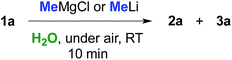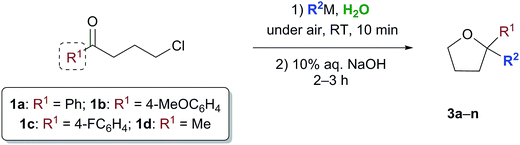Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water opens the door to organolithiums and Grignard reagents: exploring and comparing the reactivity of highly polar organometallic compounds in unconventional reaction media towards the synthesis of tetrahydrofurans†‡
Luciana
Cicco§
 ,
Stefania
Sblendorio§
,
Stefania
Sblendorio§
 ,
Rosmara
Mansueto§
,
Filippo M.
Perna§
,
Rosmara
Mansueto§
,
Filippo M.
Perna§
 ,
Antonio
Salomone§
,
Saverio
Florio
and
Vito
Capriati
,
Antonio
Salomone§
,
Saverio
Florio
and
Vito
Capriati
 *
*
Dipartimento di Farmacia-Scienze del Farmaco, Università di Bari “Aldo Moro”, Consorzio C.I.N.M.P.I.S., Via E. Orabona 4, I-70125, Bari, Italy. E-mail: vito.capriati@uniba.it
First published on 3rd November 2015
Abstract
It has always been a firm conviction of the scientific community that the employment of both anhydrous conditions and water-free reaction media is required for the successful handling of organometallic compounds with highly polarised metal–carbon bonds. Herein, we describe how, under heterogeneous conditions, Grignard and organolithium reagents can smoothly undergo nucleophilic additions to γ-chloroketones, on the way to 2,2-disubstituted tetrahydrofurans, “on water”, competitively with protonolysis, under batch conditions, at room temperature and under air. The reactivity of the above organometallic reagents has also been investigated in conventional anhydrous organic solvents and in bio-based eutectic and low melting mixtures for comparison. The scope and limitations of this kind of reaction are discussed.
Introduction
For more than one hundred years since their discovery, the life of organometallic compounds of s-block elements has been crippled by the manacles of segregation in an inert atmosphere, and generations of organic chemists have been trained to handle them under rigorously anhydrous conditions. Isn't there any hope of routinely carrying out reactions of highly polar organometallic reagents in aqueous/protic media?1In the last few years, the environmental impact of chemical processes has posed severe and compelling demands for sustainable chemistry, and the development of cost-effective and environmentally benign reaction systems, especially in drug product manufacturing, has become one of the main topics of modern synthetic chemistry.2 Green technologies actively look for new solvents to replace conventional harsh organic solvents that present inherent toxicity and high volatility.3 The field of aqueous organic synthesis, in particular, is rapidly growing, engaging and attracting, and excellent papers/reviews are being continuously written year in, year out.4 This is because water is the prototypical green solvent, being abundant, cheap, non-toxic for living organisms, non-flammable, and with unique physical and chemical properties such as, for example, a large heat capacity, thereby allowing exothermic processes to be operated safer and at room temperature (RT). Moreover, reactions of water-insoluble substrates usually lead to the formation of water-insoluble products whose isolation can be easily carried out by conventional filtration (in the case of solids) or by phase separation (in the case of liquids).
Organometallic chemistry has become a cornerstone of modern organic synthesis, and in recent years there has been growing interest towards aqueous organometallic reactions over those taking place in conventional organic solvents.1b However, although water is increasingly being used (both mixed with organic solvents and in bulk) in the chemistry of d-and p-block elements,1b,5 its employment in the chemistry of s-block elements (mainly organolithiums and Grignard reagents) is still limited to catalytic or stoichiometric amounts with surprisingly beneficial effects on reaction rate, product yield, and regio- and stereochemistry.1b,6
Interestingly, Barbier–Grignard-type reactions run in water are also taking to the stage today. The first magnesium-mediated Barbier–Grignard allylation of aldehydes in water was reported in 1998 by Li and Zhang.7a Such reactions also succeeded when performing the direct carbonyl alkynylation, phenylation, alkylation, and arylation using non-activated halides in the presence of various metals (e.g. Zn, CuI) and with the assistance of In(I) salts or transition metals such as Rh.7b–f
The potential impact of unconventional reaction media on the chemistry of s-block elements has recently been independently investigated by Hevia, García-Álvarez and co-workers8 and by our group9 employing the so-called “deep eutectic solvents”, which are fluids generally composed of two or three safe and inexpensive components that can undergo self-association through hydrogen-bond interactions, thereby forming an eutectic mixture with a melting point lower than either of the individual components and with unusual solvent properties.10 Both nucleophilic additions and substitutions8,9a promoted by Grignard and organolithium reagents proved to be effective in such unconventional solvents, thereby providing the expected adducts in good yields and competitively with protonolysis. Novel organometallic transformations have been also successfully explored and carried out directly in a glycerol-containing bio-based mixture.9b In a recent paper, Madsen and Holm showed that once solutions of highly reactive Grignard reagents (allylmagnesium bromide or benzylmagnesium chloride) and of substrates (acetone or benzaldehyde) were prepared separately in syringes and pressed against one another by means of polyethylene capillary tubes in the presence of water, the rate of carbonyl addition efficiently competed with that of protonation.11 Such an intermolecular competition in flow, however, failed in the case of the less reactive alkyl Grignard reagents. We herein investigate for the first time the potential benefits of using a heterogeneous solvent mixture in the nucleophilic addition of both Grignard and organolithium reagents to carbonyl derivatives “on water”, under air, at room temperature and in batch conditions.
