Open Access Article
Open Access ArticleThe fabrication of a supra-amphiphile for dissipative self-assembly†
Guangtong
Wang
a,
Bohan
Tang
a,
Yang
Liu
b,
Qingyu
Gao
b,
Zhiqiang
Wang
a and
Xi
Zhang
*a
aKey Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Haidian District, Beijing 100084, China. E-mail: xi@mail.tsinghua.edu.cn
bSchool of Chemical Engineering and Technology, China University of Mining & Technology, Xuzhou, Jiangsu 221116, China
First published on 28th October 2015
Abstract
Dissipative self-assembly is a challenging but attractive field of supramolecular science, because it generally concerns complex systems but is more close to the self-assembly of living bodies. In this article, we realized dissipative self-assembly by coupling a supra-amphiphile with a chemical oscillator. The supra-amphiphile was fabricated with iodine and a double hydrophilic block copolymer containing PEG segments, as the non-covalent interaction between PEG and iodine could turn PEG hydrophobic, leading to the formation of the supra-amphiphile. The self-assembly and disassembly of the supra-amphiphile could be controlled by varying the concentration of iodine. Therefore, the dissipative self-assembly of the supra-amphiphile was realized when it was coupled with the IO3−–NH3OH+–OH− chemical oscillator, which was able to produce iodine periodically. Meanwhile, the kinetic data of the self-assembly and disassembly of the supra-amphiphile could be estimated by the theoretical simulation of the chemical oscillations. This line of research promotes the self-assembly of supra-amphiphiles one step forward from thermodynamic statics to a dissipative system, and also suggests a new strategy to investigate the kinetics of stimuli-responsive molecular self-assembly.
Introduction
Self-assembly is one of the most essential issues in chemistry nowadays, because it is an important bridge between chemistry and biology. Most life activities are closely linked with molecular self-assembly. Although great progress has been made in the field of self-assembly in the last several decades, artificial self-assemblies are still far from living bodies in nature. One of the important reasons is that most self-assemblies achieved until now are actually at a static state of thermodynamic equilibrium. However, unlike these self-assemblies which are under thermodynamic control, self-assemblies of living bodies in nature are typically dissipative (or dynamic, non-equilibrium) self-assemblies, since they are open systems and their activities are realized or maintained by continuously exchanging materials and energy with the external environment.1 Recently, increasing efforts have been made to promote self-assembly from equilibrium to non-equilibrium, which may produce a new approach to understand or even imitate the self-assembly in living bodies.2 Many methods have been used to achieve dissipative self-assembly, such as UV light, chemical oscillations, electric fields and even magnetic fields.3 Among these methods, chemical oscillations4 are powerful tools to realize dissipative self-assembly due to its non-equilibrium nature, because it can provide a non-equilibrium environment in which the amount of components vary continuously and periodically.We wondered if a supra-amphiphile could be used for dissipative self-assembly. Supra-amphiphiles5 refer to amphiphiles fabricated by non-covalent interactions or dynamic covalent bonds. Generally, because of the existence of these dynamic and controllable interactions, supra-amphiphiles and their self-assembly can usually respond to external stimuli,6 which makes supra-amphiphiles promising building blocks for dissipative self-assembly. Dissipative self-assembly may be achieved when stimuli-responsive supra-amphiphiles are coupled with a chemical oscillator.
