Transfer of the pedagogical transformation competence across chemistry topics
Elizabeth
Mavhunga
Marang Centre for Maths and Science Education, School of Education, University of the Witwatersrand, Johannesburg, South Africa. E-mail: Elizabeth.Mavhunga@wits.ac.za
First published on 13th August 2016
Abstract
Pedagogical Content Knowledge (PCK) observed in one topic is commonly understood not to be transferable to another topic. This study asked, what can then be transferred in the context of learning and acquiring PCK? The study firstly posits the existence of a generic pedagogical competence that is developed in pre-service teachers to pedagogically transform content knowledge, thereby developing their PCK exclusively in the topic, distinguished from general PCK as Topic Specific PCK (TSPCK). It then investigated the transferability of the competence learnt through an intervention on particulate nature of matter for the development of TSPCK in a subsequent, much harder topic chemical equilibrium. The location of the study was in a chemistry methodology class with 36 pre-service teachers. Mixed methods were employed in the research design. The intervention focused on developing the competence to transform concepts in the topic of particulate nature of matter. The primary data comprised a set of completed pre- and post-TSPCK tests in both topics, measuring the extent of the shifts in the quality of the construct. Additional qualitative data in the topic of the intervention was collected from class activities and Content Representations (CoRes) during the intervention, while a vignette was used in the second topic. The findings show the success of the intervention in enabling pedagogical transformation of the content knowledge in both topics, thus demonstrating a successful transfer of the learnt competence. Implications for the development of PCK in core topics in a discipline through transfer of the pedagogical transformation competence are provided.
Introduction
Learning to understand chemistry is traditionally considered a difficult experience (Rollnick et al., 2001), learning to teach it is even more challenging. Pedagogical Content Knowledge (PCK) has emerged as an important form of knowledge for pre-service teachers to develop during their training. PCK was first introduced by Shulman (1986) as a knowledge base for teaching that is unique to the profession, and which enables teachers to bridge difficult content for students' understanding. According to Shulman (1987), a key feature of PCK is the transformation of content knowledge. Teachers generally transform their content knowledge through drawing interactively on other teacher knowledge bases to formulate effective teaching strategies. The importance of the interaction of different teacher knowledge bases rather than a single knowledge base for the development of PCK has been strongly emphasised by a number of researchers, such as Aydin and Boz (2013, p. 615), who state that “PCK is formed through the transformation of many different knowledge bases for teaching; however, it is not the ordinary mixture of them”. In their study on the nature of interactions of components of PCK, Park and Chen (2012, p. 939) pointed out thatPCK is not a straightforward process related primarily to simple possession of those components. Rather, the constructions of PCK are largely influenced by the interaction of different components.
Therefore, learning to transform content knowledge is a central element of the teaching of PCK to pre-service teachers. In learning PCK, pre-service teachers need to learn both the knowledge of the components of PCK, as well as their combined, interactive use in formulating explanations and responses to questions on teaching a topic (Mavhunga and Rollnick, 2013). This ability constitutes the competence in transforming content knowledge and is referred to in this study as pedagogical transformation competence (PTC). PTC is not PCK per se but rather one side of the PCK coin, and is seen as the generic competence to be acquired. Once acquired, the competence is to be applied to engage with the content knowledge of the specific topic (the other side of the coin). A previous study (Mavhunga, 2014) found the process of application of the PTC to be characterized by trial and error engagement with the content knowledge. The product of PTC as a result of its application to the relevant content can then be described as PCK. Since it is developed exclusively in a specific topic, it is regarded as topic-specific PCK (TSPCK), a construct linked to PCK but exclusively located at a topic level rather than discipline PCK, in terms of Veal and MaKinster's (1999) multilevel taxonomy of PCK. The distinction between these forms is discussed in detail in the Literature Background.
In this paper, the aim of the study is to explore how the use of PTC to develop TSPCK, once taught in one chemistry topic, can be applied to a different topic for the same purpose. The importance of this study lies in proposing the existence of a generic pedagogical competence employed to develop PCK from topic to topic. The proposition thus explored in this study is that PTC, once learnt in one topic is a competence that is transferrable for application in another.
The impetus for the development of PTC was the realisation that the initial development of TSPCK takes weeks (Mavhunga and Rollnick, 2013) and makes it impossible for pre-service teacher programmes to invest the required time in developing PCK one topic at a time, across the various topics of a given discipline. The existence of PTC would offer pre-service teachers a framework to use when faced with the planning and teaching of a new topic. Furthermore, the understanding of such competence would offer teacher educators a clearer insight into the kind of knowledge and the corresponding skills that ought to constitute the content of an initial teacher development programme, with the fundamental aim of developing PCK as a goal.
Literature background
PCK is widely taken to be topic-specific, meaning that it is not automatically transferable when it is acquired in a given topic (Aydin et al., 2014). Furthermore, it is further developed through experience in practice, although practice alone is not enough (Halim and Meerah, 2002). However, its early development is desirable in pre-service teachers while they are learning to teach. For pre-service teachers, it is possible for PCK to be developed through coursework, which is in a planned form of PCK (referred to as planned PCK) at a thinking and planning level, rather than in an enacted format (referred to as enacted PCK) (Park and Oliver, 2008). Furthermore, PCK is probably developed in one topic at a time, as in this study, rather than in the discipline as a whole. It is therefore important to distinguish this form of topic-situated PCK, as explored below. The main point is the recognition that planned PCK is linked to reasoning about teaching. According to Shulman (1987), reasoning about teaching is as important as the actual act of teaching, and it is important to influence the thinking that pre-service teachers draw upon in justifying their actions in practice. Therefore, it is argued in this paper that the teaching of PCK through reasoning is useful, and particularly the development of PTC through exposure to transformation of content knowledge.What distinguishes PCK in a topic from PCK in a discipline? Revealing the topic specific nature of PCK
In science education, the understanding that PCK is topic-specific emerges from several independent studies. Van Driel et al. (1998) have explored the development of PCK of pre-service teachers in chemical equilibrium, through a workshop intervention. Their data suggests that pre-service teachers' PCK in the topic improved by focusing on their knowledge of representations and instructional strategies that help to overcome student misconceptions of the topic. In a later study, Park et al. (2011) developed a rubric to measure the quality of PCK, based on two components of their pentagon PCK model (Park and Oliver, 2008), knowledge of learners, and knowledge of instructional strategies. In their rationale for the choice of these components, the authors point to the agreement between the work of PCK science education scholars and that of Shulman (1986), regarding the link between these two components and the topic-specific nature of PCK.Recently, more empirical studies have shed light on the specific knowledge that gives PCK its topic-specific nature. Park and Chen (2012) explored the integration among PCK components for two different biology topics, namely photosynthesis and heredity. The authors counted the frequency of connections among the PCK components. The results of the study showed that the PCK components of knowledge of learner and knowledge of instructional strategies were found to have the strongest interactions, and were more explicit in the teachers' PCK for both topics. However, knowledge of curriculum, which could be argued to be more relevant at discipline than at topic level, had the least connection with other components, and therefore had little influence on teachers' practice. The component of orientation towards science, also more meaningful at discipline level, was less evident, however, where its influence was evident it controlled the component of knowledge of representations and teaching strategies, causing the latter to interact less with other components. Another study on chemistry topics using a similar PCK pentagon model was conducted by Aydin and Boz (2013). The authors observed the interaction of the PCK components in two experienced chemistry teachers' teaching of electrochemical cells and redox reactions. Their findings confirmed the observations by Park and Chen (2012) that the PCK components of knowledge of learner and knowledge of instructional strategies played a more prominent role in the PCK of the teachers in both topics. A study based on the Magnusson et al.'s (1999) model, widely used with studies exploring PCK at a discipline level viz. science, was initiated by Aydin and his colleagues (2014) to identify which components of the model reveal the topic-specific nature of PCK. The study examined the teaching of two experienced teachers' PCK, using a case study methodology pertaining to the topics of electrochemistry and nuclear reactions. The findings also pointed to the components of knowledge of the learner, as well as knowledge of the curriculum. The component of assessment was found to be generic.
The realisation that the topic-specific nature of PCK emerges from components that are content-specific is common across the studies mentioned above. Content-specific components identified include knowledge of learners, and instructional teaching strategies. Components not found to emerge frequently are from those that particularly pertain to a discipline, such as assessment.
These findings provide empirical evidence for the idea that PCK is experienced at different levels, as hinted at by the PCK taxonomies of Veal and Makinster (Veal and MaKinster, 1999; Nezvalová, 2011).
In a previous study (Mavhunga and Rollnick, 2013), PCK at a topic level was conceptualised selectively from components of PCK that are content-specific, and reveal its topic specific nature. In this model, PCK at a topic level is referred to as TSPCK, and defined from a set of five content-specific components listed by Geddis and Wood (1997) as: (i) the knowledge about prior knowledge of learners; (ii) most important core concepts to be understood and their relations to prior concepts in the discipline (which they called curricular saliency); (iii) areas likely to pose potential difficulty for understanding by learners; (iv) representations specific to the topic; and (v) conceptual teaching strategies for the topic that take all of the above into consideration.
These components include those identified by the studies cited above, as they exclude components found in PCK models that are relevant at a discipline level, such as assessment. Thus, from the perspective of teaching PCK to pre-service teachers through the topic specific model, it is necessarily to remain alert to the fact that PCK developed in this manner is exclusively located in the given topic and cannot be claimed for more than that. This accounts for the use of the term TSPCK, not to be tautological but to distinguish the situationally of the acquired PCK.