Results and discussion
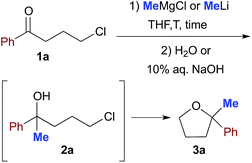
A. Reactions in anhydrous THF
To tackle this problem, we initiated our study by using 4-chloro-1-phenylbutan-1-one (1a) as a model substrate for the preparation of 2,2-disubstituted tetrahydrofuran 3a12via intramolecular cyclization of the intermediate chlorohydrin 2a upon reaction with commercially available MeLi (1.6 M Et2O solution) or MeMgCl (3.0 M THF solution) in anhydrous THF and under a nitrogen atmosphere for comparison (Table 1). The preparation of this substrate from an enolizable ketone also offers the possibility to gain more information about the less/more pronounced nucleophilic/basic character exhibited by the employed organometallic reagent, under certain experimental conditions, by monitoring the competitive formation of other potentially attainable products.| Entry | RM (equiv.) | T (°C) | Time | 1a yield% | 2a yield% | 3a yield% |
|---|---|---|---|---|---|---|
| a Upon quenching with H2O. b Determined by 1H NMR analysis of the crude reaction mixture. c From −40 °C to RT. d Upon treatment with 10% aq. NaOH, 3 h. e Isolated yield after column chromatography. f A mixture of unidentified products also formed. g Neat conditions. h Same result at −40 °C. | ||||||
| 1 | MeMgCl (3)a | −40 | 10 min | 72b | 25b | 3b |
| 2 | MeMgCl (3)a | −40c | 12 h | 20b | 35b | 35b |
| 3 | MeMgCl (3)d | −40 | 12 h | — | — | 60e |
| 4 | MeMgCl (6)d | −40 | 12 h | — | — | 80e |
| 5 | MeMgCl (3)d | RT | 12 h | — | — | 10b,f |
| 6 | MeMgCl (3)d,g | −40 | 12 h | — | — | <5b,f,h |
| 7 | MeLi (3)a | −40 | 10 min | 40b | 60b | — |
| 8 | MeLi (3)a | −40c | 12 h | — | 38b | 38b |
| 9 | MeLi (3)d | −40 | 12 h | — | — | 70e |
| 10 | MeLi (6)d | −40 | 12 h | — | — | 85e |
| 11 | MeLi (3)d | RT | 12 h | — | — | 30e,f |
When a THF (1 mL) solution of 1a (0.5 mmol) was reacted with MeMgCl (3 equiv.) or MeLi (3 equiv.) at −40 °C, and quenched after 10 min reaction time with H2O, mainly a mixture of unreacted substrate (up to 72% in the reaction with MeMgCl) and chlorohydrin 2a (up to 60% in the reaction with MeLi) was detected in the crude product (Table 1, entries 1 and 7). The spontaneous intramolecular cyclization of 2a to 3a in the presence of the organometallic reagent, however, proved to be slow in THF because a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2a and 3a was still present after 12 h stirring at RT (Table 1, entries 2 and 8). Upon further treatment with 10% aq. NaOH (3 h), THF derivative 3a could finally be isolated in 60 and 70% yields in the reactions with MeMgCl and MeLi, respectively (Table 1, entries 3 and 9). By increasing the amount of the organometallic reagent to up to 6 equiv., the corresponding yields of 3a were found to increase to up to 85% (Table 1, entries 4 and 10). Conversely, upon running the reaction at RT, the yield of 3a considerably decreased to 10–30% (Table 1, entries 5 and 11), whereas only a trace of product (<5%) was detected both at RT and at −40 °C under neat conditions (Table 1, entry 6).
1 mixture of 2a and 3a was still present after 12 h stirring at RT (Table 1, entries 2 and 8). Upon further treatment with 10% aq. NaOH (3 h), THF derivative 3a could finally be isolated in 60 and 70% yields in the reactions with MeMgCl and MeLi, respectively (Table 1, entries 3 and 9). By increasing the amount of the organometallic reagent to up to 6 equiv., the corresponding yields of 3a were found to increase to up to 85% (Table 1, entries 4 and 10). Conversely, upon running the reaction at RT, the yield of 3a considerably decreased to 10–30% (Table 1, entries 5 and 11), whereas only a trace of product (<5%) was detected both at RT and at −40 °C under neat conditions (Table 1, entry 6).
B. Reactions in deep eutectic solvents and low melting mixtures
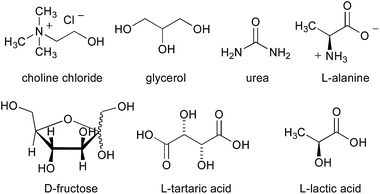
The next investigation was to study the effect of different bio-based deep eutectic solvents (DESs) and low melting mixtures (LMMs)13 based on carbohydrates/urea (Fig. 1) on the chemoselectivity of the addition reaction of the aforementioned organometallic reagents to γ-chloroketone 1a. The addition of a solution of MeMgCl (3 equiv.) to 1a (0.5 mmol) in a D-fructose–choline chloride (ChCl) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) eutectic mixture, at RT and under air, gave 66% conversion to 2a and 18% conversion to 3a after just 10 min reaction time (Table 2, entry 1). It should be noted that a higher conversion was detected both in a D-fructose–urea LMM (3
1) eutectic mixture, at RT and under air, gave 66% conversion to 2a and 18% conversion to 3a after just 10 min reaction time (Table 2, entry 1). It should be noted that a higher conversion was detected both in a D-fructose–urea LMM (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (up to 81% of 2a) (Table 2, entry 3) and in a ChCl–glycerol (Gly) (1
2) (up to 81% of 2a) (Table 2, entry 3) and in a ChCl–glycerol (Gly) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES mixture (up to 85% of 2a) (Table 2, entry 5). On the other hand, if the acidity of the reaction medium increases considerably, as with the employment of L-tartaric acid–ChCl (1
2) DES mixture (up to 85% of 2a) (Table 2, entry 5). On the other hand, if the acidity of the reaction medium increases considerably, as with the employment of L-tartaric acid–ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and L-lactic acid–L-alanine (9
2) and L-lactic acid–L-alanine (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) eutectic mixtures, essentially complete protonation of the Grignard reagent is observed with substantial or quantitative recovery of the unreacted ketone (Table 2, entries 7 and 8).
