Organic chemistry students' fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis
Mary E.
Anzovino
and
Stacey Lowery
Bretz
 *
*
Miami University, Department of Chemistry & Biochemistry, Oxford, OH, USA. E-mail: bretzsl@miamioh.edu
First published on 15th July 2016
Abstract
Organic chemistry students struggle with multiple aspects of reaction mechanisms and the curved arrow notation used by organic chemists. Many faculty believe that an understanding of nucleophiles and electrophiles, among other concepts, is required before students can develop fluency with the electron-pushing formalism (EPF). An expert concept map was created to depict an understanding of nucleophiles and electrophiles ideally held by undergraduates. Second year organic chemistry students were interviewed and asked to give examples of nucleophiles and electrophiles and to identify them in reactions. A cognitive map was created to represent each student's understanding. The students' maps were compared to the expert map, revealing that students possess fragmented ideas about the structure and function of nucleophiles and electrophiles.
Introduction and background
Organic chemistry students often have difficulty seeing the field as a coherent whole, instead taking the perspective that each individual reaction, its reagents, and its mechanism must be memorized (Grove and Bretz, 2012). The sheer number of reactions discussed makes this rote approach to learning very difficult, and therefore students are prone to developing incorrect ideas, many of which persist beyond their time as an undergraduate chemistry student. In particular, crafting plausible reaction mechanisms has been investigated as knowledge that presents a particular challenge to students (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012).Chemistry students' difficulties with reaction mechanisms and related concepts
A variety of previous studies have examined the difficulties that students encounter with mechanisms. When undergraduate chemistry majors within a semester of graduation were asked to select the most likely product of a given set of reagents and reaction conditions in multiple choice items, they focused preferentially on product stability rather than mechanism feasibility (Rushton et al., 2008). Similar results were observed in a sample of introductory organic chemistry students who were explicitly directed to use mechanistic thinking in product prediction tasks (Grove et al., 2012). Rather than proposing and evaluating a mechanism and determining what product(s) would be the outcome of such a pathway, these students predicted a product first, most likely from memorization, and then added in some arrows that may or may not have corresponded to the atom and electron pair placement in the products. Bhattacharyya and Bodner (2005) reported similar findings with organic chemistry graduate students who were provided with complete reactions (starting materials, reagents and conditions, and products) and asked to provide mechanisms for the given transformations. When undergraduate chemistry majors were presented with similar tasks, they encountered a variety of barriers to meaningfully using the curved arrow formalism, including poorly understood content (for example, a weak understanding of acids and bases interfered with the students' abilities to even identify these species in reactions) and the inability to properly apply or understand recalled information (for example, invoking an electrophilic aromatic substitution mechanism when the reaction conditions would not permit such a pathway) (Ferguson and Bodner, 2008). The body of literature reporting on students' use and understanding of reaction mechanisms suggests that the students view the curved arrows, which are so meaningful to organic chemists, as meaningless symbols to be applied in a pattern that they have memorized rather than deeply understood.Bhattacharyya (2013) surveyed faculty regarding what they considered to be the conceptual prerequisites that students needed to master before they could meaningfully engage with the electron-pushing formalism (EPF) and curved arrow notation. The faculty identified a number of concepts, including electronegativity, bond polarity, Lewis acid–base theory, and identification of nucleophilic and electrophilic sites or functional groups in organic molecules. Senior chemistry majors in their final semester struggled to perform this last type of task, demonstrating a weak understanding of the relationship between structure and function, of both the nucleophilic/electrophilic and acidic/basic varieties (DeFever et al., 2015). Prior work by Cartrette and Mayo (2011) found that students didn't consider the relationships between nucleophiles/electrophiles and bases/acids unless explicitly asked to do so. The students in that study also struggled to relate nucleophilicity and electrophilicity to the appropriate model of acid–base behavior (i.e., Lewis) and indiscriminantly applied the labels of nucleophile and electrophile to both Lewis and Brønsted–Lowry acid–base reactions. Students have also struggled to recognize strong bases (e.g., methoxide) in alkyl halide reactions, along with confusion regarding the concerted or step-wise nature of SN2 or SN1 reactions (Cruz-Ramírez de Arellano and Towns, 2014).
Recently, we investigated what second-semester organic chemistry students consider to be the essential characteristics of nucleophiles and electrophiles (Anzovino and Bretz, 2015), as well as their strategies to determine whether twelve reactions involved nucleophiles and electrophiles (or not). Students focused on surface features such as charge to identify nucleophiles, and were more successful at identifying nucleophiles than electrophiles. Perhaps most surprising, though, was the importance of mechanisms to the students. In contrast to the literature summarized above, the students in this study struggled to identify nucleophiles and electrophiles in the absence of a mechanism. The presence of a mechanism was so essential that students would first craft a mechanism to in order to complete the interview task, and if they could not do so, declare that they could not tell if the reaction contained a nucleophile and electrophile because they did not know the mechanism of the reaction.
