polyethylene glycol) hydrogel (DexPEG) substrates.30 The hydration of the lipid film was performed with a solution of 225 mM NaNO3, 10 mM Tris, 200 mM sucrose and 0.8 mM lucigenin at room temperature. The DexPEG hydrogel allows both the efficient encapsulation of the lucigenin fluorophore and the growth of GUVs under the high ionic strength conditions which are required to perform chloride/nitrate exchange.
Lucigenin-loaded GUVs were subsequently functionalized with CP4K4 by incubation in a solution containing lipopeptide 1. Even though it is possible to grow GUVs with lipopeptide 1 pre-incorporated directly into the lipid mixture, we chose to make plain GUVs and modify them a posteriori with lipopeptide 1 because our aim is to deliver the transporter to cellular membranes, which do not contain 1 as a specific recognition motif. As mentioned above, we showed previously that similar cholesterol modified lipopeptides can be inserted efficiently into liposomal membranes31 and the plasma membrane of cancer cells26 by simple incubation. In the present work, we followed the same procedure for the peptide functionalization of GUVs. Briefly, after the formation of lucigenin loaded GUVs, 300 μL of the solution containing the GUVs was transferred to 700 μL of solution containing 225 mM NaNO3, 10 mM Tris, 200 mM glucose and 1 μM CP4K4 (1). GUVs were incubated for one hour at room temperature to allow the incorporation of molecule 1 into the membrane of GUVs. The higher density of the sucrose lucigenin solution encapsulated in GUVs compared to external glucose solution caused the GUVs to sink to the bottom of the micro centrifuge tube. Transfer of sedimented GUVs to fresh external solution in further steps also enabled the removal of the excess of non-encapsulated lucigenin fluorophore.
Liposome–GUV membrane fusion was initiated by treating peptide 1-functionalized GUVs (∼20 μm diameter) containing the chloride-sensitive lucigenin fluorophore with peptide 2-decorated liposomes (∼150 nm diameter) containing 3 pre-incorporated in the lipid membrane (Fig. 1). Briefly, CP4K4 membrane-functionalized GUVs (200 μL) and CP4E4 membrane-functionalized liposomes (100 μL) were combined in 700 μL of 225 mM NaNO3, 10 mM Tris and 200 mM glucose solution. After gentle mixing for 15 minutes using a rotary shaker, the mixture was incubated for 105 minutes more at room temperature to allow fusion of liposomes with GUVs and concomitant delivery of transporter 3 to the lipid bilayer of GUVs. Finally, 200 μL of the sedimented sucrose-containing GUVs were taken from the bottom of the micro centrifuge tube and transferred to a chamber on the stage of a confocal microscope with 100 μL of 225 mM NaNO3, 10 mM Tris and 200 mM glucose. The integrity of GUVs following membrane fusion was verified by confocal fluorescence (excitation at 488 nm) and bright field imaging.
To test for delivery of transporter 3 to the GUV membranes, the chloride-permeability of the GUVs was assayed through lucigenin fluorescence. NaCl (25 μL, 1 M solution) was added with a microsyringe to the microscope chamber containing the GUVs, and the lucigenin emission intensity was observed to decay markedly over a period of ∼3 minutes (Fig. 2, blue triangles). The quenching of lucigenin fluorescence after delivery of transporter 3 (76% after 3 minutes) was significantly stronger than the effect of photobleaching (9% after 3 minutes; Fig. 2, black squares). This result agrees well with previous experiments where transporter 3 was pre-incorporated into the lipid bilayer of GUVs for direct visualization of chloride transport into GUVs.29 Thus, membrane fusion efficiently delivered transporter 3 to the membrane of peptide-functionalized GUVs.