For teacher educators, a clear understanding of the components that makes PCK topic-specific, and linking such knowledge to pedagogical transformation of the content, is important for the development of PTC.
The link between pedagogical transformation competence (PTC) and TSPCK
Shulman (1987) argued for the recognition of teaching as a profession whose competence is complex and cannot be merely described by a combination of basic skills, content knowledge, and general pedagogical skills. He highlighted the sophistication of the knowledge base from which teachers draw their knowledge, and the complex process of reasoning that they undergo as they plan, deliver and reflect on their lessons afterwards. Like Fenstermacher (1986), Shulman (1987) declared reflective teaching to require sound reasoning through the teacher's comprehended understanding and skilful performance. He ascribed six teacher actions to the process of reasoning, which he termed pedagogical reasoning and action. He indicated that these steps are not necessarily always in the given sequence, they however have a cyclic nature. They begin with comprehension of facts, principles, connections of ideas within and outside the discipline, and purposes. They proceed to transformation of the knowledge into re-structured bits of knowledge that are sequenced, and represented in ways that foster learner understanding. The process continues into the actual instruction, evaluation, reflection and finally new comprehension by the teacher. In this study, there is particular interest in the teacher act of transformation of content knowledge. It is conceptualised as pedagogical transformation, which has been defined as “the instructional principle in which scientific ideas are simplified and reconstructed into what can be readily accessible to and understood by students without distorting the essential features of the ideas” (Oh and Oh, 2011, p. 1124). The notion of pedagogical transformation is analogous to that of didactic transposition suggested by Chevallard (1988) in the context of mathematics education. Pedagogical transformation is a step within the pedagogical reasoning process, considered to be “the essence of the act of pedagogical reasoning of teaching” (Shulman, 1987, p. 16). According to Shulman, transformation of content knowledge happens long before the actual teaching, and is central to the presence of PCK. He also suggested that PCK emerged and developed, as teachers transformed their content knowledge for the purpose of teaching students (Wilson et al., 1988). A number of researchers have supported the view that the transformation of subject matter knowledge is the essence of teachers' PCK (e.g.Van Driel et al., 1998; Abell, 2008). Pedagogical transformation of comprehended knowledge is further endorsed by Geddis and Wood (Geddis and Wood, 1997, p. 612) as seen in their statement as follows:The value of a focus on the transformation of subject matter is that it directs attention simultaneously to subject matter, learners and educational purposes, and to the interactions among these different kinds of teacher knowledge in the pedagogical encounter.
Geddis and Wood (1997) explored the idea further and identified different kinds of knowledge which we have listed earlier as components of TSPCK, which are the source of transformation of content knowledge. In this study, the competence needed to carry out the act of pedagogical transformation is singled out, for the purposes of teaching it to pre-service teachers. This competence, as mentioned earlier, has been termed PTC. It refers to the understanding of the relevant kinds of knowledge, the components of TSPCK, and the ability to create ‘interactions’ that need to be drawn into a pedagogical encounter, as averred by Geddis and Wood (1997) above. PTC is to be learnt and applied on a specific content knowledge so as to result in PCK for the topic of concern. The process would need to be repeated for the development of TSPCK in each different topic.
In our previous study (Mavhunga and Rollnick, 2013), it was demonstrated that the explicit discussion of transformation of content knowledge as a teaching strategy in a pre-service programme, lead to a significant improvement in the quality of TSPCK. The present study, therefore, builds on this finding, by repeating the previous intervention, using the topic of particulate nature of matter. Additionally, we investigate the transfer of the learnt PTC for its application to a new topic, in this case, chemical equilibrium.
Method
The study employed a mixed methods research design that allowed for quantitative analysis of the shifts in the quality of TSPCK in both topics and for qualitative analysis of portrayed TSPCK. The choice of the research method was based on the advantages that this approach traditionally offers in view of the complex and tacit nature of the theoretical concept of PCK. The study adopted case study as a research strategy, due to its benefits in enabling in-depth interactions with the participants and consideration of the issues under investigation (Stake, 2005).Participants and the context
Merriam (2002, p. 8) describes a case study as “an intensive description and analysis of a social unit”. The ‘social unit’ in this study is a chemistry methodology class of 36 third-year pre-service teachers. These are pre-service teachers in their third year of study towards a teacher qualification – the B Ed degree, with Chemistry and Physics as their major subject. In the institution, the teacher preparation programme is structured such that the content and the methodological components are delivered as separate, but parallel courses, by the same department. Integration across the two courses is promoted by teaching a topic at about the same time across the two courses. The institution is located in Johannesburg, a city known to be the major economic hub of the province. Students drawn to the science education degree come primarily from both nearby townships, urban suburbs (radius of 80 km) and distant rural areas across different provinces (up to 400 km distant). The townships and rural areas are generally populated by previously disadvantaged communities, whose provisioning and delivery of education, particularly when it comes to science education, is historically known to be poor (Zhang et al., 2015). Most of the science education students from previously disadvantaged communities are on a national bursary programme with a requirement that they provide service in state schools, preferably in their home towns, in order to improve the quality of science education in the Secondary Schools. Thus, there is a national, socially-driven pressure on them to give back to the community, and to do so proficiently.The 3rd year methodology Chemistry class were purposely selected due to the nature of the intervention implemented in this particular course. The course has, as one of its goals, to develop PCK in specific topics that are contained in the Curriculum and Assessment Policy Statement (Department of Basic Education, 2011). Thus, one of the interventions implemented was an intervention aimed at developing TSPCK in the particulate nature of matter. In this particular methodology class, 90% of the pre-service teachers were from previously disadvantaged communities, with the remaining 10% from affluent suburbs with good quality schools. Their ages ranged from 20–30 years old.
All the research activities were carried out with the approval of the institution's Ethics Committee, as well as the Head of School, so as to ensure the rights and protection of the participants. Participation was voluntary, and pseudonyms and codes are used throughout the manuscript to preserve confidentiality.
Intervention
The intervention happened over a period of six weeks, with three one-hour periods in each week. Two of the periods made up a double period early in the week, with a single period often used as tutorial discussion, held towards the end of the week. The intervention was conducted by the author. It consisted of explicit explanations of the idea of transformation of content knowledge as a step in the process of pedagogical reasoning (Shulman, 1987). The five content-specific components of TSPCK identified earlier namely: (i) Learner Prior Knowledge; (ii) curricular saliency; (iii) what is difficult to understand; (iv) representations; and (v) conceptual teaching strategies, were introduced as knowledge from which transformation of content knowledge emerges, and thus, were central to the content of the intervention.The five content-specific components of TSPCK were discussed one at a time, using the topic of particulate nature of matter. The topic was chosen in the course because it is fundamental to the understanding of all other topics in chemistry, and is also contained in the National Secondary School Curriculum, where pre-service teachers will be expected to teach it once qualified. Also, pre-service teachers would be familiar with the content knowledge of the topic, as the advanced aspects of the topic were being taught in the separate content course, at about the same time as the intervention. This is one of the advantages of offering both the content and the methodology courses within a single School of Education Division, as it allows alignment and timing of teaching of different emphasis in the same topic.
The discussion on each TSPCK component was focused on strategically selected concepts of particulate nature of matter, linked to the component. For example, the component of Learners' Prior Knowledge was introduced using learner statements displaying common misconceptions about the size of particles of a substance when heated. That is, learners often think that the size of particles increases when a substance is heated (Ayas et al., 2010). This enabled an aspect of content knowledge, kinetic particle theory in this case, to be dealt with from a point of a common misconception, rather than from providing definitions or explanations, as may traditionally be the case in a content course.
The discussions on the second component of curricular saliency focused on identifying the core, most important concepts to be understood by learners in this topic. The theoretical idea of formulating ‘Big Ideas’ of the topic was introduced (Loughran et al., 2004). A concept map presenting the main and sub-concepts of the topic was used to pictorially share a common structuring of the topic. The main concepts in the map were used as anchors to formulate Big Idea statements that describe their meaning. Statements formulated included the existence of particles in all substances, the constant motion of particles, the arrangement of particles in different phases, etc. These were confirmed as the ‘Big Ideas’ of the topic (Loughran et al., 2004), and their difference from the enlisted main concepts, in the concept map, from which they were derived, were pointed out. So, the discussion on Big Ideas naturally assisted in identifying the main concepts in the topic and distinguishing them from their subordinate concepts. Once the discussion on Big Ideas was in place, conceptual sequencing of the identified Big Ideas when planning to teach the topic was discussed.
The key element in deciding on the sequence was the identification of pre-concepts that would be required prior to the fruitful discussion of a particular Big Idea. The next discussion centred on identifying potential areas that may be difficult for learners to understand. For example, understanding the existence of empty spaces between particles in a substance is potentially difficult to understand against the backdrop of the existence of intermolecular forces between particles such as molecules.
The discussion was followed by the introduction of the use of representations in three distinct levels to explain concepts in chemistry (Davidowitz and Chittleborough, 2009). These were listed as macroscopic, symbolic and sub-microscopic levels. At this point, reference was made to examples of representations used in the discussions of the components much earlier in the intervention. A typical example was the reference to the discussion on Learner Prior Knowledge, to the common misconceptions held by learners such as about the size of particles, as well as about arrangement of particles in different phases. Emphasis was placed on the benefit derived from using representations at all three levels at about the same time, when explaining concepts.