The idea behind dissipative self-assembly based on a polymeric supra-amphiphile is shown Scheme 1. The coordination between polyethylene glycol (PEG) and iodine has been well known for several decades7 and used to analyze PEG in aqueous solution, because the complex formed by PEG and iodine is insoluble in water (shown in Fig. S1†). Herein we used a double-hydrophilic block copolymer, methoxy-poly(ethyleneglycol)113-block-poly(L-lysine hydrochloride)200 (PEG-b-PLKC) to fabricate a supra-amphiphile with iodine. After coordination with iodine, the amphiphilicity of the PEG segment changed from hydrophilic to more hydrophobic. Considering that the PLKC segment still remained hydrophilic, it led to the formation of a supra-amphiphile, PEG(I2)-b-PLKC, as shown in Scheme 1. Due to the iodine-responsiveness, a dissipative self-assembly could be realized when PEG-b-PLKC was coupled with a IO3−–NH3OH+–OH− chemical oscillator since the oscillator could generate iodine periodically.8
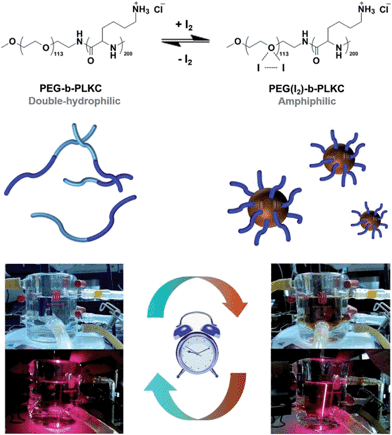 | ||
| Scheme 1 Dissipative self-assembly realized by a supra-amphiphile based on PEG-b-PLKC and iodine coupled with a chemical oscillator. | ||
Results and discussion
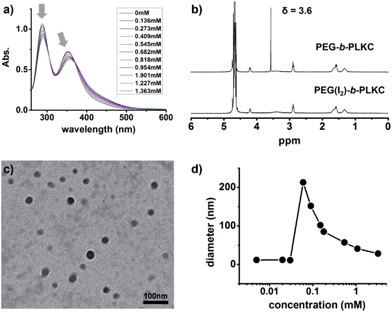
UV/Vis spectrometry and 1H-NMR were used to investigate the formation of the supra-amphiphile, PEG(I2)-b-PLKC. The change in the UV/Vis spectra of iodine after adding PEG-b-PLKC indicated the interaction between PEG-b-PLKC and iodine. As shown in Fig. 1a, both of the absorption bands of iodine (λmax = 288 nm and 352 nm) decreased, meanwhile, one of the absorption bands (λmax = 352 nm) exhibited a slight red shift, making the brown solution even darker. In addition, 1H-NMR was used to further confirm the formation of PEG(I2)-b-PLKC. As shown in Fig. 1b, after the addition of iodine, the sharp peak of the CH2 group in the PEG segment at δ = 3.6 ppm weakened and broadened significantly. The change of the peak not only indicates the interaction between the PEG segment and iodine, but also suggests the self-assembly of PEG(I2)-b-PLKC. In other words, the change of the peak suggests the PEG segment turned hydrophobic, and self-assembled into the interior of the aggregates with the addition of iodine.To further characterize the self-assembly behaviour of PEG(I2)-b-PLKC, transmission electron microscopy (TEM) and dynamic light scattering (DLS) were applied. The samples for TEM observation were prepared by drop-coating the solution on a carbon-coated copper grid without any staining. As shown in Fig. 1c, approximately spherical aggregates were formed by PEG(I2)-b-PLKC. The ζ-potential of the aggregates was measured to be +22.5 mV, indicating the positively charged hydrophilic surface of the aggregates mainly consisted of the PLKC segment. As shown in Fig. 1d, the diameter of the aggregates varying with the concentration of iodine was also measured by DLS. The aggregates could be detected only when the concentration of iodine was increased to 0.06 mM (6.6% of the repeating units of the PEG). Meanwhile, it can be found that the diameter of the aggregates got smaller with the increase of iodine. It may be ascribed that more iodine turns the PEG segment more hydrophobic, making the aggregates more compact.
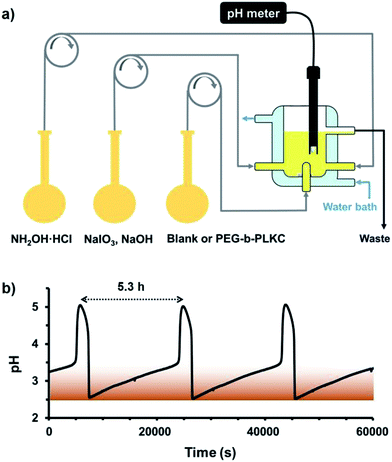
Since the self-assembly of the supra-amphiphile varied with the concentration of iodine, dissipative self-assembly can be achieved by coupling the supra-amphiphile with a chemical oscillator in which the amount of iodine is time-dependent. Among the numerous well-studied chemical oscillators, an IO3−–NH3OH+–OH− oscillator was chosen because it can not only generate iodine periodically but the pH value of the reaction mixture is also oscillatory, allowing the oscillations to be easily tracked. Moreover, its oxidant, IO3− was not as strong in acidic environment as to destroy the PEG-b-PLKC.3c As a dissipative system, sustained oscillations need a continuous supply of reactants and energy. Therefore, a continuous stirred tank reactor (CSTR) should be employed (Fig. 2a). Stock solutions of reactants were injected into the CSTR by peristaltic pumps and the waste produced by the chemical oscillations overflowed from the reactor. A thermostatic water bath was used to keep the temperature at 303 K. As shown in Fig. 2b, oscillations over a period of about 5.3 h could be achieved when the reciprocal residence time, k0 was 1.78 × 10−4 s−1, and the stirring rate was 800 rpm. The generation of iodine can be clearly observed during the low-pH state of the oscillations by the brown colour of the reaction mixture.