1) eutectic mixtures, essentially complete protonation of the Grignard reagent is observed with substantial or quantitative recovery of the unreacted ketone (Table 2, entries 7 and 8).
| Entry | RM | DES/LMMa | 1a yieldb% | 2a yieldb% | 3a yieldb% | 4a yieldb% |
|---|---|---|---|---|---|---|
a 1 g per 0.5 mmol of 1a; DES A: D-fructose–ChCl (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol mol−1); LMM A: D-fructose–urea (3 1, mol mol−1); LMM A: D-fructose–urea (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w); DES B: ChCl–Gly (1 2, w/w); DES B: ChCl–Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, mol mol−1); DES C: L-tartaric acid–ChCl (1 2, mol mol−1); DES C: L-tartaric acid–ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, mol mol−1); DES D: L-lactic acid–L-alanine (9 2, mol mol−1); DES D: L-lactic acid–L-alanine (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol mol−1).
b Determined by 1H NMR analysis of the crude reaction mixture.
c Reaction run at 65 °C.
d Reaction run at 50 °C. 1, mol mol−1).
b Determined by 1H NMR analysis of the crude reaction mixture.
c Reaction run at 65 °C.
d Reaction run at 50 °C.
|
||||||
| 1 | MeMgCl | DES A | 16 | 66 | 18 | — |
| 2 | MeLi | DES A | 12 | — | 28 | 55 |
| 3 | MeMgCl | LMM Ac | 19 | 81 | — | — |
| 4 | MeLi | LMM Ac | 15 | 26 | 26 | 33 |
| 5 | MeMgCl | DES B | 15 | 85 | — | — |
| 6 | MeLi | DES B | 10 | 63 | 10 | 12 |
| 7 | MeMgCl | DES Cd | 70 | 30 | — | — |
| 8 | MeMgCl | DES D | 100 | — | — | — |
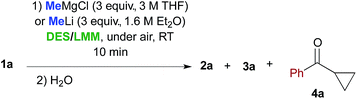
When performed in the above DES/low melting mixtures using MeLi, such addition reactions proved to be less effective. The remaining mass balance in this reaction is mostly accounted for by the formation of cyclopropyl phenyl ketone 4a in variable amounts as the main by-product, which probably arises from an intramolecular displacement of the chloride ion by the enolate intermediate (Table 2, entries 2, 4 and 6). These results suggest that in suitable DES media, the basic character of the organolithium reagent is much more pronounced than its nucleophilic character compared with those of the corresponding Grignard reagent, the latter also being able to promote the formation of compounds 2a and 3a at a higher conversion rate than in THF.14
Cognizant of the above achievements, we were pleased to find that the nucleophilic addition of other Grignard reagents (i-PrMgCl, EtMgCl, 4-MeOC6H4MgBr and 4-ClC6H4MgBr) to the above enolizable ketone (1a), run in the ChCl–Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) eutectic mixture, and followed by treatment with NaOH, straightforwardly furnished the expected 2,2-disubstituted tetrahydrofurans 3b–e in 65–80% yield under air and at RT (Scheme 1). These results are thus in agreement with previous findings by Hevia and García-Álvarez.8 It is worth noting, however, that the employment of carbohydrate urea melts (e.g. LMM A, Table 2) can also be useful for successfully carrying out the above nucleophilic additions (Table 2, entry 3).
2) eutectic mixture, and followed by treatment with NaOH, straightforwardly furnished the expected 2,2-disubstituted tetrahydrofurans 3b–e in 65–80% yield under air and at RT (Scheme 1). These results are thus in agreement with previous findings by Hevia and García-Álvarez.8 It is worth noting, however, that the employment of carbohydrate urea melts (e.g. LMM A, Table 2) can also be useful for successfully carrying out the above nucleophilic additions (Table 2, entry 3).