Knowledge structures and novice-expert comparisons
Organic chemistry is indeed a complex subject, encompassing a wide variety of concepts, skills, and connections between ideas (Graulich, 2015). Concept maps have been used extensively in chemistry education research to explore students' knowledge structures, or the ways in which students organize and connect these concepts in their minds. The majority of published studies have involved students constructing their own concept maps to explore their understanding of areas including chemical bonding (Stensvold and Wilson, 1992; Burrows and Mooring, 2015), functional groups (Akkuzu and Uyulgan, 2016), coordination chemistry (Donovan and Nakhleh, 2001), nanotechnology (Moyses et al., 2010), and gases (Bak Kibar et al., 2013). In some cases, concept maps have been used as instructional tools, e.g. for units on complex-ion equilibria and thermochemistry (Francisco et al., 1998). Lopez et al. (2014) used concept mapping as one task in a study to explore the factors that contribute to first-semester organic chemistry students' problem-solving performance. The students were asked to construct concept maps for four units: structure and bonding, stereochemistry, alkyl halide reactions, and reactions of alkenes. Notably, the list of terms (provided by the researchers in that study) for alkyl halide reactions included nucleophile but not electrophile, suggesting greater emphasis on the former, which in turn shapes students' understandings (Anzovino and Bretz, 2015).Comparisons of novices and experts in chemistry have primarily focused on problem-solving processes. Camacho and Good (1989) found that novices rarely invoked “important theoretical concepts,” including heat of reaction, molar enthalpy, and free energy ideas when explaining problems involving chemical equilibrium. In a comparison of strategies for solving open-ended problems, Randles and Overton (2015) observed that exclusion of evaluative reflection during the problem-solving process set novices (students) apart from industrial and academic chemists. Other work in this area has compared novice and expert characterizations and interpretations of visualizations in chemistry, finding that novices tended to use surface features to make smaller categories in which to group visualizations (where experts used more conceptual understandings to create larger groups), and that novices were less able to describe and derive meaning from the visualizations than the experts were (Kozma and Russell, 1997). Some researchers have assessed how “expert-like” students' thinking is about chemical substances (Stains and Talanquer, 2007) and chemical reactions (Stains and Talanquer, 2008). Deviations from expert-like thinking frequently arose from faulty connections between concepts or an overreliance on surface features, similar to the findings of Kozma and Russell (1997). To our knowledge, no explicit comparison of expert and novice knowledge structures has been reported thus far in the chemistry education research literature.
Research questions
Our previous findings regarding the importance of charges and mechanisms when identifying nucleophiles and electrophiles (Anzovino and Bretz, 2015) were the result of constant comparative analysis (an inductive analysis looking for themes and patterns in the students' responses without imposing any a priori categories (Strauss and Corbin, 2008)). Although that analysis answered questions about how students identify nucleophiles and how students identify electrophiles, it raised additional questions, such as how do students understand the relationship between nucleophiles and electrophiles? Examining the structure of students' knowledge could yield important insights about which concepts regarding nucleophiles and electrophiles are understood, which are misunderstood, and which might be missing altogether. Therefore, additional analysis was conducted through the theoretical lens of meaningful learning. Specifically, the research questions discussed herein were:(1) What concepts comprise the cognitive structures of second-semester organic chemistry students with regard to nucleophiles and electrophiles?
(2) How do the cognitive structures of organic chemistry students with regard to nucleophiles and electrophiles compare to those of experts?
Methods
Theoretical framework
This research was guided by the theory of meaningful learning. When students engage in meaningful learning, they make significant connections between the new knowledge and their relevant prior knowledge (Bretz, 2001; Novak, 2010; Grove and Bretz, 2012); rote learning involves primarily memorizing new information without making such connections. Meaningful learning requires three components: relevant prior knowledge (the student must already possess knowledge that can be related to new knowledge in a non-trivial manner), meaningful material (the new knowledge must be relevant to other information and contain substantive concepts and propositions), and the choice of the learner, who must approach learning as consciously being receptive to the idea of connecting the new knowledge to his or her prior knowledge (Ausubel, 1961). The underlying premise of meaningful learning is that all knowledge is concept-propositional. That is to say, all knowledge is constructed via concepts (“a perceived regularity or pattern in events or objects, or records of events or objects, designated by a label” (Novak, 2010, p. 25)) and propositions (sets of concepts joined by linking phrases that explain their relationships). Most concepts require numerous propositions to be fully described (Novak, 2010). The relationships between ideas are of central interest and the focus of this manuscript. In order to characterize the relationships that exist in the minds of the students, and in order to examine how those knowledge structures compare to those of experts, a cognitive map was created for each student, as described below. (Note that the phrase concept map typically refers to a valid, expert perspective of the knowledge structure, whereas a cognitive map describes the cognitive structure of a learner (Novak and Gowin, 1984).)Concept maps
Concept maps are visualizations intended to illustrate “meaningful relationships between concepts in the form of propositions” (Novak and Gowin, 1984, p. 15). They allow the depiction of a set of ideas as well as any and all relationships between those ideas, providing a rich description of a particular content area, broad or specific. The existence of a rich, interconnected knowledge structure can may suggest that meaningful learning has occurred. The identification of faulty or missing propositional relationships in student ideas can inform future instruction related to the relevant concepts (Novak, 2002).Although most research has focused on analysis of concept maps as constructed by students (vide supra), the origin of the concept map was in fact as a tool for post-hoc analysis of interview data (Novak and Gowin, 1984). A few instances of such use are reported in the chemistry education research literature. Pendley et al. (1994) used concept map analysis of pre- and post-instruction interviews to assess changes in students' understanding of chromatography concepts. Nicoll et al. (2001) used concept maps as a means of analyzing data from student interviews (one per student) about the topics of electronegativity, bonding, and molecular structure. The methodological decision of whether the students construct their own concept maps or the researcher constructs cognitive maps to represent students' understandings depends upon the specific goals of the research. Asking students to construct concept maps requires training each student and consideration that the act of creating a concept map may catalyze changes in the very cognitive structure of interest. As this research was not investigating the pedagogical benefit of asking students to construct concept maps as a way to improve their understanding, the cognitive maps in this research were constructed by the authors in order to synthesize the findings of the interviews.
Sample and setting
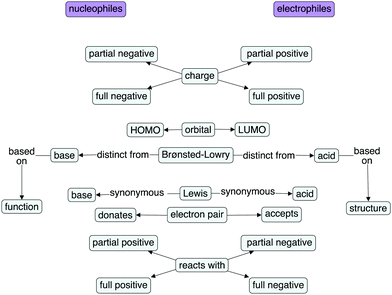
This study was conducted at a medium-sized public research university in the Midwestern United States. Participants were recruited from two, second-semester, organic chemistry lecture courses. The majority of students enrolled in these courses were in the second year of their undergraduate studies. Purposeful sampling (Patton, 2002) (employed to interview students from a variety of majors and with a range of grades in previous chemistry courses) resulted in 11 interviews. Four of the participants were enrolled in a course for chemistry and biochemistry majors; seven were enrolled in a course for majors in other science disciplines. Of the seven students in the nonmajors' course, five were chemical engineering majors. The students in the sample had earned a range of grades (from As to Cs) in their previous chemistry courses (first-year university chemistry I and II and organic chemistry I). Eight of the students in the sample self-identified as male and three as female. As the students were enrolled in their second semester of organic chemistry, they had been taught a wide variety of reactions (including, but not limited to, unimolecular and biomolecular substitution and elimination reactions, radical processes, addition reactions to alkenes, electrophilic aromatic substitution reactions, reactions of aldehydes and ketones, nucleophilic acyl substitution reactions, oxidation reactions, and reduction reactions), many of which involve nucleophiles and electrophiles.The majors' course used the textbook Organic Chemistry, by Bruice (2011), and the nonmajors' course used the textbook Organic Chemistry, by Klein (2012). The authors reviewed the relevant chapters of the Klein textbook and created a concept map of the ideas presented in the text regarding nucleophiles and electrophiles (Fig. 1). The left side of the concept map presents the description of nucleophiles, while the right side presents electrophiles. The textbook for the nonmajors' course was chosen because the nonmajors' course is significantly larger than the majors' course, so the Klein textbook is much more widely used. Additionally, the majority of students (7 of 11) in this study were enrolled in the nonmajors' course. All maps were created with CmapTools (Novak and Cañas, 2006).
 | ||
| Fig. 1 Author-generated concept map based on the nonmajors' course textbook presentation of nucleophile and electrophile structure and function. | ||
The two textbook chapters that focused primarily on introducing nucleophiles, electrophiles, and their fundamental reactions described these characteristics: charge, orbitals (including HOMOs (highest occupied molecular orbitals) and LUMOs (lowest occupied molecular orbitals)), electron pairs, and their relationships to Brønsted–Lowry and Lewis acids and bases. Oddly, the textbook concept map lacked cross-links between these characteristics that would indicate a rich, connected knowledge structure. Rather, the map resembled a ladder, merely listing the characteristics of nucleophiles on one side and the electrophiles on the other, much as the text had done in paragraph form. For this study, the textbook concept map was organized such that structural ideas appear toward the top and functional concepts toward the bottom. The distinction between Brønsted–Lowry bases and nucleophiles is based on function (with what a species reacts), whereas the distinction between Brønsted–Lowry acids and electrophiles is based on structure (the presence or absence of an acidic proton). Therefore, the Brønsted–Lowry “rung of the ladder” serves as the transition point between structural and functional considerations.
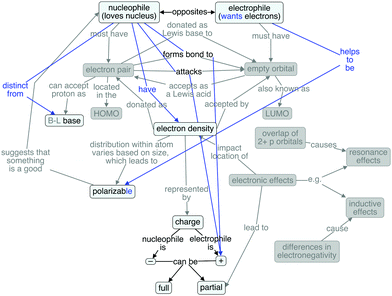
Because the textbook concept map lacked links between the characteristics, a more connected expert concept map (Fig. 2) was constructed and iteratively edited by the authors. The map in Fig. 2 was validated as containing the important concepts and descriptive linking words by two faculty members who teach the introductory organic chemistry courses. The faculty members suggested the addition of bond formation and the addition of resonance effects and their basis, as a complement to inductive effects and their basis. One of the faculty members suggested including steric effects as a complement to electronic effects. However, as steric effects are more related to the relative nucleophilicity or electrophilicity of a molecule or ion, rather than absolute ability to function as a nucleophile or electrophile, they were excluded to be consistent with the semi-structured interview protocol (Anzovino and Bretz, 2015), which did not explore relative nucleophilicity/electrophilicity.
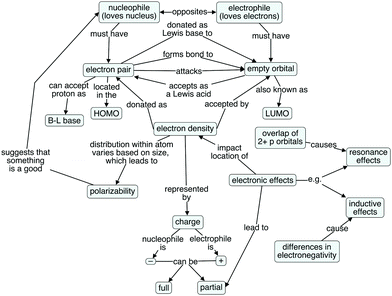 | ||
| Fig. 2 Author-generated concept map developed to convey expert perspectives of nucleophile and electrophile structure and function. | ||
The expert map is arranged such that ideas pertaining more to nucleophiles and their behavior appear on the left side of the map, while concepts related more closely to electrophiles and their behavior are on the right. This organization is not dissimilar to that of the textbook map, but notably, this map has many more connections between the concepts than does the textbook map. The expert map contains both more nodes (concepts) and more linking phrases (connections between concepts) than does the textbook map, giving a more complete view of the ideas important to considering nucleophilic and electrophilic structure and function. Of interest for this research was how students' ideas were connected, and the extent to which their knowledge structures mirrored expert ideas. To explore this notion, a cognitive map was created for each student, starting with the expert map and adding, changing, or omitting (graying-out) items as appropriate.
Data collection and analysis
Each student completed one interview, typically lasting about 60 minutes, approximately two-thirds of the way through the semester. A Livescribe smartpen (Linenberger and Bretz, 2012; Livescribe, Inc.) was used to collect audio recordings and students' writing/drawings. Clarification of students' use of “this” or “that” while pointing at a particular item on a page was accomplished via videorecording of their gestures. The Institutional Review Board approved the research protocol for this study, and each student provided informed consent prior to the interview. Each participant received a $20 amazon.com gift card as compensation for completing the interview. Pseudonyms are used to refer to the students in this manuscript.The interviews were transcribed verbatim and data was managed using NVivo 10 for Mac (QSR International Pty Ltd, 2014). The transcripts from phase 1 of the interviews (open-ended questions about students' definitions of and ideas about nucleophiles and electrophiles) were analyzed using the framework provided by the expert concept map. A cognitive map was created for each student, starting with the expert map. The use of the expert map structure as a common starting point for each student to which modifications (additions or deletions) were made, rather than creating a unique cognitive map for each student, could be viewed as a limitation of this analysis. However, doing so facilitated comparisons across students using a common frame of reference and did not require omission of any significant student ideas. Themes that emerged from an inductive, open coding of students' definitions and examples, as well as an assessment of their success in evaluating reactions for the presence of absence of nucleophiles, have been described elsewhere (Anzovino and Bretz, 2015).
Description of interview prompts
The interviews followed a semi-structured interview protocol to permit deeper exploration of students' ideas as they emerged. This manuscript addresses student ideas from the first phase of the interview, which included questions such as:• Can you tell me what you know about nucleophiles and electrophiles? What comes to mind when you hear those words?
• What are some defining characteristics of nucleophiles [electrophiles]?
• Can a nucleophile [electrophile] also be a base? What, if any, is the relationship between these groups?
• Can a nucleophile [electrophile] also be an acid? What, if any, is the relationship between these groups?
Results and discussion
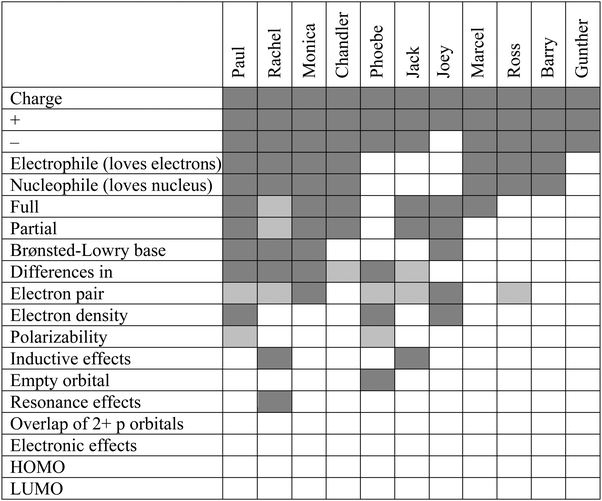
The expert concept map contains 19 nodes, or concepts, including the foundational nodes of nucleophile and electrophile. These foundational nodes also involve the etymological root of each term's name. The frequency with which each of the 19 concepts appeared in the students' cognitive maps can be found in Table 1. A dark gray cell indicates that the student explained the concept correctly, while a light gray cell indicates that a student discussed the concept, but incorrectly (e.g., “lone pair” instead of “electron pair,” since the reacting electron pair in a nucleophile need not be a nonbonding one). Brønsted–Lowry base is shaded only for the students who explicitly talked about this model of basicity, either by name or description (i.e., discussing proton transfer in some way). A white cell means that the student did not discuss the concept. The concepts that were most frequently discussed are at the top of the table, with the students who discussed the largest number of concepts to the left.The mean number of concepts discussed per student was 7.7, with individual students' values ranging from as few as 3 to as many as 12. The mode number of concepts discussed without errors was 6. Even the students with the most detailed understandings of nucleophiles, electrophiles, and related concepts only correctly discussed 11 of the 19 concepts appearing in the expert concept map.
The concepts appearing in the knowledge structures of all or nearly all of the students involved charge. This finding is unsurprising because charge is one of the fundamental characteristics of nucleophiles and electrophiles. All students talked about electrophiles being positively charged, as exemplified by Barry's (second year biology major) comment: “And electrophile is usually just something with a positive charge.” All but one student, Joey (second year biochemistry major), described nucleophiles as negatively charged. Joey referred to nucleophiles as compounds “with a higher density of electronegative charge.” Though it is possible that his mental model of a nucleophile included a formal or partial negative charge, he never spoke of it as such.
Just as some concepts were discussed by all (or nearly all) the students, there were also concepts that were rarely or never mentioned. No one spoke of frontier molecular orbitals (i.e., the HOMO and the LUMO) involved in reactions between nucleophiles and electrophiles nor orbital overlap. Phoebe (second year zoology major) was the only student who even mentioned orbitals:
“But, they [electrophiles] don't necessarily need to have a positive charge, they just need to, um, have, like an orbital that can be, um, like an empty orbital, um, that can take the electrons, uh, of a nucleophile.”
Similarly, Rachel (second year chemical engineering major) was the only student to invoke both resonance and inductive effects to explain the electrophilic nature of the carbon in a carbonyl group:
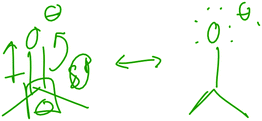
“Well right now, we're doing like ketones and aldehydes, so like the carbon of the carbonyl group has a slightly negative charge because of induction and resonance. Or a slightly positive charge because of induction, resonance with the carbonyl group, so that carbon would be an electrophile.”
However, she did not explain the origin of that alternative resonance form (that is, the overlap of two p orbitals), and her drawing (Fig. 3) actually lacked the positive charge to which she referred. Neither did she discuss any relationship between resonance and induction, failing to speak about them as members of the broader category of electronic effects. The other students who brought up resonance or induction did not discuss a relationship between induction and resonance.
The concepts appearing in many of the students' cognitive maps were consistent with the features that appear in the sparser textbook map (Fig. 1). These include ideas about the roots of the names of the types of species, and concepts related to charge, which were heavily emphasized in instruction. The concepts that appeared in few to no student cognitive maps pertain more to the components of the nucleophile/electrophile model that possess explanatory power. For example, polarizability, inductive effects, and resonance effects all help to explain why a particular site in a molecule or ion can (or cannot) function as a nucleophile or electrophile, rather than simply describing a surface feature of that molecule or ion.
Representative student cognitive maps
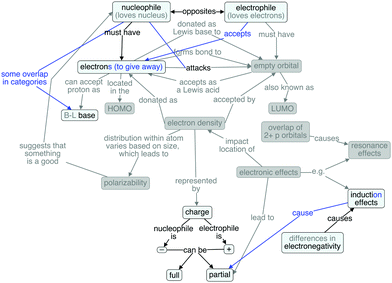
Although Table 1 is useful for examining the relative frequency of concepts included in students' responses, it does not provide information about the specific ways in which students linked the concepts in their explanations. In order to present and discuss the variety of students' understandings with regard to both the quality and quantity of concepts and links between them, five student cognitive maps have been chosen to represent their varying degree of overlap with respect to both the concepts and connections of the expert map. It is important to keep in mind that each cognitive map was not constructed by the student but by the authors. Furthermore, we do not claim that each map depicts the student's entire knowledge structure, but rather each cognitive map is a representation of the student's knowledge through the analytic lens described earlier. These cognitive maps are presented in order of decreasing overlap with the expert map. Paul (second year chemical engineering major) and Rachel were among the students with the most overlap, Jack's (junior chemical engineering major) and Chandler's (second year biochemistry major) cognitive structures exhibited a moderate (based on the range present in the sample) amount of overlap, and Gunther's (second year microbiology major) map includes very few of the ideas present in the expert map. Ideas that were consistent between the student map and the expert map appear in black. If a concept or connection was discussed by the student using different language from that appearing in the expert map, this discrepancy is noted using blue text and lines. Finally, if a concept or connection was not mentioned by the student, that concept or connection appears in gray.Paul's knowledge structure (Fig. 4) had the most overlap with the expert map in terms of the number of concepts discussed. He defined nucleophiles as loving the nucleus and electrophiles as wanting more electrons. He was also one of the few students to explicitly talk about electron density, although he did not connect it directly to charge. Rather, he spoke of nucleophiles as having electron density. Bond formation and attack were both important in his ideas, but they both were between a nucleophile and a positive charge. This use of attack differs from the expert map, where an electron pair attacks an empty orbital. There was no mention of donation/acceptance of electrons or the Lewis base/acid comparisons. He also failed to identify any distinguishing feature other than charge for either a nucleophile or an electrophile. With respect to charge, nucleophiles are negative and electrophiles are positive; he used the words ‘partial’ and ‘completely’ to describe the possibilities. Partial charges are the result of differences in electronegativity.
Paul did mention that it helps for “one of them” (meaning either nucleophiles or electrophiles) to be polarizable. He incorrectly recalled that it was the electrophile, suggesting that he did not understand what it means for something to be polarizable, but rather was just verbalizing a vocabulary term he remembered to be relevant to the general idea of nucleophiles and electrophiles.
Paul's ideas about nucleophiles and bases were different from those of many other students. In his mind, they were distinct from one another, and which category a species belonged to was a function of the type of reaction (substitution for nucleophiles, elimination for bases). Therefore, his categorization of negatively-charged species depended on his knowledge of the mechanism of a reaction and/or its product(s); this suggests that he does not look for features in the starting materials to help make this categorization. Although his ideas are somewhat the reverse of what would be ideal, he was the most articulate when explaining the difference between a nucleophile and a base. (Though again, like all of the other students, he did not explicitly refer to the Brønsted–Lowry definition of bases.)
Although Paul discussed many of the concepts appearing in the expert concept map, many of his linking phrases were novel. He noted that a pair of electrons is required for a nucleophile, but did not consider what feature, beyond a positive charge, would be required of an electrophile. The reliance on charge demonstrated here, and prevalent in other students' ideas as well, suggests that these symbols are merely that and nothing more – just symbols. He recognized that nucleophiles possess electron density, but did not explicitly connect that knowledge to the presence of an electron pair. Although he possessed a number of ideas related to the concepts of nucleophiles and electrophiles, the propositional nature of his knowledge differed from an expert perspective.
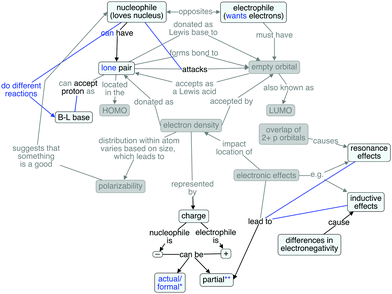
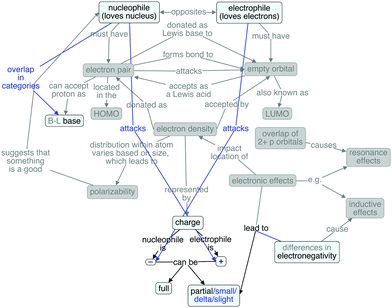
Rachel's cognitive map (Fig. 5) also reflects a great deal of overlap when compared against the expert map. She invoked the etymological roots of the terms of interest, but did not define them as opposites. She articulated the most thorough description of electronic effects, even though she did not explicitly refer to them as such. She mentioned both resonance and inductive effects and attributed partial charges to both. Furthermore, she understood that differences in electronegativity lead to inductive effects. However, she did not explain the origins of resonance nor mention polarizability, but she did thoroughly explain the electronic effects and their impacts on partial charges.
Interestingly, with respect to charge, Rachel spoke about partial charges and actual/formal charges (using those terms synonymously), but only in reference to positive and negative, respectively. Like Paul, Rachel distinguished bases from nucleophiles based on what they do: bases accept protons, whereas nucleophiles attack. However, her explanation of the difference between bases and nucleophiles is not as clear as Paul's. Like many other students, Rachel described a lone pair as being a common characteristic of a nucleophile, but she recognizes that it is not an essential characteristic. She did not describe any analagous structural requirements for an electrophile.
The additional connections present in Rachel's map are the vague distinction between bases and nucleophiles, the connection from nucleophile to attacks (and there is no object for the attack), and the direct connections between resonance/induction effects and partial charges. However, the middle part of her map is grayed out, suggesting a largely fragmented knowledge structure.
Jack (Fig. 6) defined nucleophiles and electrophiles as opposites but did not invoke the roots of either word to explain why they could be considered “opposites.” Nucleophiles must have electrons “to give away,” but he could not describe how he would determine whether electrons were available to be given away. In his view, electrophiles accept the electrons that the nucleophiles give, but again he could not provide any information about how he would decide if a molecule or ion were capable of accepting electrons. There was some overlap between the categories of nucleophile and base, though he did not specify the Brønsted–Lowry model of basicity, nor did he articulate the differences and similarities between those groups. His discussion of electronic effects (which he did not identify as such) was limited to what he referred to as induction (sic) effects due to the presence of electronegative atoms. Jack's failure to realize that a difference in electronegativity between two atoms (rather than simply the presence of electronegative atoms) leads to the polarization of sigma bonds known as induction, is consistent with previous research (Taagepera and Noori, 2000). However, he did recognize that induction leads to partial charges, and that nucleophiles can be either fully or partially negative as can electrophiles be fully or partially positive. The idea of nucleophilic attack was important to him, but he never specified what the nucleophile was attacking. Although his map contains a number of concepts consistent with the expert map, his knowledge structure lacks the expert connectivity between those ideas.
Chandler (Fig. 7) did not discuss any essential features of nucleophiles and electrophiles aside from charges: nucleophiles are negative and electrophiles are positive. His initial definitions derive from the meanings of the words, so he spoke of nucleophiles as looking for something positive to attack and electrophiles looking for something negative to attack. He did not use the word ‘attack’ to link any other pair of concepts. Chandler did not explicitly use the word “full” charge (or any synonyms), but implied that nucleophiles are negative while electrophiles can be partially positive or positive. He also used a variety of imprecise words to refer to partial charges; in addition to “partial,” he referred to these sites as “slightly,” “small,” or “delta.”
The only comment Chandler made about the relationship between nucleophiles and bases was that there is some overlap between the two, but he could not articulate what characteristics were common to both or what characteristics distinguished the two. The middle of his map is very sparse; he did not mention anything about the frontier molecular orbitals (HOMO or LUMO), or either type of electronic effect. The closest he came was mentioning that the electronegativity of oxygen leads to a partial positive on the carbon bonded to it in a carbonyl, without explicitly explaining that it is the difference in electronegativity that causes that partial positive charge. In this way, his explanation was similar to Jack's. Chandler also did not discuss polarizability, donation/acceptance of electrons, or bond formation. Perhaps most notably, there was no discussion of an electron pair being necessary for a nucleophile (and by extension, no mention of an empty orbital required of an electrophile). Also absent was the comparison of nucleophiles and electrophiles to Lewis bases and acids.
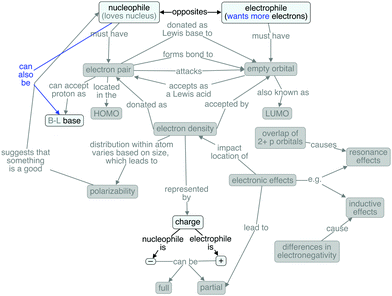
Finally, Gunther represents a student quite different from the others; that is, his map (Fig. 8) contains very few of the concepts and connections in the expert map. Gunther's ideas were limited to those about charge. He did not distinguish the ideas of partial vs. full charges, merely saying that a nucleophile is negative and an electrophile is positive. He also did not rely upon the etymological origin of nucleophile, but he did define electrophile as wanting more electrons. He defined nucleophile and electrophile as opposites, but could not offer any additional explanation as to why.
Conclusions
The expert concept map contained a number of ideas that arose in the student interviews, but it also included concepts and linking ideas that were not discussed by any of the students in the study. Not surprisingly, the concepts that were prevalent in the students' responses included ideas that were prominent in the textbook and instruction: structural features of nucleophiles and electrophiles, such as charges. Missing from the students' understandings, however, were discussions of those concepts having explanatory power. For example, only one student (Rachel) discussed how resonance contributed to partial charges, and even she was unable to explain the structural prerequisite for such resonance (overlap of at least two p orbitals). The students whose maps contained very few concepts (e.g., Gunther) only spoke of the prevalent concepts related to structural features and not anything more nuanced or explanatory.Even the students whose maps contained more of the concepts from the expert map (e.g., Paul and Jack) could not articulate the connectivity between those concepts. The primary focus of the students heavily favors structure, without any consideration of how structure contributes to the function of a molecule or ion. The theory of meaningful learning posits that rote learning, the opposite of meaningful learning, occurs when a learner memorizes new concepts without understanding their relationships to other concepts. The scarcity of propositional knowledge demonstrated by the students in this study (as evidenced by their fragmented cognitive maps) suggests that meaningful learning has occurred to a limited extent, if at all.
Implications for teaching and research
The expert cognitive map presented in this manuscript can be considered a representation of the core concepts pertinent to the ideas of nucleophilicity and electrophilicity. The paucity of discussion by the students regarding the concepts in the middle of the expert map, which are related mainly to explanatory power (i.e., what structural features give rise to a particular kind of reactivity, and why and how those causal relationships exist), suggests that these types of components of the model should be emphasized more in instruction. It is perhaps unrealistic to expect the students to fully discuss all of the concepts in the expert map, given that several iterations were required to produce the expert map and the students' conceptions of important knowledge is likely guided by concepts discussed in lecture. However, comparing the students' cognitive maps to the expert map is still a useful means of determining what concepts are most prevalent in students' thinking and exploring the ways in which students discuss those concepts.The framework of meaningful learning requires that the student not only possesses relevant prior knowledge, but also makes a conscious choice to relate this knowledge to new information being learned. Although it is possible that students held ideas about the relationships between the structural features and the reactivity of molecules or ions, the fact that some of them did not discuss those ideas suggests that reactivity is not essential to those students' conceptions of nucleophiles and electrophiles. The fragmented knowledge structures possessed by the students in this study could also impede their ability to engage in meaningful learning of subsequent concepts in organic chemistry or subsequent courses that build upon those ideas (e.g. biochemistry), since the potential for new information to be meaningful to a student depends upon his or her unique cognitive structure (Ausubel, 1961).
Paul's claim that “it helps if one of them” (meaning nucleophiles or electrophiles) is polarizable, and then stating it would help if the electrophile is polarizable, paired with his lack of elaboration on the concept, suggests that he lacks a deeper understanding of what it means for something to be polarizable and how that polarizability contributes to chemical reactivity. He has seemingly memorized that polarizability is important, without engaging in meaningful learning about the concept. The misalignment between the propositional nature of his knowledge and that in the expert map could interfere with future meaningful learning, as his prior knowledge structure for subsequent concepts could be inadequate. Rachel's use of the phrases “actual charge” and “formal charge” synonymously is potentially problematic, given that the formal charge of an atom in a structure is rarely, if ever, an accurate representation of its true calculated charge, a distinction that is not often emphasized in introductory organic chemistry. To fully comprehend the concepts of nucleophiles and electrophiles, students must have an understanding of the underlying factors (e.g. differences in electronegativity leading to polarized bonds and therefore partial charges) that contribute to an excess or lack of electron density in a particular part of a molecule or ion. With this prior knowledge in place, the organic chemistry instructor's role is to facilitate the connection between these structural considerations and the nucleophilic, electrophilic, acidic, and/or basic reactivity of the species in question. Engaging students in a prior knowledge assessment could aid instructors in determining what ideas students bring with them, allowing the instructor to target incorrect or missing concepts or propositions in the course of teaching. In order to emphasize the connections between concepts, instruction and assessments should focus on the questions that ask why: why is hydroxide acting as a nucleophile in a particular reaction? Why does a ketone with α hydrogens act as an electrophile with certain reagents (e.g. Grignard reagents) but as a Brønsted–Lowry acid with other reagents (e.g. lithium diisopropyl amide)? Focusing on the how the underlying structural features contribute to certain kinds of chemical reactivity should result in student cognitive maps containing more (and more correct) connections between ideas than those exhibited by the students in this study.
Although this research did not ask the students themselves to create concept maps, such an activity could provide answers to other research questions complementary to those answered here. It is possible that providing a list of relevant terms to students creating concept maps could cue them to recognize that they do possess knowledge about some of the concepts not elaborated upon. Future research could also explore what types of research protocols best cue students to their knowledge relevant to this or other sets of concepts. These prompts could either involve asking about the reactions differently (“what kind(s) of reactive species are involved in this reaction?”) or asking students to engage in different kinds of tasks (comparing reactivity of molecules or ions).
Acknowledgements
This material is based upon work supported by the Volwiler Distinguished Research Professorship at Miami University (SLB).References
- Akkuzu N. and Uyulgan M. A., (2016), An epistemological inquiry into organic chemistry education: exploration of undergraduate students' conceptual understanding of functional groups, Chem. Educ. Res. Pract., 17, 36–57.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Ausubel D. P., (1961), In defense of verbal learning, Educ. Theory, 11, 15–25.
- Bak Kibar Z., Yaman F. and Ayas A., (2013), Assessing prospective chemistry teachers' understanding of gases through qualitative and quantitative analyses of their concept maps, Chem. Educ. Res. Pract., 14(4), 542–554.
- Bhattacharyya G., (2013), From source to sink: mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: how students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bretz S. L., (2001), Novak's theory of education: human constructivism and meaningful learning, J. Chem. Educ., 78(8), 1107–1116.
- Bruice P. Y., (2011), Organic Chemistry, 6th edn, Upper Saddle River, NJ: Prentice-Hall.
- Burrows N. L. and Mooring S. R., (2015), Using concept mapping to uncover students' knowledge structures of chemical bonding concepts, Chem. Educ. Res. Pract., 16(1), 53–66.
- Camacho M. and Good R., (1989), Problem solving and chemical equilibrium: successful versus unsuccessful performance, J. Res. Sci. Teach., 26(3), 251–272.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Cruz-Ramírez de Arellano D. and Towns M. H., (2014), Students' understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental rolodexing: senior chemistry majors' understanding of chemical and physical properties, J. Chem. Educ., 92(3), 415–426.
- Donovan W. J. and Nakhleh M. B., (2001), Students' use of web-based tutorial materials and their understanding of chemistry concepts, J. Chem. Educ., 78(7), 975–980.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Francisco J. S., Nicoll G. and Trautmann M., (1998), Integrating multiple teaching methods into a general chemistry classroom, J. Chem. Educ., 75(2), 210–213.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16, 9–21.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13(3), 201–208.
- Grove N. P., Cooper M. M. and Rush K. M., (2012), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Klein D. R., (2012), Organic Chemistry, 1st edn, Hoboken, NJ: John Wiley & Sons, Inc.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Linenberger K. J. and Bretz S. L., (2012), A novel technology to investigate students' understandings of enzyme representations, J. Coll. Sci. Teach., 42(1), 45–49.
- Livescribe, Inc., Livescribe:: never miss a word. http://www. livescribe.com/en-us/.
- Lopez E. J., Shavelson R. J., Nandagopal K., Szu E. and Penn J., (2014), Factors contributing to problem-solving performance in first-semester organic chemistry, J. Chem. Educ., 91(7), 976–981.
- Moyses D. D., Rivet J. L. and Fahlman B. D., (2010), Using concept maps to teach a nanotechnology survey short course, J. Chem. Educ., 87(3), 285–290.
- Nicoll G., Francisco J. S. and Nakhleh M., (2001), An investigation of the value of using concept maps in general chemistry, J. Chem. Educ., 78(8), 1111–1117.
- Novak J. D., (2002), Meaningful learning: the essential factor for conceptual change in limited or inappropriate propositional hierarchies leading to empowerment of learners, Sci. Educ., 86(4), 548–571.
- Novak J. D., (2010), Learning, Creating, and Using Knowledge, 2nd edn, New York, NY: Routledge Taylor & Francis Group.
- Novak J. D. and Cañas A. J., (2006), The Theory Underlying Concept Maps and How to Construct and Use Them (No. 2006-01). Institute for Human and Machine Cognition (IHMC).
- Novak J. D. and Gowin D. B., (1984), Learning How to Learn, Cambridge: Cambridge University Press.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, 3rd edn, Thousand Oaks, CA: Sage Publications, Inc.
- Pendley B. D., Bretz R. L. and Novak J. D., (1994), Concept maps as a tool to assess learning in chemistry, J. Chem. Educ., 71(1), 9–15.
- QSR International Pty Ltd, (2014), NVivo qualitative data analysis software (Version 10).
- Randles C. A. and Overton T. L., (2015), Expert vs. novice: approaches used by chemists when solving open-ended problems, Chem. Educ. Res. Pract., 16(4), 811–823.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9(2), 122–130.
- Stains M. and Talanquer V., (2007), Classification of chemical substances using particulate representations of matter: an analysis of student thinking, Int. J. Sci. Educ., 29(5), 643–661.
- Stains M. and Talanquer V., (2008), Classification of chemical reactions: stages of expertise, J. Res. Sci. Teach., 45(7), 771–793.
- Stensvold M. and Wilson J. T., (1992), Using concept maps as a tool to apply chemistry concepts to laboratory activities, J. Chem. Educ., 69(3), 230–232.
- Strauss A. and Corbin J., (2008), Basics of Qualitative Research, 2nd edn, Thousand Oaks, CA: Sage Publications, Inc.
- Taagepera M. and Noori S., (2000), Mapping students' thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77(9), 1224–1229.
| This journal is © The Royal Society of Chemistry 2016 |