The last component discussed was conceptual teaching strategies. Emphasis was placed on distinguishing this component from the general pedagogical knowledge teaching methods. The component referred to conceptual teaching strategies that are formulated specific to the topic, based on knowledge, including consideration of that knowledge generated from the other content-specific components discussed earlier. For example, when teaching about intermolecular forces, it is useful to identify a single substance as an example, like water, and to discuss how the arrangement of particles differs across the different phases by using representations at all three levels at the same time. The use of sub-microscopic representations bring the added benefit of pointing to the size of the particles across the different phases, emphasizing equal size, as well as to the intermolecular forces between the particles, and the empty spaces between the particles, all possible to do because of the nature of the representations used. The discussions on the components of representations and conceptual teaching strategies, allowed the demonstration of the natural interaction and influence of the components into one another.
After the discussions of all five components, the interaction of the components was explicitly pointed out in the specific examples used to discuss the different components at different times of the intervention. It was at this point that the Content Representation (CoRe) adapted from the original version by Loughran and his colleagues (2004) was introduced to pre-service teachers as a tool to collate and organize one's thoughts when planning to teach a topic. This was explained as a tool that allows consideration of the content from the perspective of the five content-specific components, allowing these considerations to be captured in a written manner, and subsequently pulled together to suggest effective teaching strategies. This approach facilitates the transformation of content knowledge, through prompts that requires specific content considerations to be made.
The sequence followed in the intervention is summarized in Table 1 below.
| Component (week) | Intervention | Specific concepts of CK used |
|---|---|---|
| Learners’ prior knowledge (1) | Discussions were on widely researched common misconceptions of the topic found in the literature. |
– Size of particles under different conditions (heat and pressure)
– Phase change |
| Curricular saliency (2) | Discussions were geared towards identifying the ‘Big Ideas’ and the corresponding subordinate concepts in a topic; sequencing big ideas; awareness of the foregrounding concepts, and knowing what is most important to understand in a big idea. |
Big ideas:
– All substances are made of tiny particles – Particles are in constant motion – Molecules have forces between each other Prior knowledge needed: – Knowledge of the periodic table |
| What is difficult to teach (3) | Exploration of concepts considered difficult to learn, and identifying the actual issues that make understanding difficult. This focused on the pin-pointing the actual difficulty. |
– There is an empty space between particles of matter
– There are different types of small bits of substances. |
| Knowledge of representations (4) | Introduction of the three levels of explanations in chemistry at macroscopic, symbolic and sub-microscopic levels. Emphasis was placed on the power of using all three representations side by side in explaining a phenomenon. | – Phase change |
| Conceptual teaching strategies (5) | Conceptual teaching strategy would consider the generated knowledge from the other four components. |
– Intermolecular forces
– Phase change |
| Pulling it together (6) | Introduction of Content Representations (CoRe) as a tool to capture thoughts as one thinks about content knowledge of a topic through the knowledge components of TSPCK. |
The key feature of a CoRe is the explicit nature of the prompts that help a teacher to access and think through the teaching of a topic in a coherent manner (Cooper et al., 2015). In this study, the prompts of the CoRe were modified to highlight the five components of TSPCK explicitly (Fig. 1).
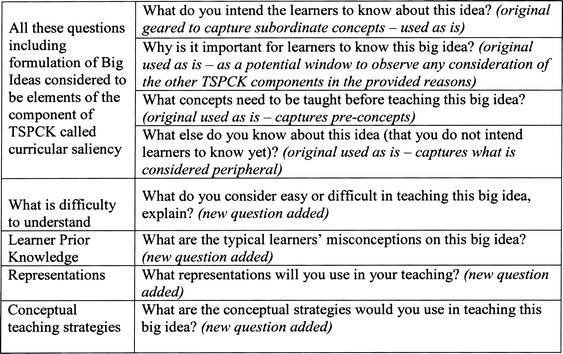 | ||
| Fig. 1 An adapted CoRe highlighting the five components of TSPCK (adapted from Loughran et al., 2004). | ||
Once familiar with the structure of the adapted CoRe, pre-service teachers were asked to complete the adapted CoRe, using three of the Big Ideas on the topic of particulate nature of matter.
Data collection
Two sets of data were collected. The first set was based on the topic of intervention mainly to establish the impact of the intervention in improving the quality of TSPCK in the topic (particulate nature of matter). A TSPCK tool in particulate nature of matter was administered before the intervention as (pre-TSPCK) and after the intervention as (post-TSPCK). The tool was developed and validated in a study by Pitjeng (2015). It is structured into five sections that correspond to the five content-specific components of TSPCK, respectively. Each section has a few sub-questions that are presented as teacher tasks, formulated to allow open-ended responses. An example of the one of the questions on the test item on Learner Prior Knowledge is provided in Fig. 2. The test item is comprised of two questions that bear learner prior knowledge, one in the form of a misconception as shown in Fig. 2 below, and another reflecting a doubt that a learner may have. Both questions are in a classroom setting, and require the pre-service teachers to formulate a response. The emphasis of the tool is on knowledge for teaching the topic, rather than on understanding content knowledge.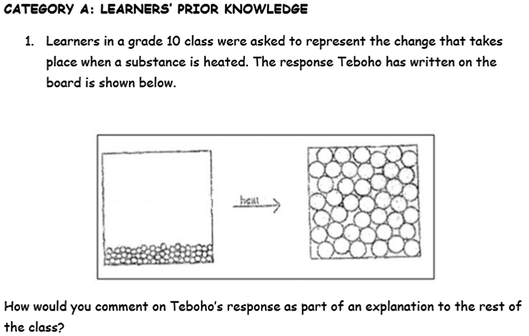 | ||
| Fig. 2 TSPCK test item for the Learner Prior Knowledge component in the particulate nature of matter topic. | ||
In addition to the pre-post TSPCK tests, the adapted CoRes completed by the pre-service teachers towards the end of the intervention, as well as class activities from the tutorials, were collected as supplementary data.
The second set of data was on the topic of transfer, chemical equilibrium. The topic of chemical equilibrium was chosen as a topic, because it is one of the major core topics in chemistry, yet perceived to be difficult and abstract (Bergquist and Heikkinen, 1990). It is part of our National Secondary School Curriculum, thus, pre-service teachers would be expected to teach it effectively on qualifying. Furthermore, it was considered that any evidence of success in PTC would illustrate the nature of the effort involved, due to the distance of the topic from that of the intervention.
At the time of intervention, pre-service teachers had not been exposed to a similar intervention on teaching chemical equilibrium, but relied on their knowledge of the topic acquired from the content course during their second year of study, and from their high school background. In order to establish transfer, a tool measuring the quality of TSPCK in chemical equilibrium was similarly administered, before and after the intervention. The tool is structured in a similar manner to that of particulate nature, with five categories corresponding to the TSPCK components. An extract of one of the questions on the test item on the component of Learner Prior Knowledge tool is shown in Fig. 3 below. As in the case of particulate nature of matter, the test item contains two questions on learner prior knowledge based in a classroom setting; one question based on a misconception, and the other investigating doubt expressed by a learner. Pre-service teachers were expected to formulate teacher responses to each question.
Additional supplementary data on the topic of transfer included a vignette that sought to determine whether pre-service teachers could identify TSPCK for the topic when acted out, and whether they were able to recommend TSPCK-informed improvements, where these were found lacking. The vignette used a similar idea to the study by Brovelli et al. (2013), where the PCK of pre-service teachers in a topic was determined through the evaluation of a recorded video lesson, where evidence of PCK was being sought. Exposing pre-service teachers to a vignette with a classroom context was done based on the understanding that a measurement of a tacit construct like PCK in a topic, would be best undertaken from both the context of planning and enactment (Aydeniz and Kirbulut, 2014). Thus, while the evaluation of a recorded lesson is not similar to the actual act of teaching the topic by the pre-service teachers themselves, it is the nearest action to the classroom context. It offered a window into examining the extent of transfer in recognizing pedagogical transformation competence or lack thereof, when demonstrated in classroom teaching.
The video-recorded lesson was a 40 minute lesson by a beginner teacher, on the dynamic nature of chemical equilibrium. The beginner teacher was chosen based on the judgement that his/her TSPCK in the topic was still developing through experience in the classroom (Halim and Meerah, 2002), where the lesson would exhibit both extant TSPCK, and opportunities for improvement. Pre-service teachers were requested to capture their evaluations by identifying TSPCK episodes in the recorded video lesson. The pre-service teachers were given an operational definition of an episode of TSPCK, after Park and Chen (2012), who describe it as a teaching segment where there is evidence of the use of two or more components of TSPCK in an interactive manner. Such a case would demonstrate a moment of pedagogical transformation of the concepts concerned.