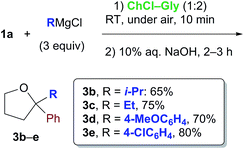 | ||
| Scheme 1 Formation of THF derivatives 3b–evia nucleophilic addition of Grignard reagents to γ-chloroketone 1a in a ChCl-based eutectic mixture at RT and under air. | ||
C. Reactions on water
Can we replace a bio-based eutectic mixture with water? A preliminary experiment showed that when 1 equiv. of MeMgCl (3.0 M THF solution) or MeLi (1.6 M Et2O solution) was rapidly spread over a suspension of 1a (0.5 mmol) in water (1 mL) at RT and under air, a very poor conversion of starting material into chlorohydrin 2a resulted after 10 min (up to 20% yield, Table 3, entries 1 and 2). By employing 2 equiv. of either MeMgBr or MeLi, chlorohydrin 2a was detected in up to 50% yield in the crude reaction mixture (Table 3, entries 3 and 4). Pleasingly, upon switching to 3 equiv., γ-chloroketone 1a underwent nucleophilic addition by MeMgBr and MeLi affording chlorohydrin 2a in 70 and 72% yields, respectively (Table 3, entries 5 and 9). It should be noted that the corresponding percentage conversions in anhydrous THF at −40 °C after 10 min were only 25% (MeMgCl) and 60% (MeLi) (compare with Table 1, entries 1 and 7). Treatment of the crude reaction mixtures with 10% aq. NaOH finally led to the direct isolation of THF derivative 3a in up to 75% yield (Table 3, entries 6 and 10). This latter value could be further improved to up to 82% by using 6 equiv. of the above organometallic reagents (Table 3, entries 7 and 11). The employment of a larger volume of water (3 mL), however, produced a considerable decrease in the yield of 3a from 75% to 45% further to the addition of MeLi (3 equiv.) (Table 3, entry 12).| Entry | RM (equiv.) | 1a yield% | 2a yield% | 3a yield% |
|---|---|---|---|---|
| a Determined by 1H NMR analysis of the crude reaction mixture. b Upon treatment with 10% aq. NaOH, 3 h. c Isolated yield after column chromatography. d Ketone 4a could also be isolated in 15–20% yield. e After removing most of the THF under vacuum from a commercial solution of MeMgCl. f Water: 3 mL. | ||||
| 1 | MeMgCl (1) | 82a | 18a | — |
| 2 | MeLi (1) | 80a | 20a | — |
| 3 | MeMgCl (2) | 71a | 29a | — |
| 4 | MeLi (2) | 50a | 50a | — |
| 5 | MeMgCl (3) | 20a | 70a | 5a |
| 6 | MeMgCl (3)b | — | — | 72c,d |
| 7 | MeMgCl (6)b | — | — | 80c,d |
| 8 | MeMgCl (3)b | — | — | 35c,d,e |
| 9 | MeLi (3) | 18a | 72a | 5a |
| 10 | MeLi (3)b | — | — | 75c,d |
| 11 | MeLi (6)b | — | — | 82c,d |
| 12 | MeLi (3)b | — | — | 45c,d,f |
With satisfactory conditions found for MeLi and MeMgBr, we sought to capitalize on this by exploring the scope of the reaction with a variety of substrates and organometallic reagents. Assorted aliphatic and aromatic Grignard and organolithium reagents such as i-PrMgCl/i-PrLi, EtMgCl/EtLi, n-BuLi, allylMgCl, p-anisylMgBr and p-chlorophenylMgBr all proved to be effective in the nucleophilic addition to a suspension of 1a in water, at RT and under air, straightforwardly providing the expected THF derivatives 3b–g in satisfactory yields (3 equiv.: 50–75%; 6 equiv.: 58–85%) upon final treatment with 10% aq. NaOH (Table 4).
| a 3 equiv. of R2M (isolated yields). b 6 equiv. of R2M (isolated yields). c Compound 3d could also be obtained (70% yield) by reacting γ-chloroketone 1b with PhLi (3 equiv.). d After removing most of the hexanes under vacuum from a commercial solution of n-BuLi. e Compound 3m could also be obtained in 80 and 85% yield by reacting γ-chloroketone 1d with 3 and 6 equiv. of 4-FC6H4MgBr, respectively. |
|---|
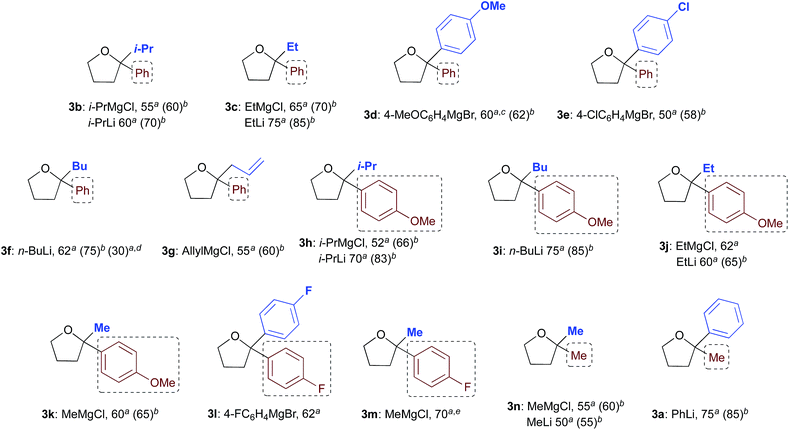
|
Similarly, when an aryl-substituted ketone with an electron-donating group (1b) was used as a substrate, adducts 3h–k were isolated in 52–75% yield with 3 equiv. and in up to 85% yield employing 6 equiv. of RMgCl/RLi (Table 4). The presence of a fluorine atom on the aromatic ring (1c) was well tolerated in the addition reactions with both fluorinated and aliphatic Grignard reagents (3 equiv.), thereby affording products 3l and 3m in 62 and 70% yields, respectively (Table 4). Finally, it was interesting to observe that both aliphatic and aromatic Grignard and organolithium reagents again worked well in the addition reaction to a suspension of the aliphatic γ-chloroketone 1d in water, thus leading to the corresponding adducts 3a and 3n in 50–75% (3 equiv.) or 55–85% yields (6 equiv.). It should be noted that compounds 3d and 3m could also be obtained in 60–85% yield by reacting γ-chloroketones 1b and 1d with PhLi (3 equiv.) and 4-FC6H4MgBr (3 or 6 equiv.), respectively.