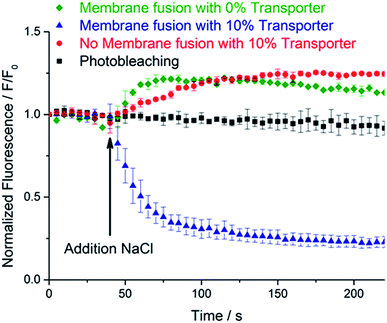 | ||
| Fig. 2 Averaged normalized lucigenin emission intensity after the addition of NaCl (t = 40 s) to: CP4K4-functionalized GUVs treated with CP4E4 liposomes containing the transporter 3 (blue triangles); plain GUVs treated with CP4E4 liposomes containing the transporter 3 (red circles); CP4K4-functionalized GUVs treated with CP4E4 liposomes without transporter 3 (green diamonds). The background photobleaching of CP4K4-decorated GUVs (no NaCl added) is shown as black squares. The normalized fluorescence traces plotted are the averages of three independent membrane fusion experiments on three different individual GUVs. Data are means ± SEM. For individual experiments, see Fig. S5–S8, ESI.† The arrow indicates the addition of NaCl after 40 seconds of time lapse imaging. | ||
As a control, plain GUVs without CP4K4 were mixed with CP4E4-functionalized liposomes containing 10 mol% of transporter 3. After the addition of the NaCl solution, the lucigenin emission intensity inside the GUVs did not decrease (Fig. 2, red circles), proving that transporter 3 was not delivered to the GUV membrane. Instead, there was a small increase in the fluorescence intensity. We attribute this increase to the difference in osmotic pressure between the inside and the outside of the GUVs following NaCl addition. This results in a decrease in the diameter of the GUVs (as detected by bright field microscopy) and hence, an increase in lucigenin concentration. We conclude that omission of the lipopeptide CP4K4 from the membrane of GUVs inhibits the delivery of transporter 3 to GUVs. Thus, membrane fusion induced by the lipopeptides 1 and 2 is required for the targeted delivery of transporter 3 to the membrane of GUVs.
In a second control experiment, we omitted transporter 3 from the CP4E4 liposomes. After membrane fusion, we added the NaCl solution and monitored the lucigenin emission intensity. Again the fluorescence of GUVs increased (Fig. 2, green diamonds), presumably due to GUV shrinkage. This result suggests that the fusion of peptide-decorated liposomes and GUVs neither makes the lipid membrane permeable to chloride ions nor induces leakage of the encapsulated lucigenin fluorophore from GUVs. We conclude that coiled-coil driven membrane fusion is a specific and highly efficient system to deliver anionophores to GUVs.
To determine whether the lipopeptides 1 and 2 can also be used to deliver the lipophilic transporter 3 to live cells, we used cells engineered to express a halide-sensitive fluorophore. We selected for this study Fischer Rat Thyroid (FRT) cells expressing the halide-sensitive fluorophore yellow fluorescent protein (YFP) variant H148Q/I152L, which is highly sensitive for iodide vs. chloride (hereafter termed YFP-FRT cells);32,33 FRT cells are a model system used to investigate epithelial ion transport.34 We demonstrated recently that YFP-FRT cells can be used to study chloride/iodide exchange by anionophores, by monitoring iodide-induced fluorescence quenching.19 Herein, we use our membrane fusion system for the targeted delivery of transporter 3 to the plasma membrane of YFP-FRT cells. Using the same protocol as that presented in Fig. 1 to deliver 3 to GUVs, the plasma membrane of YFP-FRT cells was functionalized with CP4K4 by incubating the cells for 2 hours at 37 °C with the CP4K4 lipopeptide 1 in phosphate-buffered saline (PBS), followed by the addition and incubation with CP4E4 liposomes containing 10 mol% anion transporter 3 for 1 h at 37 °C. The YFP-FRT cells were then transferred to a perfusion chamber mounted on the stage of a fluorescence microscope and perfused with PBS.
After several minutes the PBS flow was changed to a PBS solution containing NaI (10 mM) for 5 minutes. This change of the external buffer led to a rapid and robust quenching of cell fluorescence (Fig. S9 in the ESI†). This decrease in cell fluorescence was almost completely reversed when NaI was washed from the extracellular solution with fresh PBS, indicating the efficient and reversible exchange of chloride and iodide by anionophore 3. Repeating the exposure to NaI after an interval of 20 minutes elicited a further rapid quenching of cellular fluorescence followed by a recovery after washing NaI from the extracellular solution once more with PBS. We performed two control experiments by treating the YFP-FRT cells with either plain POPC liposomes containing transporter 3, the delivery method used in our previous work,19 or with the targeted delivery system without 3 (Fig. 3).