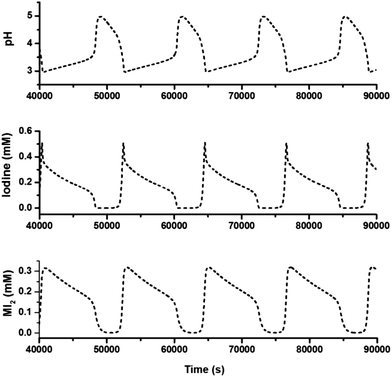
For realizing dissipative self-assembly, 24 μM (2.71 mM, calculated by repeating units of PEG) PEG-b-PLKC was injected into the CSTR as shown in Fig. 2a. Before the injection, the aqueous solution of PEG-b-PLKC was treated with ultrasonic sound for more than 10 min to make the polymer sufficiently disperse in water. The period of the oscillations dwindled from 5.3 h to about 3.2 h with the addition of PEG-b-PLKC as shown in Fig. 3a. A laser beam at λ = 655 nm was applied to detect the formation of the aggregates. During the low-pH state, a significant “Tyndall phenomenon” can be observed (shown in Scheme 1 and the supplementary video, the video is played at a 1000× speed for convenience of the observation), indicating the self-assembly of PEG(I2)-b-PLKC in the CSTR. However, the scattering light turned significantly weak and the reaction mixture became colourless during the high-pH state, as the iodine disappeared and the supra-amphiphile disassembled. For further quantitative investigation, a micro-spectrometer with optical fibre was used to measure the scattering light as exhibited in Fig. 3c. The time-dependent intensity of the scattering light was recorded synchronously with the chemical oscillations (Fig. 3b). It can be found that the scattering light varied with the oscillations regularly. During the high-pH state, the scattering light was too weak to be recorded, because the concentration of iodine was too low to form the supra-amphiphile. However, the intensity of the scattering light increased steeply when the oscillations suddenly switched to a low-pH state, because the iodine was massively produced in this switch. Then the scattering light faded during the following period of the low-pH state due to the reduction of iodine. Interestingly, the scattering increased again at the end of the low-pH state. This may be attributed to the aggregation increasing when the concentration of iodine decreases as shown in Fig. 1d. Meanwhile, it can be also observed that the intensity of the scattering light got stronger in every next period, especially in the earlier stage of the low-pH-state. This might result from the insufficient dispersion of PEG-b-PLKC re-generated by the disassembly of the aggregates of PEG(I2)-b-PLKC.2d Therefore, part of the polymers may still aggregate in a loose manner. When the iodine re-generated massively in the next period, these loose aggregates can grow into larger aggregates. The larger aggregates in the CSTR may gradually accumulate and even grow larger with repeated oscillation cycles causing the scattering light to get stronger, because the intensity of the scattering light is very sensitive to large aggregates, according to the Rayleigh equation. It could also be observed that the solution in the CSTR got slightly cloudy after several oscillation cycles (further discussion and more experimental data are included in the ESI, Fig. S3†).
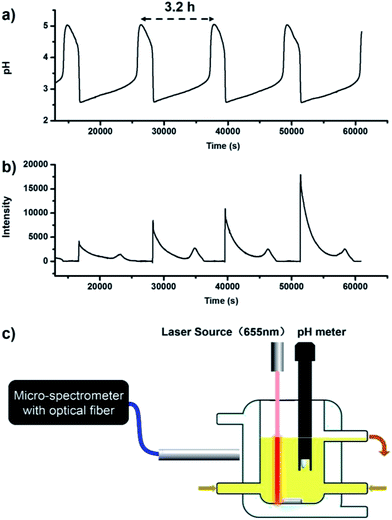 | ||
| Fig. 3 Oscillations of pH and scattering light intensity in the CSTR. (a) The pH-time curve. (b) The intensity–time curve of the scattering light. As the oscillations also generated N2O and N2, the bubbles in the CSTR would sometimes cause a random and momentary increase of the scattering light in a very short time. For convenience, the influence of the bubbles was removed. The original curve is included in ESI, Fig. S2.† Input concentration: [NH2OH·HCl]0 = 50 mM, [NaIO3]0 = 13 mM, [NaOH]0 = 40 mM, [PEG-b-PLKC]0 = 8.0 μM, k0 = 1.78 × 10−4 s−1, T = 303 K. (c) An illustration of the equipment for the quantitative measurement of scattering light. | ||
The mechanism of the IO3−–NH3OH+–OH− oscillator has been well studied by Rábai and Epstein in 1990,8a which involves eight component reactions which are listed in Table 1 (no. 1–8). In the case of supra-amphiphile PEG(I2)-b-PLKC, there should be one more reaction between PEG-b-PLKC and iodine, listed as no. 9 in Table 1. Although the kinetics of the coordination between iodine and PEG-b-PLKC should not be as simple as that of an elementary reaction, we just added the rate reaction between iodine and PEG-b-PLKC as an elementary reaction to the original mechanism suggested by Rábai and Epstein for simplicity. The apparent forward and backward rate constants were set as kM9 = 3.48 mol−1 L s−1, and k−M9 = 2 × 10−3 s−1, respectively. The results of the simulation are shown in Fig. 4. Except for the slight difference in the peak shape, both the amplitude and the period agree well with the experimental value. Meanwhile, the simulation also shows that the decrease of the period after adding PEG-b-PLKC compares with the period of the oscillations without PEG-b-PLKC (Fig. S4†), which is the same as the experimental result. Furthermore, the equilibrium constant of reaction 9 in Table 1, KM9, could be also calculated by the simulation of the oscillations: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KM9 = log(kM9/k−M9) = 3.3. This is quite close to the experimental value, log
KM9 = log(kM9/k−M9) = 3.3. This is quite close to the experimental value, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KM9 = 3.5, obtained from isothermal titration calorimetry (shown in Fig. S5†), providing further support for the above simulation. Notably, the kinetics of the stimuli-responsive molecular self-assembly remain difficult to investigate directly by the means of experiments so far, because most of the processes of molecular self-assembly are too fast to be well detected by current characterization techniques. The simulation of the kM9 and k−M9 suggests that coupling with proper chemical oscillations may provide a new approach to investigate the kinetics of stimuli-responsive molecular self-assembly.
KM9 = 3.5, obtained from isothermal titration calorimetry (shown in Fig. S5†), providing further support for the above simulation. Notably, the kinetics of the stimuli-responsive molecular self-assembly remain difficult to investigate directly by the means of experiments so far, because most of the processes of molecular self-assembly are too fast to be well detected by current characterization techniques. The simulation of the kM9 and k−M9 suggests that coupling with proper chemical oscillations may provide a new approach to investigate the kinetics of stimuli-responsive molecular self-assembly.
| No. | Reaction | Rate |
|---|---|---|
| a “M” represents the repeating units of the PEG segment. | ||
| 1 | NH2OH + H+ ⇌ NH3OH+ | k M1[NH2OH][H+] − k−M1[NH3OH+] |
| 2 | H2O ⇌ H+ + OH− | k M2[H2O] − k−M2[H+][OH−] |
| 3 | 3NH3OH+ + IO3− ⇀ 3NOH + I− + 3H2O + 3H+ | k M3[IO3−][NH3OH+] |
| 4 | IO3− + 5I− + 6H+ ⇀ 3I2 + 3H2O | k M4[IO3−][I−]2[H+]2 |
| 5 | NH3OH+ + I2 ⇀ NOH + 2I− + 3H+ | {(kM5 + kM5′/[H+])[I2][NH3OH+]}/{1 + K[I−] + Q[I−]2 + Q′[IO3−] + Q′′[I−][IO3−]} |
| 6 | 2NOH ⇀ N2O + H2O | k M6[NOH]2 |
| 7 | NH2OH + NOH ⇀ N2 + 2H2O | k M7[NOH][NH2OH] |
| 8 | I2(a.q.) ⇀ I2(gas) | k M8[I2(a.q.)] |
| 9 | Ma + I2(a.q.) ⇌ MI2 | k M9[M][I2(a.q.)] − k−M9[MI2] |
Conclusions
In summary, we have fabricated a supra-amphiphile based on the non-covalent interactions between PEG and iodine. The self-assembly and disassembly of the supra-amphiphile can be controlled by the concentration of iodine. A dissipative self-assembly is achieved successfully by coupling the supra-amphiphile with a chemical oscillator which allows the control of the concentration of iodine periodically. This has demonstrated that supra-amphiphiles are promising building blocks for dissipative self-assembly. Considering the diverse building blocks used for constructing supra-amphiphiles, our research not only develops the application of chemical oscillations, but also enriches the field of artificial dissipative self-assembly. In addition, we also try to use the theoretical modelling of chemical oscillations to estimate the kinetic data of molecular self-assembly, which are difficult to monitor directly. This line of research may provide a new strategy to investigate the kinetics of stimuli-responsive molecular self-assembly.Acknowledgements
We thank Dr Changwei Pan, Dr Chao Wang, Dr Ning Ma, and Yuetong Kang's helpful discussions. We also thank Ocean Optics and Changchun New Industries Optoelectronics for their helpful technical solutions. This research is financially supported by the National Basic Research Program of China (2013CB834502), NSFC Innovation Group (21421064).References
- (a) R. F. Service, Science, 2005, 309, 95 CrossRef CAS PubMed; (b) E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111 CrossRef CAS PubMed; (c) S. Mann, Nat. Nanotechnol., 2009, 8, 781 CAS; (d) D. K. Kumar and J. W. Steed, Chem. Soc. Rev., 2014, 43, 2080 RSC; (e) G. M. Whitesides, Angew. Chem., Int. Ed., 2015, 54, 3196 CrossRef CAS PubMed.
- (a) V. V. Yashin and A. C. Balazs, Science, 2006, 314, 798 CrossRef CAS PubMed; (b) J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2010, 49, 4825 CrossRef CAS PubMed; (c) R. Yoshida, H. Ichijo, T. Hakuta and T. Yamaguchi, Macromol. Rapid Commun., 1995, 16, 305 CrossRef CAS; (d) T. Ueki, M. Shibayama and R. Yoshida, Chem. Commun., 2013, 49, 6947 RSC; (e) H. Q. Liu, E. D. Spoerke, M. Bachand, S. J. Koch, B. C. Bunker and G. D. Bachand, Adv. Mater., 2008, 20, 4476 CrossRef CAS; (f) A. K. Dambenieks, P. H. Q. Vu and T. M. Fyle, Chem. Sci., 2014, 5, 3396 RSC; (g) T. Toyota, N. Maru, M. M. Hanczyc, T. Ikegami and T. Sugawara, J. Am. Chem. Soc., 2009, 131, 5012 CrossRef CAS PubMed; (h) S. N. Semenov, A. S. Y. Wong, R. M. van der Made, S. G. J. Postma, J. Groen, H. W. H. van Roekel, T. F. A. de Greef and W. T. S. Huck, Nat. Chem., 2015, 7, 160 CrossRef CAS PubMed; (i) Q. H. Chen, F. L. Jiang, D. Q. Yuan, G. X. Lyu, L. Chen and M. C. Hong, Chem. Sci., 2014, 5, 483 RSC.
- (a) Y. H. Wei, S. B. Han, J. W. Kim, S. L. Soh and B. A. Grzybowski, J. Am. Chem. Soc., 2010, 132, 11018 CrossRef CAS PubMed; (b) H. Nakanishi, D. A. Walker, K. J. M. Bishop, P. J. Wesson, Y. Yan, S. Soh, S. Swaminathan and B. A. Grzybowski, Nat. Nanotechnol., 2011, 6, 740 CrossRef CAS PubMed; (c) M. Orbán, K. Kurin-Csörgei and I. R. Epstein, Acc. Chem. Res., 2015, 48, 593 CrossRef PubMed; (d) T. Liedl and F. C. Simmel, Nano Lett., 2005, 5, 1984 CrossRef PubMed; (e) I. Lagzi, B. Kowalczyk, D. W. Wang and B. A. Grzybowski, Angew. Chem., Int. Ed., 2010, 49, 8616 CrossRef CAS PubMed; (f) I. Lagzi, D. W. Wang, B. Kowalczyk and B. A. Grzybowski, Langmuir, 2010, 26, 13770 CrossRef CAS PubMed; (g) G. M. Liu and G. Z. Zhang, J. Phys. Chem. B, 2008, 112, 10137 CrossRef CAS PubMed; (h) H. Nabika, T. Inumataa, H. Kitahatab and K. Unoura, Colloids Surf., A, 2014, 460, 236 CrossRef CAS; (i) J. V. I. Timonen, M. Latikka, L. Leibler, R. H. A. Ras and O. Ikkala, Science, 2013, 341, 253 CrossRef CAS PubMed; (j) S. O. Krabbenborg, J. Veerbeek and J. Huskens, Chem.–Eur. J., 2015, 21, 9638 CrossRef CAS PubMed; (k) R. Tomasi, J.-M. Noël, A. Zenati, S. Ristori, F. Rossi, V. Cabuil, F. Kanoufi and A. Abou-Hassan, Chem. Sci., 2014, 5, 1854 RSC.
- (a) V. K. Vanag, L. F. Yang, M. Dolnik, A. M. Zhabotinsky and I. R. Epstein, Nature, 2000, 406, 389 CrossRef CAS PubMed; (b) E. Poros, K. Kurin-Csörgei, I. Szalai and M. Orbán, J. Phys. Chem. A, 2013, 117, 9023 CrossRef CAS PubMed; (c) H. M. Liu, J. A. Pojman, Y. M. Zhao, C. W. Pan, J. H. Zheng, L. Yuan, A. K. Horváth and Q. Y. Gao, Phys. Chem. Chem. Phys., 2012, 14, 131 RSC; (d) H. W. Zhou, X. B. Ding, Z. H. Zheng and Y. X. Peng, Soft Matter, 2013, 9, 4956 RSC.
- (a) X. Zhang and C. Wang, Chem. Soc. Rev., 2011, 40, 94 RSC; (b) Y. T. Kang, K. Liu and X. Zhang, Langmuir, 2014, 30, 5989 CrossRef CAS PubMed; (c) Y. Yao, X. D. Chi, Y. J. Zhou and F. H. Huang, Chem. Sci., 2014, 5, 2778 RSC; (d) G. C. Yu, K. C. Jie and F. H. Huang, Chem. Rev., 2015, 115, 7240 CrossRef CAS PubMed.
- (a) C. Wang, Y. S. Guo, Y. P. Wang, H. P. Xu and X. Zhang, Chem. Commun., 2009, 5380 RSC; (b) C. Wang, Q. S. Chen, Z. Q. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 8612 CrossRef CAS PubMed; (c) G. T. Wang, G. L. Wu, Z. Q. Wang and X. Zhang, Langmuir, 2014, 30, 1531 CrossRef CAS PubMed; (d) G. C. Yu, M. Xue, Z. B. Zhang, J. Y. Li, C. Y. Han and F. H. Huang, J. Am. Chem. Soc., 2014, 53, 13248 Search PubMed; (e) K. Liu, Y. X. Yao, Y. T. Kang, Y. Liu, Y. C. Han, Y. L. Wang, Z. B. Li and X. Zhang, Sci. Rep., 2013, 3, 2372 Search PubMed; (f) X. P. Huang, R. C. Fang, D. G. Wang, J. Wang, H. P. Xu, Y. P. Wang and X. Zhang, Small, 2015, 11, 1537 CrossRef CAS PubMed.
- (a) R. S. Mulliken, J. Am. Chem. Soc., 1950, 72, 600 CrossRef CAS; (b) J.-H. Chang, M. Ohno, K. Esumi and K. Meguro, J. Am. Oil Chem. Soc., 1988, 65, 1664 CrossRef CAS; (c) N. Kaneniwa, A. Ikekawa and H. Hayase, Chem. Pharm. Bull., 1974, 22, 2635 CrossRef CAS.
- (a) G. Rábai and I. R. Epstein, J. Phys. Chem., 1990, 94, 6361 CrossRef; (b) R. T. Wang, G. Rábai and K. Kustin, Int. J. Chem. Kinet., 1992, 24, 11 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc03907j |
| This journal is © The Royal Society of Chemistry 2016 |