In a landmark paper published in 2005, Sharpless and co-workers introduced the concept of “on water” reactions when insoluble organic reactants were able to generate high yields of products with substantial rate acceleration once stirred vigorously in pure water for short periods of time.15 An interesting and important aspect of this work, often overlooked in the literature, is that a significant solvent isotope effect was also noticed by the authors: the reaction rate decreased when D2O was used in place of water. These reactions are thought to occur at the interface between the immiscible phases. The molecular origin of such a rate acceleration, however, has been a matter of dispute. Recently, Huck and co-workers succeeded in quantifying the “on water” effect by using a biphasic (water/toluene) fluidic approach,16 which supported a mechanism involving a possible stabilization of both reactants and transition state by trans-phase H-bonding according to the model proposed by Jung and Marcus.17 On the other hand, some of the well-studied “in water” effects operating in organic reactions for clear solutions of soluble organic reactants are (a) the Breslow hydrophobic effect,18 (b) hydrogen-bonding effects on reactants and transition states, and (c) water polarity effects.19
The reactions described in the present paper deal with the quick addition of an ethereal/hydrocarbon solution of the organometallic reagent miscible in varying proportions [from completely miscible (e.g. THF) to completely immiscible (e.g. hexane)] in the water medium to a suspension of the sparingly soluble γ-chloroketone (ca. 10−3 mol L−1)20 at RT, under air, and under vigorous stirring. Thus, they are unique per se in the scenario of organic transformations in aqueous media investigated so far because the observed chemoselective s-block-metal-mediated nucleophilic additions to the carbonyl derivatives are at the same time in competition with protonolysis processes. Some remarks are in order. By comparing the results of Tables 1 and 3, it transpires that, upon switching from THF to water, comparable yields in 2a and 3a can be obtained in shorter reaction times. For example, in the addition reaction of MeMgCl (3 equiv.) to 1a in dry THF, the amount of starting ketone can be reduced to 20% only after 12 h stirring at RT (overall yield in 2a and 3a: 70%) (Table 1, entry 2). Conversely, the same reaction performed “on water” needs only 10 min stirring at RT to produce a similar result and to afford 2a and 3a in an overall yield of 75% (Table 3, entry 5).
We also investigated the influence of the ethereal/hydrocarbon solution of the organometallic reagent on the final yield. After removing most of the THF or hexanes under vacuum from a commercial solution containing 3 equiv. of MeMgCl or n-BuLi and transferring each of the resulting, very reactive, concentrated solutions to a flask containing 1a, the expected THF derivatives 3a and 3f again formed after treatment with 10% NaOH, albeit in diminished yields (3a: 35%; 3f: 30%) (Table 3, entry 8 and Table 4). Therefore, solvation and dilution of the organometallic reagent is important for better yields.
Does the reaction take place within the organic solvent or at the interface with water? As was pointed out earlier, while organolithium compounds are generally prepared and sold in hydrocarbon solvents which are immiscible with water, most of the Grignard reagents employed in the present study are in a THF solution, which is totally miscible with water. Thus, at least for the latter case, nucleophilic addition should occur at the oil–water phase boundary, with the starting ketone being the only insoluble organic component in the aqueous medium.
May the trans-phase H-bonding be playing a role in this context? Two sets of reactions were run in parallel on 1a (0.5 mmol) with MeMgCl (3 equiv., 3.0 M in THF) and EtLi (3 equiv., 0.5 M in benzene/cyclohexane) employing either H2O (1 mL) or D2O (1 mL) as the aqueous phase (Scheme 2). After 10 min reaction time, each reaction was worked up. The 1H NMR analysis of the crude reaction mixtures revealed the presence of chlorohydrins 2a and 2c (66–75% yield) and very small amounts (up to 5% yield) of the corresponding THF derivatives 3a and 3c as the only products, with the rest being identified as the starting ketone.21 Interestingly, a small deuterium kinetic isotope effect (KIE) was observed. Both the reactions run on D2O proved, indeed, to be slightly slowed down, the overall yields in the final adducts 2a/3a and 2c/3c decreasing to up to about 8% (Scheme 2).