The magnitude of the fluorescence decay in both control experiments was significantly smaller than that elicited by the use of the membrane fusion lipopeptides 1 and 2 for the delivery of anionophore 3. The result of the first control experiment is in agreement with the inability of 3 to be exchanged between membranes without membrane fusion (Fig. S1 in ESI†). The data are also consistent with the control experiments performed using liposomes and GUVs (Fig. 2). Thus, the highly lipophilic anion transporter 3 can be successfully delivered to CP4K4-functionalized YFP-FRT cells via coiled-coil-driven membrane fusion, where it efficiently transports anions across the plasma membrane of YFP-FRT cells.
Conclusions
In conclusion, we demonstrate that the lipidated coiled-coil forming peptides 1 and 2 function as a highly specific molecular recognition system that facilitates membrane fusion. This synthetic model system can be applied as a fast and efficient tool in drug delivery studies. We use a supramolecular approach to solve the deliverability problem of a lipophilic anionophore, with powerful anion transport activity by leakage-free membrane fusion between cell-sized GUVs and liposomes. Similar results were observed using cells engineered to express a halide-sensitive fluorophore. We envisage the topical delivery of the fusogenic lipopeptides and anionophore to the lungs in which peptide 1 and subsequently liposomes carrying peptide 2 and anionophore 3 are inhaled. This raises the hope that the system can be used to deliver anionophores to the apical membrane of airway epithelia, the key target tissue in cystic fibrosis. There is also potential for extending the method to deliver other poorly soluble molecules to biological membranes.Acknowledgements
N. L. M. and A. K. acknowledge CONACyT and the ERC (grant 240394) for financial support. H. V., H. L., D. N. S., and A. P. D. acknowledge the EPSRC (grant. EP/J00961X/1) for financial support. We thank AS Verkman for the generous gift of FRT cells expressing YFP-H148Q/I152L, the Wolfson Bioimaging Facility (University of Bristol) and MA Jepson and AD Leard for help and advice.Notes and references
- B. Gupta, T. S. Levchenko and V. P. Torchilin, Adv. Drug Delivery Rev., 2005, 57, 637–651 CrossRef CAS PubMed.
- T. Lian and R. J. Y. Ho, J. Pharm. Sci., 2001, 90, 667–680 CrossRef CAS PubMed.
- G. Gregoriadis, Trends Biotechnol., 1995, 13, 527–537 CrossRef CAS PubMed.
- R. A. Schwendener, Adv. Exp. Med. Biol., 2007, 620, 117–128 CrossRef PubMed.
- B. S. Pattni, V. V. Chupin and V. P. Torchilin, Chem. Rev., 2015, 115, 10938–10966 CrossRef CAS PubMed.
- V. P. Torchilin, Pharm. Res., 2007, 24, 1–16 CrossRef CAS PubMed.
- M. E. Brewster and T. Loftsson, Adv. Drug Delivery Rev., 2007, 59, 645–666 CrossRef CAS PubMed.
- D. Ma, G. Hettiarachchi, D. Nguyen, B. Zhang, J. B. Wittenberg, P. Y. Zavalij, V. Briken and L. Isaacs, Nat. Chem., 2012, 4, 503–510 CrossRef CAS PubMed.
- P. A. Gale, R. Perez-Tomas and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef CAS PubMed.
- A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348–357 RSC.
- H. Valkenier and A. P. Davis, Acc. Chem. Res., 2013, 46, 2898–2909 CrossRef CAS PubMed.
- J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843–3862 RSC.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- D. A. Stoltz, D. K. Meyerholz and M. J. Welsh, N. Engl. J. Med., 2015, 372, 351–362 CrossRef PubMed.
- J. M. Rommens, M. C. Iannuzzi, B. S. Kerem, M. L. Drumm, G. Melmer, M. Dean, R. Rozmahel, J. L. Cole, D. Kennedy, N. Hidaka, M. Zsiga, M. Buchwald, J. R. Riordan, L. C. Tsui and F. S. Collins, Science, 1989, 245, 1059–1065 CAS.
- G. R. Cutting, Nat. Rev. Genet., 2015, 16, 45–56 CrossRef CAS PubMed.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Chem. Rev., 2014, 5, 1128–1134 CAS.
- C. J. Haynes, N. Busschaert, I. L. Kirby, J. Herniman, M. E. Light, N. J. Wells, I. Marques, V. Felix and P. A. Gale, Org. Biomol. Chem., 2014, 12, 62–72 CAS.
- H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- J. E. Rothman, Angew. Chem., Int. Ed., 2014, 53, 12676–12694 CrossRef CAS PubMed.
- T. C. Sudhof, Angew. Chem., Int. Ed., 2014, 53, 12696–12717 CrossRef PubMed.
- T. Weber, B. V. Zemelman, J. A. McNew, B. Westermann, M. Gmachl, F. Parlati, T. H. Sollner and J. E. Rothman, Cell, 1998, 92, 759–772 CrossRef CAS PubMed.
- H. R. Marsden and A. Kros, Angew. Chem., Int. Ed., 2010, 49, 2988–3005 CrossRef PubMed.
- H. R. Marsden, N. A. Elbers, P. H. H. Bomans, N. A. J. M. Sommerdijk and A. Kros, Angew. Chem., Int. Ed., 2009, 48, 2330–2333 CrossRef PubMed.
- H. R. Marsden, A. V. Korobko, T. T. Zheng, J. Voskuhl and A. Kros, Biomater. Sci., 2013, 1, 1046–1054 RSC.
- H. R. Zope, F. Versluis, A. Ordas, J. Voskuhl, H. P. Spaink and A. Kros, Angew. Chem., Int. Ed., 2013, 52, 14247–14251 CrossRef CAS PubMed.
- J. Voskuhl, C. Wendeln, F. Versluis, E. C. Fritz, O. Roling, H. Zope, C. Schulz, S. Rinnen, H. F. Arlinghaus, B. J. Ravoo and A. Kros, Angew. Chem., Int. Ed., 2012, 51, 12616–12620 CrossRef CAS PubMed.
- H. Valkenier, L. W. Judd, H. Li, S. Hussain, D. N. Sheppard and A. P. Davis, J. Am. Chem. Soc., 2014, 136, 12507–12512 CrossRef CAS PubMed.
- H. Valkenier, N. L. Mora, A. Kros and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 2137–2141 CrossRef CAS PubMed.
- N. L. Mora, J. S. Hansen, Y. Gao, A. A. Ronald, R. Kieltyka, N. Malmstadt and A. Kros, Chem. Commun., 2014, 50, 1953–1955 RSC.
- F. Versluis, J. Voskuhl, B. van Kolck, H. Zope, M. Bremmer, T. Albregtse and A. Kros, J. Am. Chem. Soc., 2013, 135, 8057–8062 CrossRef CAS PubMed.
- A. S. Verkman and L. J. V. Galietta, Nat. Rev. Drug Discovery, 2009, 8, 153–171 CrossRef CAS PubMed.
- L. J. V. Galietta, P. M. Haggie and A. S. Verkman, FEBS Lett., 2001, 499, 220–224 CrossRef CAS PubMed.
- D. N. Sheppard, M. R. Carson, L. S. Ostedgaard, G. M. Denning and M. J. Welsh, Am. J. Physiol., 1994, 266, L405–L413 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc04282h |
| ‡ Present address: Engineering of Molecular NanoSystems, Université libre de Bruxelles, 50 avenue F.D. Roosevelt, B-1050 Brussels, Belgium. |
| This journal is © The Royal Society of Chemistry 2016 |