Analysis
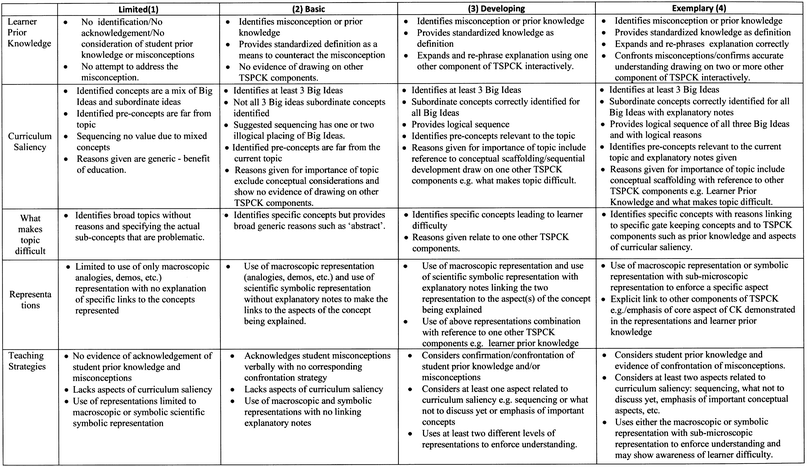
The pre-service teachers' responses in the completed set of pre- and post TSPCK tests in the topic of intervention, and that of transfer (chemical equilibrium) were first scored, using a rubric. The rubric is criterion-based, with four quality-related categories reflecting the degree to which a response engages interactively with a component of the TSPCK. These categories are: ‘limited’, assigned a score of 1; ‘basic’, assigned a score of 2; ‘developing’, assigned a score of 3; and ‘exemplary’, assigned a score of 4 (Park et al., 2011). A response demonstrating poor understanding of a component, and with no evidence of drawing on other components interactively, was given a score of 1, falling into the ‘limited’ quality category. A response that reflects standard understanding of a component based largely on textbook definitions, and drawing interactively on two components, was assigned a score of 2, falling in the ‘basic’ category, while a response that expands on concepts more than providing standard definitions and draws on three components interactively was assigned a score of 3 in the ‘developing’ category. An engagement with the topic drawing interactively from more than three of the five components would be considered ‘exemplary’, and be assigned a score of 4. A TSPCK rubric is provided in Fig. 4 below. The scores for pre-service teachers in each test were averaged for each TSPCK component, and these in turn were averaged to represent the overall score for the group. The overall score was compared to the corresponding category of the quality of TSPCK in the rubric. It is important to highlight the limitation in the sensitivity of the scale of the TSPCK rubric, as fractional average scores were rounded up or down to whole numbers in order to locate the score in the appropriate category. It is important to note that the interaction of the components is not directly related to the mathematical calculation of an average score, but that each category of TSPCK in the rubric requires interaction with other components. Thus, the calculated group average score is used as a proxy for a measure of the possible overall effect of the influence of the individual component interactions represented in the categories involved in the calculation of the average score. This is in line with the understanding that PCK (Abell, 2008), even at topic level (Aydin et al., 2015) is not the sum of the individual components, but rather of their interactions.Two raters scored the completed pre- and post-TSPCK tests independently and had an agreement calculated using the Cohen's Kappa inter-rater reliability, found to fall within the acceptable range of 0.80/0.85 and 0.79/0.80 for the pre-/post-tests in the topics of intervention and transfer, respectively. Where the scores differed, the individual raters met face-to-face to discuss, and come to a final resolution.
The scores generated from the respective tests using the TSPCK rubric were translated into person probability measures, using a Rasch statistical model (Winsteps). Rasch performs statistical analysis that describe connective likelihood, in which both item difficulty and person ability are placed along a single continuum with a similar scale (Bond and Fox, 2001). Boone and colleagues (2014) described the benefit of placing the scores of items and persons on the single continuum with a similar scale in the statement:
because persons and items have the same unit, and because logits are equal-interval units, persons can be compared to other persons, items can be compared to other items […] also items and persons can be compared” (Boone et al., 2014, p. 70).
This implies that by using Rasch measurement, one would be able to determine the item difficulty rank order in a test, which reflects the extent of difficulty experienced by the pre-service teachers in answering each item in relation to another. Furthermore, the Rasch statistical model allows anchoring of the tests for a clearer measurement of the shift from the one experience of the test items, such as before the intervention, to another experience after the intervention. This allows for a more convincing quantitative calculation for significant difference, if present between the means of the tests.
The calculation of validity in Rasch analysis is based on the idea that the recorded performances are reflections of a single, underlying construct, which is made explicit by the relationship of items to the human ability in the sample measured (Bond and Fox, 2001). Item difficulty and person ability measures that fall within the fit statistic range of −2 to +2 are considered a good match, coherent and measuring a single construct, which therefore constitutes a valid measure.
Following the normalization of the scales, and the anchoring of the pre- and post-TSPCK tests item scores, the means of the tests were compared for significance difference using the Wilcoxon paired signed rank test for non-parametric data, appropriate for small sample data as in the present study (N = 36). Qualitative data, in the form of submitted CoRes in the topic of intervention, and the written evaluations from the vignette used in the topic of transfer, were all subjected to content analyses for evidence of TSPCK episodes. The identification and analysis of the TSPCK episodes was performed independently by the same two raters mentioned above, and all disputes were resolved through discussions with evidence. The Identified TSPCK episodes were first extracted, analysed for the number of TSPCK components contained, and how they complement one another in the formulated response.
Findings
The focus in this study was on the transferability of the PTC targeted for development in the intervention. This is the competence needed to transform content knowledge in a given topic, thereby developing TSPCK. Three salient findings are suggested from the data with respect to the aim of the study, namely: to explore how the use of PTC, once taught in one chemistry topic, can be applied to a different topic.Firstly, pre-service teachers applied their learnt competence to transform content knowledge in the topic of intervention successfully, thereby improving their TSPCK in the topic. Secondly, they also successfully applied their learnt competence to significantly develop TSPCK in the topic of transfer. Thirdly, the extent of the shift in the improvement of the quality of TSPCK in both topics was found to be the same. Each of these findings is unpacked in detail below.
Improved quality of TSPCK in the topic of intervention
When comparing the overall group average score for the pre-test and that in the post-test, it was observed that pre-service teachers experienced an improvement in the quality of their TSPCK. The quality of their TSPCK shifted from the ‘basic’ to the ‘developing’ category, see Table 2 below.| Pre-service teachers | Pre/post-TSPCK scores in particulate nature of matter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Learner prior knowledge | Curricular saliency | What is difficult to understand | Representations | Conceptual teaching strategy | ||||||
| Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | |
| Average group score for pre-test: 1, corresponding to a Limited quality category of TSPCK. Average group score for post-test: 3, corresponding to a Developing quality category of TSPCK. | ||||||||||
| BFA01 | 2 | 4 | 2 | 4 | 1 | 3 | 2 | 4 | 1 | 3 |
| CMT02 | 3 | 3 | 2 | 2 | 1 | 3 | 2 | 2 | 1 | 1 |
| JMQ03 | 2 | 4 | 1 | 3 | 2 | 2 | 1 | 3 | 1 | 3 |
| KMT04 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 2 |
| KFT05 | 2 | 4 | 1 | 4 | 1 | 3 | 2 | 4 | 1 | 3 |
| KMT06 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 2 |
| KFA07 | 2 | 4 | 1 | 4 | 1 | 3 | 1 | 4 | 1 | 3 |
| LMS08 | 2 | 3 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 |
| LML09 | 2 | 3 | 2 | 3 | 1 | 2 | 1 | 3 | 2 | 3 |
| MFL10 | 1 | 4 | 2 | 4 | 1 | 3 | 1 | 4 | 1 | 3 |
| MFR11 | 2 | 3 | 2 | 4 | 1 | 3 | 2 | 2 | 1 | 3 |
| MFV12 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 2 | 1 | 2 |
| MMM13 | 1 | 4 | 1 | 3 | 2 | 3 | 1 | 3 | 1 | 3 |
| MME14 | 2 | 4 | 1 | 3 | 1 | 2 | 1 | 3 | 1 | 3 |
| MMI15 | 2 | 4 | 2 | 3 | 2 | 3 | 1 | 3 | 1 | 2 |
| MAM16 | 2 | 4 | 1 | 4 | 1 | 3 | 1 | 4 | 2 | 3 |
| MMA17 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 3 | 1 | 2 |
| MFS18 | 2 | 3 | 1 | 4 | 1 | 3 | 1 | 2 | 1 | 3 |
| MMT19 | 3 | 3 | 1 | 3 | 1 | 2 | 2 | 3 | 1 | 2 |
| MMA20 | 2 | 4 | 1 | 3 | 1 | 2 | 1 | 3 | 1 | 3 |
| MMP21 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 |
| MMB22 | 1 | 3 | 1 | 3 | 1 | 2 | 2 | 3 | 1 | 2 |
| MFN23 | 1 | 4 | 1 | 4 | 1 | 3 | 1 | 4 | 1 | 3 |
| MMT24 | 2 | 4 | 1 | 4 | 1 | 4 | 2 | 4 | 1 | 3 |
| MMT25 | 2 | 4 | 2 | 3 | 2 | 2 | 2 | 3 | 1 | 2 |
| MFP26 | 2 | 4 | 2 | 4 | 2 | 4 | 1 | 4 | 1 | 3 |
| MFM27 | 2 | 3 | 2 | 4 | 2 | 3 | 2 | 2 | 1 | 3 |
| NMS28 | 1 | 4 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 3 |
| NMT29 | 2 | 4 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 2 |
| NMS30 | 2 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 1 | 3 |
| NML31 | 1 | 3 | 1 | 4 | 1 | 3 | 2 | 2 | 1 | 3 |
| PFM32 | 1 | 3 | 2 | 4 | 1 | 3 | 2 | 2 | 1 | 3 |
| RMJ33 | 1 | 3 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 2 |
| SMT34 | 1 | 3 | 1 | 3 | 2 | 2 | 1 | 2 | 2 | 2 |
| SMS35 | 2 | 3 | 2 | 2 | 2 | 3 | 1 | 3 | 1 | 2 |
| SML36 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 3 | 1 | 2 |
| Average/component | 2 | 3 | 1 | 3 | 1 | 3 | 2 | 3 | 1 | 2 |
According to the criteria in the rubric, in the ‘developing’ category, pre-service teachers showed evidence of expanded explanations of the main concepts, rather than providing mere textbook definitions. They identified correctly three ‘Big Ideas’ in the topic that are not mixed with ‘subordinate ideas’. They further identified specific concepts that may cause potential difficulty for learners, rather than broad statements, used representations at multiple levels of explanation rather than a single representation. Three of the components above were present in their explanations, working together to provide depth and support meaning.
An example of such an improvement is shown by the comparison of the pre- and post-responses of pre-service teacher Jabu (KFT05 in Table 2) to the test item on Learner Prior Knowledge. The test item from the TSPCK tool required pre-service teachers to provide a response to a learner's misconception shown earlier in Fig. 2. Jabu responded to the test item in the pre-test as follows:
When substances are heated their molecules do not expand in size. The size of the molecules stays the same.
While the above response is technically accurate, and serves as evidence that Jabu had identified the misconception of the learner, the response is at the level of a standard definition that lacks reasoning, as well as an effort to correct the identified misconception. It also lacks strong evidence of interaction with other components TSPCK. The response given in the post-test, however, is evidently confronting the misconception identified, by drawing on multiple TSPCK complementary components and affording depth of response. This is shown in the extract below:
Particles gain kinetic energy when heated, which result [sic] in weakening or breaking of forces of attraction that keep the particles together. These forces are not visible but they exist between particles. As a result, molecules move further apart as heat is administered; and eventually solids may turn to liquids and then to gases. The change of phase does not change the size of the particles. In liquid form, the particles take up the shape of the container without necessarily filling it up (changing the volume they occupy), but in gaseous state, the particles fill up the whole container with lots of [sic] empty spaces between them and the size of molecules would be the same as they were in solid or liquid phase. The material expands as a result of particles moving further apart, and not that the articles expand [sic].
At face value, Jabu's response may be easily attributed to a display of improved content knowledge in the topic. While that is welcomed, there is evidence in his response of increased awareness of what concepts are difficult for the learners to understand. These are pinned down and sequenced in the explanation in manner that builds up to confronting the identified misconception. We are further able to see a repeated emphasis of the most important concepts needed to explain change in phase, which is followed by a clear statement disputing the misconception about a change in the size of particles across all phases, including the phase change from liquid to gas. Furthermore, Jabu's response contains an explanation of several important facts describing the effect of particles gaining energy, as seen in the sentence regarding the way in which particles take the shape of their container, etc. These facts are most important for establishing an understanding that corrects the misconception as they provide sub-microscopic reasons for an observed macroscopic phenomenon. Knowing what concepts are core and most important at an instance of teaching is an element of the component of curricular saliency. In the above extract, Jabu's response shows the use of TSPCK components of ‘what is difficult to understand’ and that of ‘curricular saliency’, working together to provide an expanded explanation, aiming at confronting an identified misconception, which is a good thing as it shows knowledge of the component of learner's prior knowledge.
Another example indicating improved quality of TSPCK in the topic of intervention, is seen in the way the component of ‘representation’ is used. The test item in the tool required pre-service teachers to choose a representation that they would use in explaining kinetic theory in relation to change of phase of matter. Given below is a comparison of pre- and post-test responses from Musa (NMT29 on Table 2):
(Pre-test): I will use the representations that show visual demonstration of water as ice cubes, liquid water in a glass, and a boiling kettle. This will help learners understand that it is the same substance in all three phases.
(Post-test): I will show a demonstration of a colourful solid substance in a closed system that I can heat to different phases in front of the learners. Each phase will be accompanied by sub-microscopic drawings of the particles. The reason is that learners will see the change of phases [sic], but at the same time, know what is happening sub-microscopically; that the forces are weakened.
Musa's response in the post-test, similarly reflects depth of understanding regarding the value of the use of sub-microscopic representations to match macroscopic observations, thus promoting learner understanding (Davidowitz and Chittleborough, 2009). The response further expands on most important concepts to be understood, the weakening of forces between particles allowing the particles to separate, and a filling up of the volume of the container, while retaining their colour. The reference to colour further shows awareness of a common misconception in relation to the specific concept of change in phases that learners may have. This misconception is planned for, ahead of its emergence. This response displays evidence of the interaction of TSPCK components in bringing depth to an explanation.
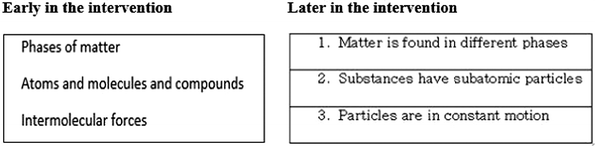
From the complementary data, which were class activities and the submitted CoRes, there was evident improvement in the manner pre-service teachers formulated Big Ideas. For example efforts to formulate Big Ideas in a class activity from early on in the intervention indicated good content knowledge of the particulate nature of matter, but weak pedagogical statements for Big Ideas (Fig. 5). They were constructed as phrases, rather than as statements, that reflect meaning of the concepts crucial to understanding the topic (Loughran et al., 2006). While there is no single correct way of formulating and reflecting ‘big ideas’, the evidence of understanding the meaning behind most important concepts that are structural pillars of a topic ought to be evident. Such evidence was lacking in earlier effort, see Fig. 5.
An improvement was, however, noted in the CoRes submitted towards the end of the intervention. The newly formulated Big Idea statements, as shown in Fig. 5 below, are conceptually and pedagogically sound, as they reflect deeper understanding of content reproduced with consideration for understanding by learners. According to Loughran et al. (2006), the identification of Big Ideas in a topic is considered an element of professional practice associated with the broader concept of PCK, and distinguishes it from content knowledge.
It was further noticed that the teaching strategies suggested in the CoRes generally reflected consideration of potential learning difficulties, which were original ideas of the pre-service teachers, such as demonstrated in the extract by Sipho (MFP26):
Learners learn that what is observed macroscopically has its foundation in the microscopic. Students therefore believe that if sulphur is yellow it means that the atoms of sulphur are yellow. […] I will explain for example that atoms do not have colour on themselves, but the colour comes from their behaviour when excited by light (even natural sunshine light [sic]) or heat to higher energy states and emit a particular light characteristic of the amount of energy their loosing when they fall back to their lower energy levels. I will draw on the board a diagram that shows energy levels and link the calculation of energy gained or lost to the physics equation on energy and wavelength.
What is special about the extract above is the display of evidence of an emerging contemplation, in ways that learners may be linking concepts to make meaning. The issue of colour was not discussed explicitly in the intervention, however, it is brought into the discussion as a potential difficulty to learning, by virtue of a prior understanding about the importance of links between the sub-microscopic and macroscopic explanations, possibly shown to learners in a previous instruction. Thus, the extract displays an example of an emerging, original ability to anticipate potential learner difficulty.
The extracts shown above from the TSPCK tools and those from the submitted CoRes showed complementary evidence of a shift in the quality of TSPCK experienced by the pre-service teachers as a result of the intervention.
In order to determine the quantitative extent of the observed qualitative improvement in the quality of TSPCK, the scores of the pre- and post-TSPCK tests generated from the TSPCK rubric, were converted into probability measures that were normalized using the Rasch statistical model (Boone and Rogan, 2005). The generated Rasch score measures for both persons and items in each test fell within the conventional fit statistical range of (−2; +2) range for each test, indicating that a single construct was being measured as desired, and thus that both the pre- and the post-TSPCK tests were valid (see Table 3). For the pre-test, the model generated a calculated reliability index of 0.5 and 0.92 for persons and items, respectively. At 0.5, the reliability for persons was slightly lower than desired as it was just below the border of values considered acceptable (>0.5). For the post-test, the reliability values for persons and items were found to be within the acceptable range, with values of 0.81 and 0.95, respectively.
| Pre-test TSPCK | Post-test TSPCK | |
|---|---|---|
| Item measure mean set to zero & units per logit (log-odds unit) = 1 for the entire test (UIMEAN = 0 and USCALE = 1). | ||
| Sample person measure mean | −2.84 | 2.08 |
| Person reliability | 0.50 | 0.81 |
| Item reliability | 0.92 | 0.95 |
| Value of ρ | 0.000001, which is lower than α < 0.05 thus the observed difference is very significant. | |
| Item measure difficulty order in the post TSPCK test | (Item measures) | 2.13 | 1.37 | 0.08 | −1.24 | −2.33 |
| (TSPCK components) | CTS | > WiD | > ReP | > CS | > LPK |
Having established reliability and validity in each test, the tests were anchored to allow calculation of difference between the tests (Wright, 2003). The means of person measures generated from the anchored tests were compared, using a Wilcoxon paired signed test. The difference, which was an improvement in the quality of TSPCK, was found to be significant (ρ = 0.001 ≤ 0.05). It is further observed that the pre-service teachers experienced the engagement of the topic from the perspective of the TSPCK components to varying degrees of difficulty. The TSPCK component of conceptual teaching strategy was found to be the most difficult.
To summarise, the data collected towards the completion, and at the end of the intervention, showed that pre-service teachers experienced an improvement in the quality of TSPCK in particulate nature of matter.
Improved quality of TSPCK in the topic of transfer
Chemical equilibrium, the topic of transfer was not discussed in the intervention, but was used to examine the possible transfer of the PTC learnt in the intervention with particulate nature of matter. Table 4 compares the scores between the pre- and the post-TSPCK scores in the topic of transfer. The TSPCK tools in the topic of transfer were administered at about the same time as those for the topic of the intervention.| Pre-service teachers | Pre/post-TSPCK scores in chemical equilibrium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Learner prior knowledge | Curricular saliency | What is difficult to learn | Representations | Conceptual teaching strategy | ||||||
| Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | |
| Average group score for pre-test: 2, corresponding to a Basic category of TSPCK category. Average group score for post-test: 3, corresponding to a Developing quality category of TSPCK. | ||||||||||
| BFA01 | 2 | 3 | 2 | 4 | 2 | 3 | 1 | 3 | 2 | 2 |
| CMT02 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| JMQ03 | 2 | 3 | 2 | 3 | 1 | 1 | 2 | 3 | 1 | 2 |
| KMT04 | 2 | 3 | 2 | 4 | 2 | 2 | 1 | 3 | 1 | 3 |
| KFT05 | 2 | 3 | 2 | 3 | 1 | 1 | 2 | 3 | 2 | 2 |
| KMT06 | 3 | 4 | 2 | 4 | 3 | 3 | 3 | 4 | 2 | 3 |
| KFA07 | 3 | 4 | 3 | 4 | 3 | 3 | 2 | 4 | 2 | 3 |
| LMS08 | 2 | 3 | 2 | 4 | 2 | 3 | 1 | 3 | 1 | 2 |
| LML09 | 3 | 4 | 3 | 4 | 2 | 4 | 2 | 4 | 2 | 3 |
| MFL10 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 3 | 1 | 2 |
| MFR11 | 1 | 3 | 3 | 4 | 2 | 3 | 2 | 2 | 2 | 3 |
| MFV12 | 2 | 3 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 1 |
| MMM13 | 3 | 3 | 2 | 4 | 2 | 2 | 2 | 3 | 1 | 3 |
| MME14 | 2 | 4 | 3 | 4 | 3 | 4 | 2 | 4 | 3 | 3 |
| MMI15 | 2 | 4 | 2 | 3 | 2 | 1 | 2 | 3 | 2 | 2 |
| MAM16 | 2 | 3 | 2 | 4 | 2 | 2 | 3 | 3 | 2 | 3 |
| MMA17 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 2 |
| MFS18 | 1 | 3 | 2 | 4 | 2 | 3 | 1 | 2 | 1 | 3 |
| MMT19 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 |
| MMA20 | 2 | 3 | 2 | 4 | 2 | 2 | 1 | 3 | 2 | 3 |
| MMP21 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 3 | 1 | 2 |
| MMB22 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 3 | 1 | 2 |
| MFN23 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 |
| MMT24 | 2 | 3 | 2 | 4 | 3 | 3 | 2 | 3 | 2 | 2 |
| MMT25 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 |
| MFP26 | 3 | 4 | 2 | 4 | 2 | 3 | 2 | 3 | 2 | 3 |
| MFM27 | 2 | 3 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 3 |
| NMS28 | 2 | 4 | 2 | 3 | 1 | 1 | 2 | 3 | 2 | 2 |
| NMT29 | 3 | 4 | 2 | 3 | 2 | 1 | 2 | 3 | 1 | 2 |
| NMS30 | 1 | 3 | 2 | 3 | 2 | 3 | 2 | 2 | 1 | 3 |
| NML31 | 2 | 3 | 2 | 4 | 2 | 3 | 2 | 2 | 1 | 3 |
| PFM32 | 3 | 3 | 2 | 4 | 2 | 3 | 1 | 2 | 1 | 3 |
| RMJ33 | 2 | 3 | 2 | 3 | 2 | 4 | 1 | 2 | 1 | 2 |
| SMT34 | 1 | 2 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 2 |
| SMS35 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 3 | 1 | 2 |
| SML36 | 2 | 3 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 1 |
| Average/component | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 3 | 1 | 2 |
Similar to the topic of intervention, the group average score for TSPCK had shifted from a score that denotes a Basic quality of TSPCK, to a developing score. both the pre- and post-TSPCK tests scores were found in the Rasch model to have a good statistical fit, thus being valid. The reliability index in the pre-test was calculated at 0.75 and 0.88 for the persons and items, respectively. Similar acceptable reliability indices for persons and items were calculated for the post-test at 0.85 and 0.95, respectively (see Table 5).
| Pre-test TSPCK | Post-test TSPCK | |
|---|---|---|
| Item measure mean set to zero & units per logit (log-odds unit) = 1 for the entire test (UIMEAN = 0 and USCALE = 1). | ||
| Sample person measure mean | −1.20 | 0.76 |
| Person reliability | 0.75 | 0.85 |
| Item reliability | 0.88 | 0.95 |
| Value of ρ | 0.0014, which shows α < 0.05 thus the observed difference is very significant. | |
| Item measure difficulty order in the post TSPCK test | (Item measures) | 1.81 | 1.41 | −0.13 | −1.10 | −1.99 |
| (TSPCK components) | WiD | > CTS | > ReP | > CS | > LPK |
An example of a typical excerpt in the pre-TSPCK test on the test item of Learners' Prior Knowledge is shown below:
(Test item): In a classroom setting, how will you respond to a learner who seems to be in doubt and asks you: “Do both the forward and the backward reaction actually take place if a closed system is at equilibrium?”
(Pre-test Jabu's response):
The double arrow in the reaction, A + B ⇌ C + D explains that the rate which A and B react to form C, and D is the same as that of C and D, reacting back to form A and B.
The response is a typical textbook definition of chemical equilibrium, with no expansion on what causes the reaction to be understood to be at chemical equilibrium.
However, in the newly acquired category of a ‘developing quality of TSPCK’ in the post-test, pre-service teachers provided expanded explanations to test items. They further demonstrated the ability to provide responses that draw interactively on at least three of the TSPCK components, as shown in Jabu's response in the post-TSPCK test, addressing the same test item as in the pre-TSPCK test above:
For us to say that a reaction is at equilibrium the rate of the forward reaction must be equal to the rate of the backward reaction. But again, a closed system allows us to have a situation where the products initially formed react with each other to form reactants.
While the response repeats the textbook definition of chemical equilibrium, it is however done in a manner that consciously places emphasis on the most important conditions needed for attaining chemical equilibrium. This is borne out of the use of phrases such as ‘must be’ and ‘allows us’. Knowing the most important concepts, where emphasis ought to be placed by a teacher at a particular time, is an element of curricular saliency (Geddis and Wood, 1997). The response further mentions a ‘closed system’ as an enabling factor for a reverse reaction to happen. Reversible reactions are reported to be difficult for learners to understand, partly because chemical reactions are often introduced as complete, balanced one-way reactions (Tyson et al., 1999). The reference to the role of a closed system in contributing to the attainment of the state of chemical equilibrium is also indicative of the process of discerning what is most important to be understood. The understanding of the content of chemical equilibrium from the perspective of the knowledge of curricular saliency, and that of what is difficult, all worked complementary in confirming prior knowledge displayed by the learner.
Another example can be seen in the response of Sipho for the item of conceptual teaching strategies in the pre/post-test.
Test item: Following below is a student's written response in a class test, designed to assess prior knowledge of students about Le Châtelier's Principle.
Question to learners: What is the effect of adding more water to reaction given below at equilibrium?
| CH3CO2H(aq) + H2O (l) ⇌ CH3CO2− (aq) + H3O+(aq) |
A learner responded : ‘More CH3CO2−(aq) and H3O+(aq) will be formed, to counter act the effect of adding more water to the reactants. This will happen until a new equilibrium is reached.’
Following the student's response, how will you teach a lesson on predicting the effect of factors disturbing the equilibrium?
(Sipho's response in the Pre-test): From here, I can see that the learner correctly understands what is going to happen after adding water. […]But now I need to explain how to tackle the question in a sequential form, starting from the effect: what is the effect, what it does to the reaction and which substance is favoured and then explain the effect in terms of the Le Châtelier's principle?
It is observable from Sipho's extract in the pre-test that he also shares the same misconception as the learner, and his teaching strategy will focus on sequencing the explanation to be provided.
The response given in the post-test shown in the extract below indicated an improvement in many different aspects of TSPCK.
(Post-test): Not using Le Châtelier's principle as the first way to predict the response of a reaction at equilibrium to disturbances. For example, according to the principle, the learner response would not seem particularly contradictory, because adding more water will cause, according to the Le Châtelier's principle, the equilibrium to act in such a way to counteract the addition of water and form more products. However, if the students are introduced to the usage of the Kcexpression (equation on the board) to predict the effect, then it will help them to distinguish whether the addition will have any effect on the equilibrium.
Firstly, the response indicates an improvement in correctly identifying the learner's misconception and, secondly, in suggesting a conceptual teaching strategy that acknowledges the core value and priority of a conceptual law over a conceptual principle. The extract further made reference to a representation to support the intended meaning. In contrast to the suggestion made in the pre-test, the extract demonstrated evidence of drawing interactively on different components of TSPCK to attain depth in the explanations.
Further qualitative evidence of successful transformation of content knowledge in the topic of transfer was seen in the complementary data drawn from the vignette used. As mentioned in the methodology section, a vignette to evaluate whether the pre-service teachers could recognise pedagogical transformation episodes (or lack of) when displayed in practice was preferred, as it offered opportunities for evaluating quality of TSPCK from a different pedagogical context, i.e. rather than from planning. The responses indicated that the majority of the pre-service teachers were able to recognise and make suggestions for improvements in a manner that showed evidence of drawing from several components of TSPCK interactively.
Examples of such responses are extracts from Musa and Mary, who were expressing their evaluations of the observed video lesson, where a beginner teacher was introducing the concept of dynamic chemical equilibrium. These are shown below.
(Musa): Emphasis should be put on why the equilibrium is said to be ‘dynamic’. The teacher did not explain thus far. Also, the teacher failed to put emphasis on the fact that chemical equilibrium may only be reached in a closed system.
(Mary): The teacher managed to explain to the learners that the product and reactants will not be finished in the reaction but did not mentioned that the reaction is reversible, and even when he wrote the chemical equation, he used the incorrect arrow that does not represent a reversible reaction.
The extracts above refer to two gate-keeping concepts that commonly cause difficulty in understanding, particularly that the reactions are reversible and a closed system is an enabling factor. These extracts indicate that the pre-service teachers were looking for evidence of the awareness by the teacher of most important conditions for chemical equilibrium to occur. Furthermore, the recognition of a conceptual error in the representation used, reflects an understanding that representations are required to reflect explicitly the issue to be understood (Klafki, 1958). Both these extracts reflect evidence of the knowledge of curricular saliency, and how its interaction with the component of what is difficult to understand, and that of representations (in Mary's extract), could have given rise to a much more clear explanation on the part of the teacher.
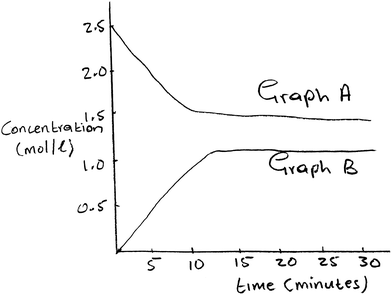
Another unique observation that emerged from the written evaluation of the video-recorded lesson is evidence of the pre-service teachers’ effort to identify the possible source of misconception in a learner's statement. This is demonstrated in a case where a learner in the recorded video lesson asked a question based on the graphical representation of the concentration vs. time graphs shown in Fig. 6.
(Learner): Do the graphs of concentration versus time of reactants and products repel each other at chemical equilibrium?
(Teacher's response): That is Physics, not Chemistry.
(Musa's evaluation of the incident): The teacher did not address the difficulty to understand shown by the learner, which I consider critical. He just dismissed the learner and said that “this [sic] is Physics, not Chemistry”. The learner likened the parallel parts of the concentration versus time graphs to a representation that would be drawn, to show the force of repulsion felt by two likely charged bodies. I think the reason for the learner to make such a suggestion is because the concentrations vs. time graphs run parallel to each other at chemical equilibrium.
In the extract above, it is clear that Musa could recognise the learning difficulty embedded in the learners' suggested analogy. However, he was disappointed by the poor response given by the teacher as it did not acknowledge the misconception. Musa's response in the extract above is focused on explaining what he perceived as the source of the learners' difficulty. In his response, one notices articulation of the exact aspects of two different concepts that could have contributed to the learner's difficulty: a representation of two parallel, non-intersecting lines that could be related to a force of repulsion experienced by like-charged bodies, compared to a graphical representation of the state of chemical equilibrium. Musa's recognition of the possible source of the learner's confusion demonstrates an ability to see through content from the perspective of the learner's prior knowledge, the connections they possibly made across similar aspects of different concepts, and how these in turn posed a learning difficulty.
The extent of the qualitative shift observed between the pre- and post-TSPCK test was similarly established through the use of the Rasch statistical model. The comparison is shown in Table 5.
The comparison shows the difference in the quality of TSPCK in the topic of transfer before and after the intervention, to be significantly different at 0.05 level. A similar observation – as with the topic of the intervention – is made, that the engagement of the new topic from the perspective of the TSPCK components was found to differ from component to component. It is also observed that the rank order differs between the two different topics (Tables 3 and 5).
To summarize, the analysis above suggested a visible shift in the overall quality of TSPCK in the topic of the intervention, as well as in that of transfer. In both cases, the shift was from a Basic to Developing category of TSPCK, suggesting an equal measure of improvement in each topic, despite their difference and perceptions of their difficulty.
Discussion and conclusion
The study began by acknowledging the common understanding that PCK observed in one topic is not transferable to another topic. The question asked was, if this is the case, what then could be transferred? The question was investigated by exploring how the use of PTC, once learnt in one Chemistry topic, can be applied to a different topic for the same purpose of developing TSPCK. The first major finding lies in the observed significant improvement in the quality of TSPCK in particulate nature of matter, the topic of intervention. Pre-service teachers were exposed to discussions in an intervention that explicitly interrogated the concepts of the topic of intervention from the perspective of the TSPCK components, and in using them interactively, to bring in-depth responses. This was the development of the PTC. Evidence of the successful application of the PTC in the topic of intervention is seen through the quality of the generated teacher responses shown in both the post-TSPCK tool and in the complimentary CoRes. The generated responses, as illustrated in the extract of student Jabu to the test item in Fig. 2, are characterized by the emphasis of what is core; the articulation of aspects of concepts that are potentially difficult to understand, all used harmonically to confront a learner misconception. Such responses demonstrated transformed content knowledge, suitable for learner understanding. The calculated rank order of difficulty of the test items in Table 3 signals a process of engagement with the components of TSPCK experienced at different extents of difficulty, rather than a simple regurgitation of the exact discussions from the intervention, even though these discussions were explicit. It is therefore reasonable to link the observed improvement in the quality of TSPCK to the extent of success in applying PTC in the topic of the intervention.The second major finding is that pre-service teachers experienced a significant improvement in the quality of TSPCK in the second topic, chemical equilibrium as the topic of transfer. This is a topic to which they had not been exposed in the course, but had to rely on courses largely targeting the achievement of content knowledge. This finding is therefore interesting in several aspects:
Firstly, chemical equilibrium is generally perceived to be a far more difficult and an abstract topic of the two (Hackling and Garnett, 1985). The significant improvement observed indicates an understanding of this topic from the perspective of teaching it, formulating responses that draw upon multiple components of TSPCK to pedagogically transform the content, thus demonstrating an improved quality of TSPCK the topic. This improvement is linked directly to the intervention as the pre-test showed a weak score in the pre-test on this regard. It could be thus stated that while the topic was not discussed explicitly in the intervention, pre-service teachers understood that they needed to reason through the content of the topic of transfer from the perspective of the PTC learnt from the intervention. Evidence of this understanding is explicitly demonstrated in the open-ended responses to the evaluation of the video recorded lesson on chemical equilibrium. The responses, such as those of Musa and Mary, outline the importance of emphasis of certain aspects of the concept of chemical equilibrium, which are regarded as difficult to understand, yet key for the conditions in which chemical equilibrium may be attained. Pre-service teachers referred to the use of components of TSPCK, and used these to attain depth of understanding by learners. The evidence demonstrated in these responses is more convincing, as it was openly and freely generated, without the prompts typically provided by test items in a tool or the CoRe.
The second interesting point is the observation that the improvement of TSPCK in the respective topics happened to the same extent, from a ‘basic’ to a ‘developing’ TSPCK level, both of which were found to be statistically significant. This observation increases the need to illustrate what entity was actually transferred. When comparing the order of difficulty in which pre-service teachers engaged with the components of TSPCK in the post-TSPCK tests in each topic (Tables 3 and 5), differences in the order of item difficulty are observed. This difference indicates that pre-service teachers experienced the engagement of the TSPCK components with the content of the respective topics differently. It further signals the existence of a rigorous process of engagement. For example, it was more difficult to engage the topic of intervention, particulate nature of matter from the perspective of the TSPCK component of conceptual teaching strategies than it was to engage the topic of transfer chemical equilibrium. In the topic of transfer it is the component of ‘What is difficult to understand” that was found to be the most difficult. Thus, the observed improvement in the quality of TSPCK in each topic was unique to the topic, although measured to be of the same extent.
The last point refers to the observation made in both topics, where pre-service teachers indicated an emerging ability to see the content of a topic through the eyes of the learners, that is, beyond the discussion in the intervention. In the topic of intervention, Sipho expresses a potential learner difficulty likely to result from the understanding of the importance of linking macroscopic observations to sub-microscopic features, by referring to the colour of elemental sulphur. This particular learner's difficulty was not discussed in the intervention, it was an original insight from Sipho himself. A similar observation is seen in Musa' extract sharing on a possible source of a learner misconception identified in a learner's question about the parallel arms of the concentration versus time graphs, representing the state of chemical equilibrium. Both these extracts signalled developing perspectives in pre-service teachers, where they can be observed to have begun to understand learners' knowledge and see through content with the learners' eyes, which is an important teacher trait advantageous to develop while still in training.
The conclusion made in this study is that, it is not enough to value PCK as an important form of teacher knowledge. But like in Mathematics education, with the theoretical concept of specialised knowledge needed for teaching the subject, there is more value in articulating pedagogical competencies that explicitly demonstrates the topic-specific nature of PCK, and how in turn these could be useful from a perspective of teaching the construct to pre-service teachers. It has been shown in this study that the explicit demonstrations in the intervention of the breaking down of the TSPCK construct into its individual components has been useful. In particular, the opportunity afforded to pre-service teachers to first learn the topic from the perspective of each component, followed by learning to pull together all the considerations generated from all the components into the process of planning for teaching. The approach has been demonstrated to be beneficial in developing pre-service teachers' TSPCK in the topic of the intervention. It offered them understanding of the professional topic specific knowledge they need, and also how to use such knowledge when planning to teach the topic. The experience from the intervention and the likely familiarity of the TSPCK components as prompts, facilitated at the very least, the needed understanding and the endurance to engage with the new topic from the same approach as in the intervention. In doing so, the pre-service teachers demonstrated the transfer of the competence to transform content of a topic. It is however, important to note that a certain amount of content knowledge in the topic of transfer was assumed and is an important factor to consider. Furthermore, the observed difference in the order of difficulty experienced by the pre-service teachers across the individual TSPCK components in both topics, reveals the pedagogical power derived from the collective and interactive use of the components of TSPCK even when the individual components have not been mastered to the same degree in a topic.
Implications
This study makes several contributions to the existing knowledge on topic specificity of PCK, and has several implications for science teacher education. First of all, the study practically models the call by Aydin and colleagues (2015) for the explicit cultivation, in a pre-service programme, of the development of both the components of PCK and the coherent interactions among them. In this case, the model is demonstrated with components of the TSPCK construct. The findings suggest that a high value is derived where TSPCK is developed from the efforts of using the components of the construct interactively even though some maybe have been experienced by pre-service teachers to be more difficult than others. Thus, pointing science teacher development programmes to the benefits derived from the interaction of the collective components that reveal the topic specific nature of PCK. Secondly, the study contributes, through the transfer of PTC, to the conundrum of the desire to develop TSPCK in several topics in a discipline, given the epistemological constraint of the construct being non-transferable. It should be noted however, that the observed transfer is largely within a context of planning, rather than observations of performances in real classroom practices, also that the findings are based on a case study, thus may not be generalized to different teachers, contexts and topics.However, despite these limitations, the findings supports the view that science teacher professional development should focus more on developing PCK in specific topics within a discipline. To do so by using the components of PCK that reveal its topic specific nature and develop PTC as a possible competence that maybe used continuously to develop TSPCK across topics in a discipline. Furthermore, the focus on a single topic in the intervention in order to place emphasis on the development of PTC makes the methodology courses more suitable a context and the time spent worthwhile. As a future study, research that investigates the transfer of the same PTC within and across topics of the different disciplines of Science, such as Physics and Biology, would expand the understanding of the nature of the transfer established in this paper.
Acknowledgements
Financial support from the National Research Foundation (NRF) of South Africa is herewith gratefully acknowledged.References
- Abell S. A., (2008), Twenty years later: does pedagogical content knowledge remain a useful idea? Int. J. Sci. Educ., 30, 1405–1416.
- Ayas A., Özmen H. and Çalik M., (2010), Students’ conceptions of the particulate nature of matter at secondary and tertiary level, Int. J. Sci. Math. Educ., 8, 165–184.
- Aydeniz M. and Kirbulut Z. D., (2014), Exploring challenges of assessing pre-service science teachers’ pedagogical content knowledge (PCK), Asia-Pacific Journal of Teacher Education, 42, 147–166.
- Aydin S. and Boz Y., (2013), The nature of integration among PCK components: a case study of two experienced chemistry teachers, Chem. Educ. Res. Pract., 14, 615–624.
- Aydin S., Friedrichsen P., Bozc Y. and Hanuscin D., (2014), Examination of the topic-specific nature of pedagogical content knowledge in teaching electrochemical cells and nuclear reactions, Chem. Educ. Res. Pract., 15, 658–674.
- Aydin S., Demirdogen B., Atkin F. N., Uzuntiryaki-Kondakci E. and Tarkin A., (2015), The nature and development of interaction among components of pedagogical content knowledge in practicum, Teach. Teach. Educ., 46, 37–50.
- Bergquist W. and Heikkinen H., (1990), Student ideas regarding chemical equilibrium, J. Chem. Educ., 67, 1000–1003.
- Bond T. G. and Fox C. M., (2001), Applying the Rasch Model: Fundamental Measurement in the Human Sciences, Mahwah, New Jersey: Lawrence Erlbaum Associates.
- Boone W. and Rogan J., (2005), Rigour in quantitative analysis: The promise of Rasch analysis techniques, African Journal of Research in SMT Education, 9, 25–38.
- Boone W. J., Staver J. R. and Yale M. S., (2014), Rasch analysis in the human sciences, Dordrecht, Netherlands: Springer.
- Brovelli D., Bölsterli K., Rehm M. and Wilhelm M., (2013), Erfassen professioneller Kompetenzen für den naturwissenschaftlichen Unterricht: Ein Vignettentest mit authentisch komplexen Unterrichtssituationen und offenem Antwortformat [Assessing Professional Competencies for Science Teaching: A Vignette Test using Authentically Complex Teaching Contexts and an Open-Ended Answer Format], Unterrichtswissenschaft, 41, 306–329.
- Chevallard Y., (1988), On didactic transposition theory: Some introductory notes.
- Cooper R., Loughran J. and Berry A., (2015), in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining pedagogical content knowledge in science education, New York: Routledge, pp. 60–74.
- Davidowitz B. and Chittleborough G., (2009), in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, Dordrecht: Springer, pp. 169–191.
- Department of Basic Education, (2011), Curriculum and assessment policy statement grades 10–12: Physical Science, Government Document, Retrieved from http://www.education.gov.za/LinkClick.aspx?fileticket=uVcOcx728Y8%3D&tabid=466.
- Fenstermacher G., (1986), in Wittrock M. C. (ed.), Handbook of research on teaching, New York: Macmillan, 3rd edn, pp. 37–49.
- Geddis A. N. and Wood E., (1997), Transforming Subject Matter and Managing Dilemmas: A Case Study in Teacher Education, Teach. Teach. Educ., 13, 611–626.
- Hackling M. W. and Garnett P. J., (1985), Misconceptions of chemical equilibrium, Eur. J. Sci. Educ., 7, 205–214.
- Halim L. and Meerah S. M., (2002), Science trainee teachers’ pedagogical content knowledge and its influence on physics teaching, Research in Science and Technological Education, 20, 215–225.
- Klafki W., (1958), Didaktik analysis as the core of preparation of instruction, London: Lawrence Erlbaum Associates.
- Loughran J. J., Berry A. and Mulhall P., (2004), In Search of Pedagogical Content Knowledge in Science: Developing Ways of Articulating and Documenting Professional Practice, J. Res. Sci. Teach., 41, 370–391.
- Loughran J. J., Berry A. and Mulhall P., (2006), Understanding and developing science teachers Pedagogical content knowledge, Rotterdam: Sense Publishers.
- Magnusson S., Krajcik J. and Borko H., (1999), in Newsome J. G. and Lederman N. G. (ed.), Examining Pedagogical Content Knowledge: the Construct and its Implications for Science Education, Dordrecht: Kluwer Academic, pp. 95–132.
- Mavhunga E., (2014), in Askew M., Venkat M., Rollnick M. and Loughran J. (ed.), Exploring Content Knowledge for Teaching Science and Mathematics: Windows into teacher thinking, Abington, UK: Routledge, pp. 29–49.
- Mavhunga E. and Rollnick M., (2013), Improving PCK of chemical equilibrium in pre-service teachers, African Journal of Research in Mathematics, Science and Technology Education, 17, 113–125.
- Merriam S. B., (2002), Qualitative research in practice: Examples for discussion and analysis, San Francisco: Jossey-Bass.
- Nezvalová D., (2011), Researching Science Teacher Pedagogical Content Knowledge, Problems of Education in the 21st Century, 35, 104–118.
- Oh P. S. and Oh S. J., (2011), What teachers of science need to know about models: an overview, Int. J. Sci. Educ., 33, 1109–1130.
- Park S. and Chen Y., (2012), Mapping out the integration of the components of pedagogical content knowledge (PCK): examples from high school biology classrooms, J. Res. Sci. Teach., 49, 922–941.
- Park S. and Oliver J. S., (2008), Revisiting the conceptualisation of pedagogical content knowledge (PCK): PCK as a conceptual tool to understand teachers as professionals, Res. Sci. Educ., 38, 261–284.
- Park S., Jang J.-Y., Chen Y.-C. and Jung J., (2011), Is Pedagogical Content Knowledge (PCK) Necessary for Reformed Science Teaching? Evidence from an Empirical Study, Res. Sci. Educ., 41, 245–260.
- Pitjeng R. J., (2015), Investigating the effect of an intervention on novice science teachers topic specific pedagogical content knowledge PhD, University of the Witwatersrand.
- Rollnick M., Green G., White M., Mumba F. and Bennett J., (2001), Profiles of First Year and Access Chemistry Students views of the study of Chemistry, Journal of the Southern African Association for Research in Mathematics and Science Education, 5, 13–28.
- Shulman L. S., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 15, 4–4.
- Shulman L. S., (1987), Knowledge and teaching: foundations of the new reform, Harvard Educ. Rev., 57, 1–22.
- Stake R. E., (2005), in Denzin N. K. and Lincoln Y. S. (ed.), The Sage handbook of qualitative research, Thousand Oaks, CA: Sage Publications, 3 edn, pp. 443–466.
- Tyson L., Treagust D. F. and Bucat B., (1999), The Complexity of Teaching and Learning Chemical Equilibrium, J. Chem. Educ., 76, 554–557.
- Van Driel J. H., Verloop N. and de Vos W., (1998), Developing science teachers’ pedagogical content knowledge, J. Res. Sci. Teach., 35, 673–695.
- Veal W. R. and MaKinster J. G., (1999), Pedagogical content knowledge taxonomies, http://www.ejse.southwestern.edu/article/view/7615, accessed 10 August 2016.
- Wilson S. M., Shulman L. S. and Richert A. E., (1988), in Calderhead J. (ed.), Exploring Teacher Thinking, Sussex, Eng: Holt Rinehart and Winston.
- Wright B. D., (2003), Rack and Stack: Time 1 vs. Time 2: Repeated Measures, Rasch Measurement Transactions, 17, 905–906.
- Zhang M., Parker J., Koehler M. J. and Eberhardt J., (2015), Understanding Inservice Science Teachers’ Needs for Professional Development, J. Sci. Teach. Educ., 26, 471–496.
| This journal is © The Royal Society of Chemistry 2016 |