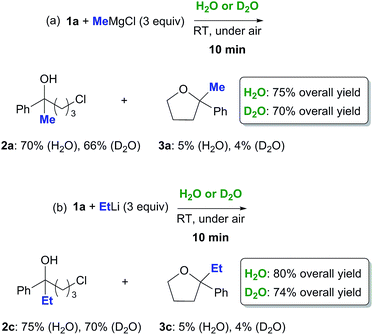 | ||
| Scheme 2 Nucleophilic addition of MeMgCl (a) and EtLi (b) to γ-chloroketone 1a on H2O or on D2O at RT and under air. | ||
In contrast to the classic KIE, these kinds of isotope effects, which are not related to the breaking of any chemical bond of water, have been neither fully understood nor closely investigated at aqueous interfaces. A possible explanation focusing on physical factors was brought forward by Marcus and Jung: the higher viscosity of D2O may affect the droplet size of the reactants, and thus reaction times.17
Pool, Nagata and coworkers, using combined theoretical and experimental approaches, recently demonstrated that the molecular organization and superstructure of water was modified when replacing water (H2O) by heavy water (D2O).22 It was shown, in particular, that the bond orientation of water at the water–vapour interface (which may also serve as a useful model system for extended hydrophobic interfaces) depends markedly on the water isotope composition with the O–H bonds tending to orient up into the vapour phase and the O–D bonds preferably pointing down into the bulk water, thereby leading to stronger hydrogen bonds. This interesting finding could provide an alternative explanation for the usually observed decrease of reaction rate in on D2O chemistry: if the number of dangling OD groups in D2O (free, not D-bonded) available at the interface reduces considerably, the efficiency of the hydrogen-bond catalysis may be affected as well. By comparing organic reactions on H2O and on D2O, Butler and Coyne have recently shown that “on water” catalysis can indeed range from weak to strong trans-phase H-bonding for reactants according to their basicity, a fact that is consistent with a chameleon-type behavior of water at hydrophobic surfaces.23
Can we replace water with another protic medium? We turned our attention to MeOH in which ketone 1a proved to be completely soluble. When trying to perform the addition reaction to a solution of 1a (0.5 mmol) in MeOH (1 mL) with MeMgCl (3 or 6 equiv., 2.0 M in THF), MeLi (3 or 6 equiv., 3% in Et2O), or n-BuLi (3 or 6 equiv., 2.5 M in hexanes), at RT and under air, a higher degree of protonation was observed, and the expected adduct 3a could only be detected in traces in the crude reaction mixture (Scheme 3). Thus, the solubility of the reactant is important and may play a role in promoting such nucleophilic additions. The ability of MeOH to engage in hydrogen bonding is also more limited as compared to water.24
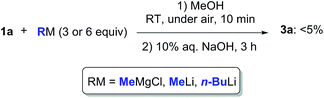 | ||
| Scheme 3 Nucleophilic addition of MeMgCl, MeLi or n-BuLi to γ-chloroketone 1a in MeOH at RT and under air. | ||
Conclusions
Returning to the main issue mentioned at the onset of this research paper, what we have learnt from the present study is : (i) that commercial ethereal/hydrocarbon solutions of organolithium and Grignard reagents (range concentration 0.5–3.0 M), preferably employed in slight excess (at least 3 equiv.) to the amount required to react with the substrate, successfully promote nucleophilic additions once added to a suspension of the enolizable γ-chloroketone in water (0.5 mmol per 1 mL of water). (ii) Both alkylation and arylation of various alkyl and aryl γ-chloroketones take place in satisfactory yields (3 equiv.: up to 75%; 6 equiv.: up to 85% yield) and can be performed under batch conditions, at RT and under air, and competitively with protonolysis. (iii) Critical to the achievement of this advance was the use of heterogeneous conditions (ketones sparingly soluble in water), which are typical of “on water” chemistry. (iv) The solvent isotope effect and the fact that water could not be replaced by alcohols suggest that strong intermolecular hydrogen bonds (and thus the creation of supramolecular clusters in solution) jointly with trans-phase H-bonding with the substrate may be playing a key role (a) in shielding the organometallic reagent from competitive protonolysis processes, and (b) in activating the carbonyl derivative towards nucleophilic addition.25 (v) Both ChCl-based DESs and low melting mixtures consisting of carbohydrate and urea were found to be similarly effective as alternative reaction media for s-block-metal-mediated nucleophilic carbonyl additions with the difference that the basicity of organolithium reagents proved to be more pronounced compared to that exhibited by Grignard reagents.Major breakthroughs can be expected in the near future by investigating the structure–reactivity relationships of highly polar organometallic compounds26 and by an in-depth understanding of their reaction mechanisms in such unconventional reaction media. It is our hope that these preliminary results will set the stage to encourage the scientific community to deepen the investigations into this amazing but still poorly understood field, so as to unveil novel aspects of reactivity, which will be both intellectually rewarding and of practical significance.
Acknowledgements
This work was financially supported by the Interuniversities Consortium C.I.N.M.P.I.S. within the framework of Project PON01_00862 (MIUR) “Una Piattaforma Tecnologica Integrata per lo Sviluppo di Nuovi Farmaci per Malattie Rare”. We are also indebted to Professor Dieter Seebach for valuable discussions and comments on the manuscript and we are grateful to both reviewers for their insightful criticisms that significantly improved the quality of our work.Notes and references
- For an introduction to the problem, see: (a) V. Capriati, F. M. Perna and A. Salomone, Dalton Trans., 2014, 43, 14204 RSC; (b) For a recent review on the reactivity of polar organometallic compounds in unconventional reaction media, see: J. García-Álvarez, E. Hevia and V. Capriati, Eur. J. Org. Chem., 2015, 6779–6799 CrossRef.
- (a) Green Chemistry: Designing Chemistry for the Environment, ACS Symp. Ser., ed. P. T. Anastas and T. C. Williamson, 1996, vol. 626, ch. 1, p. 1 Search PubMed; (b) Green Chemistry: Theory and Practice, ed. P. T. Anastas and J. Warner, Oxford University Press, New York, 1998 Search PubMed; (c) Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, ed. P. T. Anastas and T. C. Williamson, Oxford University Press, New York, 1999 Search PubMed; (d) P. T. Anastas and J. B. Zimmerrman, Environ. Sci. Technol., 2003, 37, 95 Search PubMed; (e) R. Peoples, in Green Techniques for Organic Synthesis and Medicinal Chemistry, ed. W. Zhang and B. W. Cue Jr, Wiley-VCH, Weinheim, Germany, 2012 Search PubMed.
- (a) M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807 CrossRef CAS PubMed; (b) D. S. Adams, P. J. Dyson and S. J. Tavener, in Chemistry in Alternative Reaction Media, John Wiley & Sons, Ltd, Chichester, UK, 2004 Search PubMed; (c) F. M. Kerton, in Alternative Solvents for Green Chemistry, RSC Publishing, Cambridge, 2009 Search PubMed; (d) P. J. Dunn, A. S. Wells and M. T. Williams, in Future Trends for Green Chemistry in the Pharmaceutical Industry in Green Chemistry in the Pharmaceutical Industry, ed. P. J. Dunn, A. S. Wells and M. T. Williams, Wiley-VCH, Weinheim, Germany, 2010, p. 333 Search PubMed; (e) P. G. Jessop, Green Chem., 2011, 13, 1391 RSC; (f) K. Shanab, C. Neudorfer, E. Shirmer and H. Spreitzer, Curr. Org. Chem., 2013, 17, 1179 CrossRef CAS; (g) B. H. Lipshutz and S. Ghorai, Green Chem., 2014, 16, 3660 RSC; (h) C. P. Ashcroft, P. J. Dunn, J. D. Hayler and A. S. Wells, Org. Process Res. Dev., 2015, 19, 740 CrossRef CAS.
- (a) U. M. Lindström, Chem. Rev., 2002, 102, 2751 CrossRef; (b) C.-J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (c) U. M. Lindström, in Organic Reactions in Water: Principles, Strategies and Applications, Blackwell Publishing, Oxford, UK, 1st edn, 2007 Search PubMed; (d) K. H. Shaughnessy, Chem. Rev., 2009, 109, 643 CrossRef CAS PubMed; (e) C.-J. Li, in Handbook of Green Chemistry, Reactions in Water, ed. P. T. Anastas and C.-J. Li, Wiley-VCH, Weinheim, Germany, 2010, vol. 5 Search PubMed; (f) S. Mellouli, L. Bousekkine, A. B. Theberge and W. T. S. Huck, Angew. Chem., Int. Ed., 2012, 51, 7981 CrossRef CAS PubMed; (g) T. Sela and A. Vigalok, Org. Lett., 2014, 16, 1964 CrossRef CAS PubMed.
- For Reformatsky-type reactions run in water, see: (a) C.-J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; (b) C.-J. Li, Tetrahedron, 1996, 52, 5643 CrossRef CAS; (c) Ref. 4b; for Barbier-type reactions in water mediated by indium, see: ; (d) C.-J. Li and T. H. Chan, Tetrahedron Lett., 1991, 32, 7017 CrossRef CAS; (e) Z. L. Sheng, S. Y. Wang, Y. K. Chok, Y. H. Xu and T. P. Loh, Chem. Rev., 2013, 113, 271 CrossRef PubMed.
- For a feature article, see: S. Ribe and P. Wipf, Chem. Commun., 2001, 299 RSC.
- (a) C.-J. Li and W.-C. Zhang, J. Am. Chem. Soc., 1998, 120, 9102 CrossRef CAS; (b) C. Wei and C.-J. Li, Green Chem., 2002, 4, 39 RSC; (c) C.-J. Li and Y. Meng, J. Am. Chem. Soc., 2000, 122, 9538 CrossRef CAS; (d) T. Huang, Y. Meng, S. Venkatraman, D. Wang and C.-J. Li, J. Am. Chem. Soc., 2001, 123, 7451 CrossRef CAS PubMed; (e) C. C. K. Keh, C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 4062 CrossRef CAS PubMed; (f) F. Zhou and C.-J. Li, Nat. Commun., 2014, 5, 4254 CAS.
- C. Vidal, J. García-Álvarez, A. Hernán-Gómez, A. R. Kennedy and E. Hevia, Angew. Chem., Int. Ed., 2014, 53, 5969 CrossRef CAS PubMed.
- (a) V. Mallardo, R. Rizzi, F. C. Sassone, R. Mansueto, F. M. Perna, A. Salomone and V. Capriati, Chem. Commun., 2014, 50, 8655 RSC; (b) F. C. Sassone, F. M. Perna, A. Salomone, S. Florio and V. Capriati, Chem. Commun., 2015, 51, 9459 RSC.
- For recent reviews and articles on DESs, see: (a) C. Ruβ and B. König, Green Chem., 2012, 14, 2969 RSC; (b) Q. Zhang, K. de Oliveira Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108 RSC; (c) Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550 RSC; (d) M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074 CrossRef CAS PubMed; (e) E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060 CrossRef CAS PubMed; (f) J. García-Álvarez, Deep Eutectic Solvents and Their Applications as New Green and Biorenewable Reaction Media, in Handbook of Solvents Use, Health, and Environment, ed. G. Wypych, ChemTec Publishing, Toronto, 2nd edn, 2014, vol. 2 Search PubMed; (g) A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063 CrossRef CAS; (h) P. Liu, J.-W. Hao, L.-P. Mo and Z.-H. Zhang, RSC Adv., 2015, 5, 48675 RSC; (i) For novel hydrophobic DESs, see: D. J. P. van Osch, L. F. Zubeir, A. van der Bruinhorst, M. A. A. Rocha and M. C. Kroon, Green Chem., 2015, 17, 4518 RSC; (j) For stereoselective organocatalysed reactions in DESs, see: E. Massolo, S. Palmieri, M. Benaglia, V. Capriati and F. M. Perna, Green Chem., 2015 10.1039/c5gc01855b.
- G. Osztrovszky, T. Holm and R. Madsen, Org. Biomol. Chem., 2010, 8, 3402 CAS.
- The tetrahydrofuran motif was recently proven to undergo strategic ring elaboration via direct α-lithiation ( R. Mansueto, V. Mallardo, F. M. Perna, A. Salomone and V. Capriati, Chem. Commun., 2013, 49, 10160 RSC ), ortho-lithiation (ref. 9a), and lateral-lithiation (ref. 9b) reactions.
- These “sweet” low melting mixtures based on simple carbohydrates, urea and its derivatives, and optionally inorganic salts, have recently been introduced by König and co-workers as new alternative eco-friendly solvents for organic transformations; see: (a) G. Imperato, E. Eibler, J. Niedermeier and B. König, Chem. Commun., 2005, 1170 RSC; (b) G. Imperato, S. Hoger, D. Lenoir and B. König, Green Chem., 2006, 8, 1051 RSC; (c) G. Imperato, R. Vasold and B. König, Adv. Synth. Catal., 2006, 348, 2243 CrossRef CAS; (d) F. Ilgen and B. König, Green Chem., 2009, 11, 848 RSC; (e) Ref. 10a .
- For a recent interesting paper discussing the competition between basicity and nucleophilicity of some standard organolithium reagents using DFT, see: J. Marchois, C. Fressigné, B. Lecachey and J. Maddaluno, Chem. Commun., 2015, 51, 9801 RSC.
- (a) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed; (b) For a recent review on organic synthesis “on water”, see: A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed.
- S. Mellouli, L. Bousekkine, A. B. Theberge and W. T. S. Huck, Angew. Chem., Int. Ed., 2012, 51, 7981 CrossRef CAS PubMed.
- Y. S. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492 CrossRef CAS PubMed.
- R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS.
- For a recent review summarizing “in-water” and “on-water” effects in organic synthesis, see: R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed.
- Predicted using: Advanced Chemistry Developments (ACD Labs) Software V. 9.04 for Solaris (©1994–2010ACD), http://www.scifinder.cas.org Search PubMed.
- Chlorohydrin 2c was isolated and spectroscopically characterized; see ESI‡.
- R. E. Pool, Y. Nagata, E. H. G. Backus and M. Bonn, Phys. Rev. Lett., 2012, 109, 226101 CrossRef PubMed.
- R. N. Butler and A. G. Coyne, J. Org. Chem., 2015, 80, 1809 CrossRef CAS PubMed.
- It is worth noting that although ketone 1a proved to be poorly miscible with DES A and totally miscible with LMM A, nucleophilic addition reactions successfully took place with Grignard and organolithium reagents in both the above reaction media although with some differences in reactivity (vide infra). DESs, similarly to water, are known to be engaged in a dense network of hydrogen bonds (ref. 10). Strong intermolecular interactions are also thought to be responsible of the high viscosity exhibited by LMMs (ref. 13).
- The protonolysis of organolithium compounds by water or other proton donors is often assumed to be a very simple process affording quantitatively the corresponding organic acid and LiOH. However, it may not be as simple as is commonly believed ( (a) B. J. Wakefield, Organolithium Methods, Academic Press, London, 1988 Search PubMed). The rate of “protonation” of Et2O solution of PhLi and PhCH2Li and their O-deuterated analogues by water or alcohols, for instance, shows small isotope effects (1.0–1.5), which implies that the rate-determining step is the displacement of Et2O from the organolithium compound by the oxygen of the “acid” (see: (b) Y. Pocker and J. H. Exner, J. Am. Chem. Soc., 1968, 90, 6764 CrossRef CAS). Interestingly, in the case of lithium enolates, it was also reported that the “intra-supramolecular” proton transfer from i-Pr2NH to the enolate successfully competes with the intermolecular attack of the electrophile; see: (c) T. Laube, J. D. Dunitz and D. Seebach, Helv. Chim. Acta, 1985, 68, 1373 CrossRef CAS. For an enlightening review, see: (d) D. Seebach, Angew. Chem., Int. Ed., 1988, 27, 1624 CrossRef.
- (a) V. Capriati and S. Florio, Chem.–Eur. J., 2010, 16, 4152 CrossRef CAS PubMed; (b) H. J. Reich, Chem. Rev., 2013, 113, 7130 CrossRef CAS PubMed; (c) E. Carl and D. Stalke, in Lithium Compounds in Organic Synthesis – From Fundamentals to Applications, ed. R. Luisi and V. Capriati, Wiley-VCH, 2014, ch. 1, p. 3 Search PubMed; (d) T. L. Rathman and J. A. Schwinderman, Org. Process Res. Dev., 2014, 18, 1192 CrossRef CAS; (e) V. Capriati, in Modern Lithium Carbenoid Chemistry, Contemporary Carbene Chemistry, ed. R. A. Moss and M. P. Doyle, Wiley, New York, 2014, ch. 11, p. 327 Search PubMed; (f) A. Salomone, F. M. Perna, A. Falcicchio, S. O. Nilsson Lill, A. Moliterni, R. Michel, S. Florio, D. Stalke and V. Capriati, Chem. Sci., 2014, 5, 528 RSC.
Footnotes |
| † Dedicated to Professor Paul Knochel on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data of compounds 2a, 2c, 3a–n, and 4a, and copies of 1H and 13C NMR spectra of compounds 2a, 2c, 3h, 3i, and 3m. See DOI: 10.1039/c5sc03436a |
| § L. C., S. S., R. M., F. M. P. and A. S. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |