Student oriented approaches in the teaching of thermodynamics at universities – developing an effective course structure
Lauri
Partanen
*
Laboratory of Physical Chemistry, Department of Chemistry, FI-00014 University of Helsinki, P.O. Box 55 (A.I. Virtasen aukio 1), Finland. E-mail: lauri.partanen@helsinki.fi
First published on 9th May 2016
Abstract
The aim of this study was to apply current pedagogical research in order to develop an effective course and exercise structure for a physical chemistry thermodynamics course intended for second or third year university students of chemistry. A mixed-method approach was used to measure the impact the changes had on student learning. In its final form in 2014, the course consisted of lectures following a broken lecture structure that incorporated different kinds of activating learning tasks, and a three-tiered exercise structure including qualitative and quantitative tasks with a large emphasis on collaborative problem-solving. The new lecture and exercise structures improved student learning as measured by students' exercise points, exam results, and, between 2013 and 2014, the results of a conceptual thermodynamics test the students took at the beginning and end of the course. Even though the new exercise structure increased students' motivation, positive affect and satisfaction with the course, in both 2013 and 2014, it was the interactive lecture structure that students reported to be the most beneficial part of the course. In light of these results, this study demonstrates the advantages on student learning of adopting a multifaceted approach to both lectures and exercises.
1 Introduction
In recent decades, research into the pedagogy of thermodynamics has flourished. Most of this research has been conducted by physicists (Dreyfus et al., 2015) as thermodynamics forms an integral part of the physics curriculum. On the other hand, thermodynamics is equally important for chemistry students, as it is essential for the deep and rigorous understanding of many of the most fundamental concepts in the field such as equilibrium constants, Gibbs free energy, enthalpy and entropy.While a number of research studies on thermodynamics have focused on very specific issues, such as identifying alternative conceptions regarding heat or work and then developing effective tools to improve student understanding of these issues (van Roon et al., 1994; Meltzer, 2004; Nilsson and Niedderer 2012), not many studies have combined and applied these results into larger study units such as whole courses. In this study, our aim was to apply current pedagogical research to construct a student-centered pedagogical framework for a large thermodynamics lecture course and to revise the old exercise structure to further improve student learning and create a functioning constructively aligned course unit (Biggs, 1996). Assessment and development of the student's learning objectives in different parts of the course structure was done in the framework of the revised Bloom's taxonomy (Bloom, 1956; Anderson et al., 2001).
The original course structure of 2012 was modified in two development cycles: in 2013 the focus was on the lectures, and in 2014 on the exercise structure. A summary of the changes is given in Fig. 1. The primary objective of the study was to evaluate the impact of these changes on student learning and impressions. This was done by employing a mixed-method approach (Johnson et al., 2007), with both quantitative and qualitative indicators. While the effect of the student-oriented and research-based lecture structure of 2013 and 2014 is carefully discussed in the paper, the main focus is on how the improved exercise structure affected student attitudes and learning.
The rest of the article is organized as follows: in Section 2 a short review of the main challenges of teaching thermodynamics is given. After this, the student samples and methods used in this study are described in Section 3. This is followed, in Section 4, by a detailed account of the new course structure both in terms of the lectures and exercises. The results and their possible interactions are discussed in Section 5. A summary and analysis of the reliability of our results is presented in Section 6. Finally, some general conclusions together with implications for further teaching can be found in Section 7.
2 Main challenges in the teaching of thermodynamics
Sözbilir identified several factors that make thermodynamics a challenging topic for students (Sözbilir, 2004). In addition to the abstract nature of the concepts, chief among these are instructor-centered pedagogic approaches and the failure to prioritize topics on the part of the instructor and a lack of motivation on the part of the student. Other studies have shown that a student's facility with calculus and logical thinking capabilities together with his or her study skills and attitudes towards physical chemistry affect performance in thermodynamics courses (Nicoll and Francisco, 2001; Hahn and Polik, 2004). Furthermore, understanding the particulate nature of matter (PNOM) is an essential part of a deep appreciation of thermodynamics and yet many students struggle with PNOM representations at all levels (Novick and Nussbaum, 1981; Gabel et al., 1987; Al-Balushi, 2009; Becker et al., 2013; Hernández et al., 2014).Other difficulties pertain to the student's ability to solve physicochemical problems involving algebra, which form a central part of most courses in physical chemistry. One of these is the transference of mathematical skills from the context of mathematics courses into applied subjects such as thermodynamics (Jasien and Oberem, 2002; Greenbowe and Meltzer, 2003; Thompson et al., 2006; Bucy et al., 2007; Hadfield and Wieman, 2010; Becker and Towns, 2012). For example, many students have problems with the mathematical description of the first law, claiming that w = −pΔV, q = ΔH and ΔU = q + w are all just restatements of the law (Hadfield and Wieman, 2010). More generally, connecting abstract mathematical inscriptions to information about macroscopic or microscopic systems appears to be challenging to students (Kautz et al., 2005). This seems to be true even in those cases where the students have been successful in terms of typical course metrics such as final exam scores (Thompson et al., 2006; Hadfield and Wieman, 2010; Becker and Towns, 2012), and thus represents a major challenge for teachers to overcome. The problem is further exacerbated by the heterogeneity of the student's mathematics skills at the beginning of the course which at least in Finland stems partly from the fact that chemistry typically has a mixture of students that have chosen either the long or the short mathematics curriculum in upper secondary school.
Perhaps the biggest challenge for the learning of thermodynamics is that a significant number of students enter courses holding a variety of alternative conceptions which are in opposition to the current scientific knowledge of many of the central topics of thermodynamics (Bain et al., 2014). These conceptions can be the residue of previous instruction or, perhaps more problematically, can originate from the more mundane use of these concepts in everyday life as in the case of temperature, heat, and work (van Roon et al., 1994; Thomas and Schwenz, 1998; Loverude et al., 2002; Kautz et al., 2005) and disorder in the context of entropy (Carson and Watson, 1999; Sözbilir and Bennett, 2007). The alternative conceptions are rather resistant to change and even after instruction a large number of students still come out of class with these conceptions intact (Thomas and Schwenz, 1998; Bransford et al., 2000; Tsaparlis, 2007; Christensen et al., 2009). In support of the constructivist theory of learning (Phillips, 2000), in this study we have chosen to use the term alternative conceptions instead of misconceptions, because the latter is often associated with outdated pedagogical approaches of eradicating incorrectly learned concepts and replacing them with correct ones (Maskiewicz and Lineback, 2013).
Several studies have identified students' alternative conceptions and conceptual difficulties in the central topics of thermodynamics such as the understanding of heat and work, which students often treat as state functions (van Roon et al., 1994; Greenbowe and Meltzer, 2003; Meltzer, 2004, 2006) and the first (Thomas and Schwenz, 1998; Miller et al., 2005, 2006; Hadfield and Wieman, 2010; Nottis et al., 2010; Nilsson and Niedderer, 2012, 2014), second (Carson and Watson, 1999; Meltzer, 2006; Sözbilir and Bennett, 2007; Christensen et al., 2009; Smith et al., 2009a), and third (Sreenivasulu and Subramaniam, 2013) laws of thermodynamics. For a thermodynamics course in chemistry, students' alternative conceptions about chemical equilibrium are especially important due to the central role this concept plays in the field. According to the studies by Azizoğlu et al. and Boudreaux et al., students have difficulties in correctly understanding the definitions for vaporization, condensation, sublimation or freezing points (Azizoğlu et al., 2006) and the conditions under which liquid and vapor coexist in equilibrium, as well as realizing that the vapor pressure is controlled solely by the temperature under those conditions (Boudreaux and Campbell, 2012). On a more general level, the connection between kinetic concepts and equilibrium poses problems for many students (Banerjee, 1995; Sözbilir et al., 2010; Turányi and Tóth, 2013) as they maintain, for example, that a fast reaction goes to full completion (Sözbilir, 2002). Thomas and Schwenz identified many other alternative conceptions regarding equilibrium, including the one that at equilibrium most if not all chemical reactions cease (Thomas and Schwenz, 1998).
On the affective side, a significant portion of students enter physical chemistry courses with negative perceptions and low expectations for personal success (Nicoll and Francisco, 2001). These kinds of attitudes are detrimental to student engagement with the course and may act as negative self-fulfilling prophecies (Hewstone et al., 2010) and even lead students to drop out of the course, which is why it is important to address these at the earliest possibility.
Because of the higher emphasis on expressing ideas using mathematical inscriptions and the large number of challenging concepts that regularly clash with everyday interpretations of these words, thermodynamics differs significantly from most chemistry courses. In the face of these difficulties, even the students with high self-regulation skills are more affected by the learning environment (Lindblom-Ylänne and Nevgi, 2009) which poses unique challenges also for the instructor.
3 Methods
3.1 Course background
In 2013 and 2014, at the University of Helsinki, thermodynamics was an obligatory course of four ECTS credits (where one ECTS credit corresponds to 27 hours of work) both for chemistry majors and those studying to become chemistry teachers. The course was intended for second or third year students and was the only course in thermodynamics after the general chemistry and solution chemistry courses. In general, the students had a relatively good background knowledge of many of the topics of thermodynamics as most of them had been introduced in the first year courses.In 2013 and 2014, the course consisted of 40 hours of lectures which meant that on most weeks there were two 90 minute lecture sessions. There was a set of eight exercises in 2013 and six in 2014. In 2012, the course in thermodynamics was organized together with the dynamics course as a seven ECTS credit package, but the amount of lecture hours for the thermodynamics part and the number of exercises devoted to thermodynamics was the same as in 2013. In all the years, the course was staffed by the author of this paper as the course instructor and two teacher's assistants (TAs), who were either postgraduate or graduate students at the Laboratory of Physical Chemistry.
In 2012, the course grade was almost solely determined by two partial exams: each of the exams awarded a maximum of 18 points, whereas at most 4 bonus points could be obtained from doing exercises. As shown in Fig. 1, in 2013 and 2014 the role of the exercises was much greater, contributing 25% to the course grade or 20 course points, while 30 course points were awarded for each of the two three-hour partial exams. As the style of exam questions both influences and directs student learning (Carson and Watson, 2002), in both 2013 and 2014 significantly more emphasis was put on measuring conceptual understanding of the students. In these partial exams the students were also allowed to bring with them an A4-size self written cheat sheet. The use of this kind of cheat sheet has been associated with increased learning particularly in the first three levels of the cognitive processing axis of the revised Bloom's taxonomy (Bloom, 1956; Anderson et al., 2001) and reduced test anxiety (Erbe, 2007; de Raadt, 2012). A pictorial representation of the revised Bloom's taxonomy can be found in Appendix B. It can be argued that by de-emphasizing the role of memorizing equations the use of crib sheets helps to align the exam with our course objectives which in 2013 and 2014 mostly cover the second to fourth cognitive process categories of Bloom's taxonomy. On the other hand, by emphasizing the application of knowledge instead of mere recall it also facilitates learning during the exam. In 2013 and 2014, the full set of 20 course points was awarded for doing 90% of the total number of exercises, but in order to further reward hard-working students who exceeded this limit, up to a maximum of six bonus points were given out depending on the exceeding amount. None of the students actually managed to obtain the full six bonus points.
In all of the years, the course homepage was presented on a Moodle-platform† where exercise sheets and lecture materials were made available. The course textbook was Atkins' Physical Chemistry (Atkins and de Paula, 2010), which was complemented by the course instructor's lecture notes which incorporated material from the thermodynamics education research literature and several other popular textbooks (Levine, 2008; Engel and Reid, 2014). The homepage contained a weekly updated course diary which described what topics would be covered in the next week's lectures together with the corresponding pages in the coursebook. Several discussion forums were also available via the homepage to enable the students to ask the instructor or the TAs questions, or to discuss weekly exercises.
3.2 Qualitative and quantitative instruments
The mixed-method approach employed to study the effect of the improved exercise structure between 2013 and 2014 made use of three quantitative indicators to measure student learning outcomes: the percentage of exercise points obtained during the course, the total percentage of points obtained from the partial exams, and the result of a conceptual thermodynamics test (CTT). The CTT was based in part on the instruments used to assess student alternative conceptions in the literature (Meltzer, 2004; Wattanakasiwich et al., 2013) and can be found in Appendix C. The students took the CTT both at the beginning (pre-CTT) and at the end of the course (post-CTT). The CTT was developed to on the one hand provide background information about the student level of knowledge and the prevalence of some of the most typical alternative conceptions for the lecturer and on the other to measure student improvement in conceptual understanding over the course for the purposes of this study. The CTT was reviewed by two experts in the field of thermodynamics and tested on the TAs of the course in 2013 before its implementation in the study. The first two measurement instruments were also used to get some quantitative indication of the impact that the interactive lecture structure had between 2012 and 2013.We also gathered qualitative data on student experiences about different aspects of the course in 2013 and 2014 in the form of an extensive feedback questionnaire at the end of the course. The questionnaire included open questions and it was administered at the same time as the students answered the post-CTT. The feedback questionnaire was reviewed by an expert in university pedagogy. To form a more complete picture of student reactions to the improved course unit as a whole, we conducted a series of interviews on a self-selected sample of student volunteers in 2014. The first round of the interviews was organized a few weeks into the course followed by a second round at the end of the course. The interview questions went through a series of iterations: they were reviewed by a set of two experts in university pedagogy, revised, tested on a group of four students from a previous years' courses, revised again and finally reviewed by the same two experts before being employed in the interviews.
3.3 Study sample and analysis
Most of the students enrolled in the thermodynamics course were either chemistry or chemistry education majors, but in all years the course had a handful of students majoring in other subjects such as geology, biochemistry or physics which added to the heterogeneity of the students' mathematical skills. The official number of students enrolled in the course varied between 50 and 120 depending on the time and the year. To maximize the sample size while still only accepting those students that had stayed active and involved throughout the course, we chose to include in our quantitative analysis in 2013 and 2014 only those students that had data for two out of our three indicators for learning. All of the people who participated in either one of the partial exams or took either one of the CTTs had some exercise points. Thus, these criteria excluded students who had done some exercises during the course, had participated in the first partial exam but not in the second, and had not answered one or both of the CTTs. Typically, the case was that these students had dropped out of the course somewhere in the middle and so were not relevant in terms of this study. On the other hand, our criteria did include people who for some reason had not participated in one or both of the partial exams but had done some exercises and answered both of the CTTs, which indicated that they had followed the course from the beginning to the end. Naturally, these criteria also included people who had participated in the partial exams and had some exercise points, as responding to the CTT was voluntary (the people who answered were awarded a small number of exercise points). Of all the students enrolled in the course, 69 met our inclusion criteria in 2014 and 46 in year 2013, while the number of students who responded to both pre- and post-CTT were 56 and 42, respectively. As no CTT was employed in 2012, only those people who had participated in both partial exams and returned exercises were included in the analysis, which resulted in a sample of 40 people. Analysis of quantitative results was conducted with the help of the SPSS program.‡In terms of feedback, we received 61 responses to the online questionnaire in 2014 and 48 in 2013 which in terms of the total number of people still enrolled at the end of the course was 73% and 65%, respectively. For the interviews, ten volunteers participated in the first round and eight returned for the follow-up interview. Both the relevant responses to the feedback forms and interviews were qualitatively content-analyzed (Patton, 2001). After going through the recorded interviews and responses to selected feedback questions, the responses were categorized into different themes in a process of inductive content analysis (Elo and Kyngäs, 2008) where selected categories arose from student responses and not from any theoretical framework.
3.4 Ethical perspectives
This study was conducted in the spirit of action research (Womack, 1997; Ralle and Eilks, 2002; Tripp, 2005) where the author of this paper served both as the course instructor for three years and the principal researcher. While this type of research is widely conducted in educational circles, the dual role of the author does pose a potential problem in terms of the validity of the data and the degree to which the students are aware of being participants in a research project (Zeni, 1998; Taber, 2014). To minimize these threats, the teacher was involved as little as possible in the actual grading of course tasks such as exercises and exams, providing only grading guidelines for the TAs to ensure constructive alignment in the course. However, while not being directly involved in the grading process in 2012, in 2013 and 2014 the instructor was forced to grade one of the five exam questions in both of the partial exams, due to limited resources in the course. In 2013 and 2014 the grading guidelines were also reviewed and discussed with the TAs in part to ensure that no significant discrepancies between the years existed. Although there was no formal institutional oversight, the methodology of the research was reviewed in terms of ethics by two experts in the field of university pedagogy, the research was conducted in close correspondence both with the local teaching support unit and the Helsinki University Centre for Research and Development of Higher Education.In terms of obtaining informed consent, in the first lecture the students were told that the results of the course such as grade averages and exercise points would be used as a part of doing pedagogical research to further develop the course. In terms of the CTT and the electronic feedback form, both of these contained a statement that the results would be used as part of a research project. As these were part of the course tasks, the students also had the option of not answering them, with negligible effect on their course performance. For the interviews, the students were recruited in the first CTT and were given the following information translated here from Finnish into English:
One of our goals is to do pedagogical research on the basis of the developmental work on this course. For this research we need student volunteers who are ready to share their opinions about the course in two interviews lasting about one hour. The first of these interviews will be organized at the beginning and the second at the end of the course. The lecturer will be able to access the interviews only after the course has finished, so the discussions will have no effect on the grading whatsoever. The interviews will be performed by people not associated with the course. The volunteers will be contacted via email. Your responses are vital for the continued development of the course.
In addition to the anonymity measures presented in the above quotation great care was taken to preserve anonymity also in the written feedback forms. While the feedback form contained the student ID for the marking of the exercise points that students obtained for completing it and the CTT, this was never used to connect student feedback with task performance. The students were also given the option to give feedback completely anonymously through a separate online form available in all the courses in the university, although few ever did.
4 Teaching thermodynamics in light of current pedagogical literature
4.1 Interactive lecture structure
For the course lectures in 2013 and 2014, a broken lecture structure as depicted in Fig. 2 was adopted. In this approach, the lecturing is regularly interrupted by different activating learning tasks. In addition to improving student attention during the lectures (Bunce et al., 2010), a broken lecture structure promotes a broad range of learning outcomes (Prince, 2004), including long-term retention of the material, and the perceived effectiveness and enjoyability of the lectures (Smith, 2006; Miller et al., 2013). A typical lecture in Finland consists of two 45 minute sessions with a 15 minute break in between. In the new approach, the total lecture time of 90 minutes was divided into two or three modules of 25 to 55 minutes with one or two 5 to 15 minute breaks, depending on the subject with a preference towards the three module structure. The choice to break the lecture this way was based on the findings that while student attention does not necessarily start to decline after the first 10 minutes of the lecture (Wilson and Korn, 2007), it does tend to oscillate with ever-shortening cycles of engagement towards the end of the lecture (Bunce et al., 2010). This kind of flexible and alternating lecture structure not only helps in keeping up student engagement but also aids in organizing the lecture into meaningful bite-sized study units as the lecture is interrupted whenever there is a change in topic.As depicted in Fig. 2, the different types of activating tasks varied highly depending on the lecture, including example problems and justifications of the most central results. One of the guiding principles behind these activities was to get the students to work in small groups on some aspects of the calculation thus engaging them in peer learning. The majority of modules also incorporated at least one multiple-choice discussion question where the students voted after discussing the question in small groups, followed by a general discussion and instructor explanation based on the answers. The multiple-choice questions were based on items used in the literature to probe student alternative conceptions, providing the lecturer with information about the comprehension level of the students and the prevalence of the typical alternative conceptions. This approach makes it possible to challenge these conceptions in a student engaging way immediately after they surface, and even shape the structure and topics of the coming lectures based on this information which, on the basis of the Peer Instruction literature (Mazur, 1997; Crouch and Mazur, 2001; Mellema, 2001; Meltzer and Mannivannan, 2002; Lasry et al., 2008; Turpen and Finkelstein, 2009; Smith et al., 2009b, 2011), should result in a marked improvement in student understanding of the central concepts. For some of the most widespread and fundamental alternative conceptions among students, such as the identification of entropy with disorder, the modules were crafted in the style of Module I in the two-module structure of Fig. 2. In these cases, the same multiple choice discussion question was first presented at the beginning of the class with the students responding individually and then a second time at the end of the class followed by group discussion and second voting. This allows the teacher to obtain direct feedback on the effectiveness of his or her teaching in some the most challenging and important topics of the course.
At the beginning of each module, the students were given a list of three to five review questions. The questions were visible for them for the whole module, were closely aligned with the central learning goals introduced at the beginning of each module, and served as the basis for the module-based review held at the end of the module or at the beginning of the next one. These questions together with the multiple choice discussion questions were the most important channel of direct feedback on student learning in the lectures. Additionally, the review questions allowed the students to immediately apply the material under study which facilitated open discussion and the identification of alternative conceptions and difficult concepts in the course material (Knight and Wood, 2005).
Finally, in the first lecture of the 2014 course, an attempt was made to address the negative preconceptions and bad reputation of the physical chemistry courses. This was done by assuring that support would be available to all who seek it, underlining the importance of participation in course activities, directing students to focus on understanding the concepts instead of just rote learning them, and commenting that in the past the people who committed to the course passed it almost without exception. In contrast to the interactive approach outlined above, in 2012 the course followed a more traditional lecture style where most of the time the lecturer presented the students with the information followed by some examples where the lecturer showed the students how to apply the formulae in practice. Due to the time reserved for the active learning tasks in the 2013 and 2014 lectures, only the most essential parts of the course material were discussed whereas in 2012 also some less important topics were covered. The course content and the names of the different modules in 2014 can be found in Appendix A.
4.2 Effective exercise structure
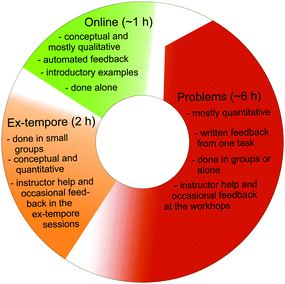
In light of the challenges to teaching thermodynamics described in Section 2, our main goal for 2014 was to develop an exercise structure based on three principles: first, the structure should aid in the rigorous understanding of thermodynamics concepts and challenge the alternative conceptions students already possess. Second, it should support the transfer of mathematical skills into the physical chemistry context. Third, it should orient the students towards a deep and multifaceted understanding of physicochemical problem-solving as opposed to just developing student's capabilities to routinely apply algorithms and formulae. Our solution was to divide the six exercise sets into three parts as summarized in Fig. 3. The first element in the new exercise structure of 2014 was two weekly bundles of online introductory exercises on Pearson's Mastering Chemistry-learning platform.§ Each of the bundles took about half an hour to go through and consisted of a series of qualitative questions and simulations based on material that had not yet been discussed in the lectures. The second weekly bundle was designated as a bonus exercise. The exercises were chosen so that by going through them the students would familiarize themselves with the key points of the upcoming lectures beforehand, and thus be more prepared to participate in the active learning tasks of the lectures. Pre-lecture activities such as quizzes and exercises that guide the students to study the material prior to the lecture have been shown to result in significant increases in learning and student ability to discuss related material in class (Dobson, 2008; Johnson and Kiviniemi, 2009; Moravec et al., 2010). Typically the exercise questions belonged to the lowest three cognitive process and knowledge categories of Bloom's taxonomy. A special focus was put on interactive simulations which in addition to being effective teaching tools also help the students to integrate the particulate nature of matter with the macroscopic aspects of thermodynamics (Podolefsky et al., 2010; Wieman et al., 2010). The Mastering Chemistry platform provides access to tips that explain the theory behind the exercises and also gives some automated feedback on student answers. Due to the introductory nature of these exercises we encouraged students to utilize these tips, refusing to penalize their use, as is the default setting in the program. Appropriate feedback and guidance are vital if one wishes to facilitate high quality practice in students as without them practice tends to focus only on familiar skills (Johnson, 2001).The second part consisted of two-hour small group ex-tempore exercise sessions for roughly twenty participants per session where the students were given a set of two tasks and a bonus task which they then solved in small groups with aid from a TA. At the end of the sessions, the groups presented their solutions followed by discussion with the students receiving immediate feedback about their performance. In addition to providing multiple channels of guidance and feedback to each student in a timely manner (Johnson, 2001), this kind of collaborative problem-solving also enables students to learn from and share information with other students within the same zone of proximal development. In addition to increasing student learning and individual problem-solving capabilities, it also enhances students' motivation, positive affect towards the subject, confidence in solving problems, and even encourages females and students at risk academically (Scott and Heller, 1991; Heller et al., 1992; Towns and Grant, 1997; Johnson and Johnson, 1999; Springer et al., 1999; Lyon and Lagowski, 2008). Furthermore, it gives the TAs a glimpse into some facets of the student's reasoning that are not available by examination of the exercise solutions, thereby improving the quality of the feedback. To facilitate the transference of mathematical skills and to ensure that the students met the mathematical background knowledge required for the course, the first of the ex-tempore sessions started off with a short math test covering the interpretation and application of the most central tools for the course such as derivatives, integrals, partial derivatives and total differentials as suggested by Hahn and Polik (2004). After the test, the TAs encouraged students to revise the topics where the students' skills were still lacking, emphasizing the importance of mastering these skills for successful performance in the course.
The exercises themselves were a mix of both quantitative and qualitative questions where students were typically required to discuss and explain some phenomenon, further encouraging group work. They were designed to probe and develop understanding at the second through fourth cognitive processing categories and first through third knowledge categories on the Revised Bloom's Taxonomy. Especially in the ex-tempore exercises, we attempted to incorporate elements that would require PNOM-level reasoning, for example, by asking the students to sketch molecular level depictions of the systems under study. By supporting the formation of multiple different-level representations for any given problem these tasks also enhance problem-solving skills in general (Gabel et al., 1987; Bodner and Domin, 2000; Madden et al., 2011; Hernöndez et al., 2014). Two sample exercises are given in Appendix B.1.
The third section of the exercises consisted of three more challenging problems, and the students had to submit their solutions before the next exercise set was made public. Typically these problems belonged to third through fifth categories in the cognitive processing dimension and second or third categories in the knowledge dimension of the Revised Bloom's taxonomy and were thus significantly more cognitively complex than either the online-exercises or the ex tempore ones (Tikkanen and Aksela, 2012). Three sample problems are presented in Appendix B.2. One of the problems awarded double points compared to the other two to increase the flexibility of the grading, allowing us to delve deeper into important aspects of some problems and still maintain a fair grading scale. This should have a positive effect on student motivation as the extra effort required by some exercises is balanced by the higher reward. Typically these double point problems would include tasks such as plotting and analyzing one or more diagrams using mathematical software. The problems were chosen to develop the understanding of the physical phenomena with the mathematical complexity kept at a minimum. The qualitative parts of the problems often required the students to interpret the answer they had obtained from calculations in some meaningful way. To encourage group work, an open two-hour problem-solving workshop was organized three times every week with a TA ready to lend guidance.
Aside from the computer generated automatic feedback given by the Mastering chemistry program and the verbal feedback provided in the ex-tempore sessions and problem-solving workshops, we decided to use the problems as our main source of formative assessment. To this effect, every week we chose one of the three exercises for which the TA was instructed to give written feedback for each student about his solution, while the rest of the problems were just marked. A weekly meeting was organized between the TAs and the instructor to discuss the focus points of this feedback, the solutions to the ex-tempore exercises and problems, and how to deal with the anticipated difficulties the students might experience when solving these.
The original exercise structure of 2013 and 2012 consisted of approximately five weekly problems. In 2013, double points were for awarded one of the problems and we added a bonus problem that dealt with material not yet discussed in the lectures. These problems were similar to the 2014 ones, although they did incorporate some easier exercises and contained almost no qualitative parts. In 2013, three two-hour workshops per week were also organized. While not specifically instructed to do so, the TAs did provide some occasional feedback on the submitted solutions to the problems. Compared to the 2013 and 2012 exercise structures, it was a conscious move in 2014 to try and put more exercises in the bonus category. This was partly in response to several students of 2013 complaining that the exercise load was too heavy, but we also wanted to enable the most motivated students to do extra exercises that were aligned with the course objectives without forcing them to do so.
5 Results and discussion
5.1 Learning outcomes
Table 1 summarizes the learning outcomes of the students in the 2013 and 2014 courses. It also displays comparative data on the exam results and the percentage of exercise points in 2012. Because the absolute value of the skewness of the distribution was less than 0.7 in all cases, we have chosen to report only the average values in Table 1. As can be seen, the new exercise mechanic had the largest effect on the results of the CTT where the average increase in the test result from the pre-CTT to the post-CTT was 14.7% in 2014 corresponding to a large effect value of d = 0.97 in terms of Cohen's d value of significance and only 8.5% in 2013 which corresponds to a moderate effect value of d = 0.57 (Cohen, 1988). There was also a 3.5 percent increase in the number of exam points obtained corresponding to a small effect size of d = 0.26. Between 2013 and 2012 the increase was 3.3 percent, corresponding to an effect size of d = 0.21. Comparing 2014 with 2012 the effect size value for the increase in exam results is d = 0.44. It should be noted that there is also a significant decrease in the standard deviations of the exam results between 2012 and 2013 whereas the deviations between 2013 and 2014 are the same. Together with the increases in the exam result, this indicates that the changes in the lecture structure between 2012 and 2013 significantly improved the learning for the people who were doing worst in the course, as in 2013 more of these students were obtaining exam results closer to the class average than in 2012. The relatively small effect size also belies the fact that as the learning objectives of the course in 2013 and 2014 came to focus on the higher dimensions of the revised Blooms' taxonomy, the exams also became significantly more difficult, so the effect seen in Table 1 between 2012 and 2013 is likely an underestimation in terms of student learning.| Exam result (%) | Exercise points (%) | Pre-CTT score (%) | Post-CTT score (%) | |
|---|---|---|---|---|
| 2014 | ||||
| Average | 56.6 | 62.3 | 46.8 | 61.5 |
| Std dev. | (13.6) | (19.4) | (15.4) | (15.0) |
| N | 67 | 69 | 56 | 56 |
| 2013 | ||||
| Average | 53.0 | 62.3 | 48.4 | 57.0 |
| Std dev. | (13.0) | (20.6) | (14.5) | (15.3) |
| N | 44 | 46 | 42 | 42 |
| 2012 | ||||
| Average | 49.7 | 63.8 | ||
| Std dev. | (18.2) | (21.5) | ||
| N | 40 | 40 | ||
| 1 | 2 | 3 | |
|---|---|---|---|
| 1. Pre-CTT result | |||
| 2. Post-CTT result |
0.297
(N = 42) |
||
| 3. Exam points |
0.251
(N = 40) |
0.527*
(N = 40) |
|
| 4. Exercise points |
0.110
(N = 42) |
0.242
(N = 42) |
0.623*
(N = 44) |
| 1 | 2 | 3 | 4 | 4a | 4b | |
|---|---|---|---|---|---|---|
| 1. Pre-CTT result | ||||||
| 2. Post-CTT result |
0.405*
(N = 56) |
|||||
| 3. Exam points |
0.172
(N = 54) |
0.419*
(N = 59) |
||||
| 4. Total exercise points |
0.136
(N = 56) |
0.407*
(N = 61) |
0.587*
(N = 67) |
|||
| 4a. Problem points |
−0.049
(N = 56) |
0.351*
(N = 61) |
0.551*
(N = 67) |
0.904*
(N = 69) |
||
| 4b. Ex-tempore exercise points |
0.141
(N = 56) |
0.238
(N = 61) |
0.417*
(N = 67) |
0.754*
(N = 69) |
0.518*
(N = 69) |
|
| 4c. Online exercise points |
0.335
(N = 56) |
0.296
(N = 61) |
0.318*
(N = 67) |
0.553*
(N = 69) |
0.358*
(N = 69) |
0.137
(N = 69) |
| 2013 (N = 42) | 2014 (N = 56) | |||
|---|---|---|---|---|
| No. of students | % | No. of students | % | |
| Strong improvement | 10 | 24 | 13 | 23 |
| Improvement | 11 | 26 | 21 | 38 |
| No or slight change | 14 | 33 | 20 | 36 |
| Decline | 7 | 17 | 1 | 2 |
| Strong decline | 0 | 0 | 1 | 2 |
| Post-CTT |
Lowest
n = 2 |
Below average
n = 8 |
Above average
n = 11 |
Highest
n = 35 |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Pre-CTT | ||||
| Lowest | 2 | 1 | 2 | 8 |
| n = 13 | 4.5 (2.1) | 6.0 | 5.5 (0.7) | 11.6 (3.1) |
| Below average | 0 | 5 | 3 | 8 |
| n = 16 | — | 0.2 (0.4) | 3.3 (1.5) | 7.9 (3.4) |
| Above average | 0 | 0 | 6 | 6 |
| n = 12 | — | — | 1.0 (0.9) | 2.5 (1.5) |
| Highest | 0 | 2 | 0 | 13 |
| n = 15 | — | −7.5 (2.1) | — | 3.1 (2.8) |
| Post-CTT |
Lowest
n = 5 |
Below average n = 4 | Above average n = 11 | Highest n = 22 |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Pre-CTT | ||||
| Lowest | 1 | 2 | 2 | 4 |
| n = 9 | −6.0 | 5.0 (1.4) | 6.5 (0.7) | 9.5 (2.1) |
| Below average | 1 | 2 | 4 | 5 |
| n = 12 | −2.0 | 0.5 (0.7) | 3.0 (1.4) | 7.6 (1.5) |
| Above average | 3 | 0 | 2 | 7 |
| n = 12 | −5.7 (0.6) | — | −0.5 (0.7) | 4.9 (4.0) |
| Highest | 0 | 0 | 3 | 6 |
| n = 9 | — | — | −3.7 (2.1) | −0.8 (2.6) |
Rather surprisingly, there was virtually no increase in the percentage of exercise points obtained moving from 2012 to 2014. Considering that the variances are very close every year and that as described in Section 4.2 in terms of actual numbers, the students had to do more exercises in 2012 and 2013 than in 2014, one can conclude that in 2014 less work resulted in better learning outcomes. This finding is in line with the research of Kim and Pak (2002) showing that conceptual understanding cannot be obtained just by solving textbook problems and Johnson (2001) highlighting the importance of the quality of practice to its effectiveness on learning.
Focusing on the effects of the change in the exercise structure, some of the notable correlations between different measuring instruments in 2013 and 2014 are shown in the correlation matrices of Tables 2 and 3, respectively. There was no significant correlation between the pre-CTT and any other quantitative learning instrument except the post-CTT in 2014, which is explained by the fact that the two tests are virtually identical. In 2013, even this correlation is absent, which may indicate that at the end of the course students had to rely more on guessing when answering the CTT questions, whereas in 2014 they were better able to recognize which of their initial answers were correct. As expected, in 2014 post-CTT results show relatively strong correlations with the other learning indicators used in this study, while even stronger correlations are observed between the exam result and the number of exercise points. These findings give confidence that our three instruments measure learning and understanding in a multifaceted and rigorous way. On the other, hand while the post-CTT was strongly correlated with the exam result in 2013, it did not show a significant correlation with the number of exercise points. This is a clear signal that in 2013 the exercises were mostly teaching computational skills, without supporting the actual understanding of what the students were doing.
In terms of the correlations between the learning indicators and the different exercise structures in Table 3 there is a moderate correlation between the post-CTT result and the problems. This is interesting because one would intuitively think that the problems have a lesser role in the development of conceptual understanding than the more qualitative ex-tempore exercises, which in fact do not show a significant correlation with the post-CTT result. As the maximum number of points obtainable from the problems was relatively large compared to that of the ex-tempore exercises (12 from problems, 4 + 2 bonus from ex-tempore), this effect might be explained by the fact that to gather a decent amount of points from the problems much more effort and time was required, which then showed in the investigation as a stronger correlation. The online exercises showed a moderately positive correlation with both the exam results and at the 0.05 level of confidence also with the post-CTT result. Unlike the other two parts of the exercises the online exercises also showed a moderate correlation with the pre-CTT result at the 0.05 level, which makes sense because these questions typically dealt with topics that had not yet been introduced in the lectures, so students with more comprehensive baseline knowledge would have an edge when doing these exercises. While the ex-tempore exercises showed relatively strong correlations with the exam results, this correlation was the strongest for the problems. As the problems were by far the most challenging and time-consuming part of the exercises it is likely that most of the learning occurred through them. It should be underlined that both the introductory and the ex-tempore exercises were partly designed to prepare students for the problems, and it is difficult to asses the kind of indirect effects the two may have on the learning.
Looking closer at the differences in the CTTs of 2014 and 2013, Table 4 shows the distribution of students into five change categories according to the change in their CTT results between the beginning and end of the two courses. The number of people in the two improvement categories was 61% in 2014 compared to 50% in 2013 which demonstrates the effectiveness of the new exercise structure. In both years, the percentage of people showing strong improvement is almost the same, but in 2014 the relative number of people in the improvement category is clearly larger than in 2013. In 2014, only two people or 4% are in the decline or strong decline categories, whereas in 2013 the number is 17%. Thus, the new approach seems to have the largest effect on the learning of students with average or below average motivation and ability, precisely as one would hope for.
The results of the CTTs are shown in a slightly different light in Tables 5 and 6 where the ranked four percentile groups of both the pre- and post-CTTs are tabulated based on the binning of the pre-CTT. The categories of the pre- and post-CTTs are represented by the rows and columns of the matrices, respectively. In both tables, the general improvement in the results can be seen in the accumulation of people in the third and fourth columns of the tables i.e. in the above average and highest-categories. However, it is clear that in Table 5 a significantly greater number of students rank into the highest percentile group relative to the starting results than in Table 6. Combined with the similar baseline means and standard deviations for the comprehensions test in both 2014 and 2013 shown in Table 4, this finding is another indication of the broader learning that took place in 2014 compared to 2013. Furthermore, the 2014 table sheds light on the two cases of decreased CTT results visible in Table 4. They are shown in Table 5 as the two lone figures on the fourth row, meaning that both of these students belonged to the highest percentile group in the pre-CTT. As the person who showed the greatest decline in skill also did not participate in the second partial exam or its retake exam, it seems plausible that these two have guessed exceedingly well in the pre-CTT, and then had some motivational problems towards the end course. However, it should be noted that most of the people who start in the highest category do stay in that category, meaning that their results either decrease only a little, stay roughly the same or increase. A clear positive signal is also that the amount of people in the first two rows of column four in 2014 is larger than in 2013. These are the people who have started the course off with very modest understanding of basic principles and central concepts of thermodynamics according to the pre-CTT, but have been able to improve significantly during the course. The role of some fortuitous guesswork cannot be ruled out, however, as some of these students in this category obtained rather mediocre results both in terms of the exams and exercise points.
The larger number of people in the decline categories in 2013 is evidenced in Table 6 by the greater number of people in the categories below the diagonal, as compared to 2014. In the categories corresponding to improvement shown above the diagonal, there is also greater spread among the different categories than there was in the 2014 case where relatively more students ended up in the highest category at the end of the course.
5.2 Qualitative results
All the selected excerpts given in this section have been translated from spoken Finnish into English. In labeling these quotes, the letter I or F followed by an integer means that the quote is taken from an interview or an open field response in the feedback form, respectively. To protect the anonymity of the respondents all of the interviewees will be referred to as female.In the 2014 feedback form we asked the students to write what they thought was the best thing in the course and the results are shown in Fig. 4. The most frequent response was the course lectures, which were also highly praised in the 2013 feedback. Of the different aspects of the lectures, the interactive elements such as the voting and discussion exercises and the concrete and practical approach of the course in general were often mentioned both in the feedback and interviews. Some shyer students felt that the use of clickers for voting lowered the threshold to participate and share their opinion in the lectures. Several of the interviewed students also reported participating more in the lectures not just in terms of actual attendance but also in terms of asking questions and being engaged in discussions. The discussion questions themselves were thought to be challenging, and in the interviews one student noted that they helped to pinpoint errors in student reasoning and thus conceivably prevent alternative conceptions from arising. In the second interview, when asked to name a positive experience from the course student I9 commented:
“I have many such experiences, for example discussion sessions where you were allowed to freely talk or even debate with the lecturer were positive and even if you were not talking yourself just following these kinds of exchanges was positive and instructive… also I have a tendency to lose focus during lectures but the discussions really wake you up and you can't just let them pass by.”
Thus, challenging student conceptions in an engaging way, as opposed to just revealing the right answer, not only benefits the student who is engaged in the discussion, but the other students as well.
In addition to directly enhancing student learning, the student-centered lecture structure and the positive attitude of the teacher also seemed to have a major motivating effect on many of the students in the course. For example, four out of the ten interviewed students reported an increase of their motivation during the course, in general moving from more outcome oriented goals and strategies to learning oriented goals and strategies as in the case of student I3:
“In the beginning I was not very motivated, I didn't even know if I was going to take the course or not… Then after making the decision that I was going to take the course I was already significantly more motivated to at least get a passing grade. When the course started progressing and so on, I was often pretty excited, at least in the lectures, writing down all sorts of things what one could do (pedagogically) and so my motivation started increasing and also the ex-tempore things that we had were inspiring and they certainly lifted my motivation, too.”
By the end of the course student I3 had done most of the course exercises and even retook her second partial exam just to get the highest grade from the course. Several interviewed students underlined the impact of the lecturer's attitudes both towards the students and subject matter on facilitating motivation and positive course atmosphere. The same number also felt that the atmosphere in this course radically differed from their previous chemistry courses. When asked about this, student I5 replied:
“I feel that it differs quite a lot, the lectures seem meaningful and progress intelligently. I know what I need to read. I enjoy going to the lectures and it seems useful.”
Student I5 was not the only person who felt that “knowing what to read”, because of the weekly updated lecture diary helped a great deal in preparing for the lectures, and also staying up to date with course progression when attending lectures was impossible.
Three interviewed students felt that the dynamic way of arranging the course breaks based on the material rather than trying to fit the material into a predetermined lecture structure enhanced their learning. In the case of two shorter breaks between modules, even a five minute break was enough for them to stretch, take a bathroom break or a cup of coffee and replenish attention. However, especially in 2013, quite a few people were critical of this approach in their feedback responses, pointing to the practical problems of breaks becoming too short to be useful when some module took up longer than expected. Certainly implementing this kind of approach requires careful planning from the lecturer and preparedness to adapt to changing situations by leaving some activating tasks out if for example previous discussion or voting sessions take longer than anticipated. However, in light of the increasingly frequent lapses in student concentration as the lecture progresses (Bunce et al., 2010), dividing the lecture into three parts with two short breaks ought to improve student concentration and should thus be the preferred option, despite these challenges. Furthermore, with regard to this question the students might not be the best judges of quality. Instead of appraising the break structure solely in terms of which approach most benefits their learning, they might prefer to have a normal coffee break, or just be more comfortable with the familiar single long break. A further improvement in the implementation of the break structure would be to direct student mobile use to the two short breaks between modules as texting during classroom has been shown to reduce comprehension by around 10–20% (Lawson and Henderson, 2015). While it was our idea that students would automatically focus their texting on the breaks, we did not make this desire explicit, which resulted in it being only partly successful. To improve the situation, according to Lawson and Henderson one possibility would be to give a research based justification for the prohibition of mobiles during lectures at the beginning of the course (Lawson and Henderson, 2015).
The student reaction to the new exercise system was also very positive. As can be seen in Fig. 4, in 2014, 25% of the respondents thought it was the best thing in the whole course citing, for example, the multifaceted nature of the exercises. Both in the interviews and feedback forms many also mentioned experiences of understanding and group work to be especially rewarding. The significance of peer-learning in the ex-tempores is nicely represented by the following quote from student F1:
“I thought that the ex-tempore exercises were very useful, because, for once I could find the courage to say that I don't know what to, when you could say it to other students and not directly to the teachers”
According to the interviews, it was a lot easier for students to ask help from a peer than from an instructor, even when the instructors were of the same age as the students. Incorporating peer-learning thus results in more students getting the help that they need. It can also encourage those students who typically work alone to discover new and fruitful learning strategies, as was the case for student I3:
“For me, the best part of the course was the second or so ex-tempore exercise. It was quite nice that we could work on and think about the problems together because previously I have always used to work through exercises alone… It decreased the time required to solve the exercises as when you found yourself going down a wrong path you were more quickly returned to the right one again so there was a clear advantage of doing together and it had no traces of one person doing all the work and the rest just copying from her.”
This quote describes exactly the kind of peer support one hopes to generate in these small group sessions. However, towards the end of the course student I3 had to change her ex-tempore group and reported seeing large differences in group cohesion between different classes. Regarding the last comment in the quote, in our implementation when going through the solutions at the end of ex-tempore session any of the students who had marked the exercise completed could be asked to present their solution to the class. This further motivates the students to understand what they are doing and not just copy the answers, minimizing free-riding within groups. However, this practice can also be a source of anxiety for the students as can be seen in the quote below from student I4:
“Originally I was a little afraid because I don't like these kinds of situations where you have to go to the blackboard to do things, but this changed along the course as I noticed that the exercises are not horribly challenging and that you could get help for the exercises.”
Thus, at least in her case the availability of instructor support and the decision of making the exercises clearly easier than the problems together with the generally encouraging atmosphere of the course were enough to alleviate preconceptions. In the interviews, two students also told that the peer pressure experienced in the ex-tempore groups encouraged them to study the topics beforehand in order not to let the group down during the session.
While most of the feedback received on the ex-tempore exercises was positive, there were some criticisms raised, and like student I3, we too observed big differences in the group dynamics between ex-tempore classes. In some cases the students formed tight groups where they actively compared answers, discussed problems and assisted one another whereas in others, despite the encouragement to do so, many of the students opted not to make contact with people they did not know. For example student F2 in the latter kind of group wrote
“It would have been nice if more emphasis had been put for example on the group work in the ex-tempore exercises. Then there would not have been so much need to struggle with the exercises alone and you would have had the support of the group to do the exercises and the whole would have worked better. This way there would have also been more time to go through the exercises at the end of the session”
In our view, this highlights the importance of carefully preparing the instructors for these classes, as they need to consciously encourage and enable group work and facilitate discussion. However, even when this is done, the truth is that not all the groups are going to function as well as one would wish. It is clearly challenging for students to work effectively in groups especially since from their previous courses they are used to working alone. For example, student F3 neatly summarized many of the problems of group work:
“The calculation of the exercises in small groups requires a lot from the students: everybody has to be able to work with people other than just their friends and the speed of going through the exercises needs to be roughly the same. When this is accomplished the idea of working in small groups is great.”
The ex-tempore sessions might also benefit from a more detailed description of the motivation and goals behind this method of instruction to give the students a better idea of what they are expected to do within these sessions. In her approach to Supervised Practice, Johnson (Johnson, 2001) assigned students with different roles for different exercises, which might also help in our case to improve peer–peer communication in the first couple of sessions. As the groups tend to stay roughly the same throughout the course, it could also be beneficial to start the first ex-tempore sessions by helping the students get better acquainted. Because willingness of the individuals to work together has an important effect on the construction of knowledge in group settings (Alexopoulou and Driver, 1996), we feel that it would be detrimental for the motivation of some of the students if we would force everyone to work in small groups and would result in them skipping the exercises altogether. Furthermore, it seems important to respect the students' right to employ study strategies that they have found to work well for them, especially because these independent students tend to have good skills for self-regulation of learning (Hadwin and Oshige, 2011; Räisänen et al., 2016). On the other hand, for those students who have problems with self-regulated learning and who habitually make use of co-regulation strategies (Hadwin and Oshige, 2011), it is important to enable their use with group exercise activities and by guiding the students to set regular small term goals such as completing the weekly exercises in due time. At its best, co-regulation can result in shared regulation, where the students not only utilize the support of others when studying but also genuinely share collective regulation processes as evidenced in the quote below from student I10 when she was asked what helped her to achieve the goals she had set for the course:
“I think that my classmates who were also motivated about the course… that it helped that you also ended up doing everything as there was always someone asking if you had done that exercise or what about this and should we attend the workshop today. When everyone else around you is enthusiastically participating you get enthusiastic yourself and do things.”
The task of selecting suitable ex-tempore exercises proved challenging and many students reported that there was not enough time to go through the exercises at the end of the session. We tried to solve this issue by selecting slightly shorter exercises after this opinion surfaced at a midpoint feedback gathering that was combined with the first partial exam. In hindsight, this problem might be better fixed by increasingly emphasizing the group aspects of ex-tempore problem solving, as the groups where the students worked actively together had less problems with timing. In terms of the difficulty of the exercises, student opinion varied widely with the majority saying that they were challenging enough.
It was our intention that the study groups formed in the ex-tempore exercises would carry over to the workshops, and thus make it easier for the students to study together. While this was true for some of the interviewed students, for others it was not the case. As student I5 noted, perhaps part of the reason for this failure was that the space reserved for the workshops was often times too crowded. This observation was corroborated by many students in both the interviews and feedback forms who also felt that one instructor was not able to help all students in a timely fashion. According to one interviewed student, this resulted in a tense and aggressive atmosphere. Thus it is not enough to just educate the instructors, one should also prepare a suitable learning environment with enough table groups and chairs for team work, and be ready to change these arrangements should the situation warrant it.
The introductory exercises divided student opinion much more than the ex-tempore ones. Many had had previous experience with the platform in other courses and while most retained neutral or positive attitudes, some had a lot of antipathy towards it. The more lenient and learning focused grading of the exercises, where mistakes and looking for hints were not punished as in previous courses, was seen as a good thing as evidenced by the quote below from student I10 when asked if her attitude towards the exercises had changed during the course:
“Well, I have to admit that originally I had a negative impression of the mastering chemistry exercises from the previous courses and I have never much cared for them. So probably in this course when they were more doable and they had changed the settings so that you wouldn't lose so much points in this course when answering incorrectly, which at some courses when you used a comma instead of a point you lost 30% of your points, which was really miserable. But in this course it was really nice which improved my attitude towards the program because the exercises were not so hard and I was not afraid of answering them.”
We feel that most of the criticism was actually directed at the platform itself while the quantitative nature of the introductory exercises was positively received. As illustrated in the quote above, a big part of the problem seemed to be that the operating language of the platform was English which in addition to issues of comprehension, led to some frustrating situations because of the differences in the handling of numbers between the languages (the Finnish language uses a comma as a decimal delimiter).
Because the problems were superficially like the written exercises of other physical chemistry courses, they did not get commented upon as much as the newer structures. As expected, several people in the interviews reported that most of the learning happened when solving the problems either in groups or alone. The workshops associated with the problems were mentioned by several students both in 2013 and in 2014 as integral for their learning. Particularly important seemed to be the questioning and discussion-oriented approach of the workshop instructor, where no ready solutions were given out and instead the instructor tried only to nudge the students in the right direction. The scoring system in which a different number of points were awarded for different tasks with bonus points being awarded for some tasks, divided student opinion. Some students thought that it had a clear motivating effect on their learning, while others felt it was unnecessarily complicated.
About half of the people interviewed mentioned that the physical chemistry courses had a bad reputation in the department and student I8 even openly stated that this predisposition had disrupted her motivation in the beginning of the course. When inquired about the origins of these predispositions student I4 replied:
“For me this predisposition had formed on the basis of what older students had said about the course… I feel that there were not so many of these people and that they were the ones who were only missing Thermodynamics from their bachelor's degree… I feel that for Thermodynamics quite a few of these were students were studying to be chemistry teachers… they were maybe a little older, too”
This is in line with the impressions we received when testing the interview form with a sample of older students, namely that the negative impressions originate from a loud minority of older peers who have not managed to pass the course in the past and are perhaps under an increased pressure to do so.
6 Summary and caveats
In this study, we attempted to develop an effective course and especially an exercise structure for a physical chemistry or physics thermodynamics course in light of the current research on thermodynamics education. We made use of three quantitative learning indicators: exercise points obtained by the students, exam results and the results of the pre- and post-CTTs to assess the effectiveness of the implemented changes in the exercise structure between 2013 and 2014 and the first two of these to estimate the impact of the changes in the lecture structure between 2012 and 2013. To obtain a deeper insight into the factors behind the success of our approach and the students' reactions to the course, feedback was gathered in electronic form in both years and in 2014 a series of 18 interviews was conducted.In the first development cycle there was a complete overhaul of the course lectures from the traditional teacher-centered approach of 2012 to the student-oriented structure of 2013 and 2014. The new structure, where instruction and activating learning tasks alternate as described in Section 4.1 and summarized in Fig. 2 clearly increased student learning. Based on the feedback in both 2013 and 2014 students felt that the lectures were the best part of the course and integral to their learning. Essential for this success was the use of active learning techniques such as discussion and voting tasks to probe for and challenge student alternative conceptions, and the incorporation of interactive elements into lecture examples and justifications. We found it helpful to pace the lecture in terms of course content rather than dividing the content according to a pre-established break structure determined either by traditional or student expectations. For about half of the lectures of ninety minutes duration, this resulted in two short breaks and three 25–35 minute modules, a structure that reportedly improved student concentration as well as making the material easier to absorb. However, the efficient implementation of this approach requires great flexibility on the part of the lecturer, as there is a risk of severely cutting the allocated break time as the module length unexpectedly increases due to spontaneous student questions or other circumstances.
In 2014, the second development cycle between 2013 and 2014 the changes in the course focused on the exercise structure. The new structure described in detail in Section 4.2 and summarized in Fig. 3 included three components: a set of introductory exercises featuring mostly qualitative exercises that served as an introduction to the next weeks' lectures, a set of ex-tempore exercises that were mostly small group work consisting of both quantitative and qualitative tasks, and a set of mostly quantitative and more challenging problems that the students could solve in workshops or on their own. We observed that this change in the exercise structure had a significant effect on student learning as seen both in the exam and CTT-scores. Particularly the learning of students of average ability was affected by the change. Furthermore, in the feedback gathered in 2014, the exercise structure was the second most frequently cited as the best thing in the course, with students pointing out its increasing effect on their learning, motivation and also satisfaction with the course.
In terms of the reliability of the findings, the use of multiple measuring instruments for learning outcomes in 2013 and 2014, and the application of qualitative data to peek behind the numbers, give credence to the claim that there was significant improvement in student learning between 2013 and 2014. As mentioned in Section 5.1, the changes in course objectives that accompanied the changes in lecture structure resulted in a substantial increase in the difficulty of the exams between 2012 and 2013. In spite of this there was a clear increase in the exam results, which underscores our claim that the new lecture structure increased student learning.
The rather low number of participants together with the relatively large variations in student gains raises some additional concerns about the reliability of the findings. On the other hand, as the number of students and the pre-CTT results were close in both 2013 and 2014, the sample demographics seem to be about the same, and it is unlikely that there has been significant variation in terms of sample starting knowledge or average student academic ability between the years. Furthermore, even though the standard deviations are relatively large for the quantitative measuring instruments, the effect sizes measured in terms of Cohen's d value indicate that the effects are significant. The complexity of the final course structure in 2014 also makes it challenging to assess which aspects of the improved course structure had the largest impacts on student learning as the number of different variables is large. This complexity also might make it difficult for other practitioners to directly implement this approach to their own courses. An additional problem associated with the study is that the CTT might have been in general too difficult for the students, as seen in the relatively low general scores of the pre-CTT. This directs more students to rely on guessing when answering the test, making it more difficult to draw conclusions. In the future, we will probably move to one of the more thoroughly tested conceptual inventories developed after the beginning of our study, such as the Thermodynamics Diagnostic Instrument (Sreenivasulu and Subramaniam, 2013) (THEDI), or the Thermodynamics Concept Inventory (Wren and Barbera, 2013; Wren and Barbera, 2014) (TCI), which better meet the standards for measurement in chemistry education research, as outlined by Arjoon et al. (2013). Finally, the dual role of the author as both course instructor and researcher is also a potential threat to the reliability of the research. As described in Section 3.4 many precautions were taken to overcome possible ethical concerns by, for example, distancing the instructor from the actual grading process as much as possible and carefully protecting the anonymity of the students for the feedback questionnaires and interviews while making sure that they were informed of the extent of their participation in this research project.
While the general results of this study are broadly applicable to any natural science courses where equations are used to communicate ideas and both quantitative and qualitative understanding is essential for mastery and challenging for students to achieve, many of the mathematical difficulties that we have touched in this study might not be relevant for physics majors, who at least in the University of Helsinki tend to possess a stronger grasp of mathematics. Most of the research cited in this paper has been conducted on university undergraduates either in chemistry or physics curriculum thermodynamics courses. Despite the difference in mathematical background, we feel that the differences between these two groups are small enough that the results obtained in one should be fairly well generalizable to the other, especially since also physics majors suffer from the problems of linking mathematical concepts to thermodynamic ones (Thompson et al., 2006; Bucy et al., 2007).
It should also be noted that in spite of all the changes implemented, according to Table 4 about one third of the students show no or only slight change in their CTT-scores, which, even in light of the difficulty of the CTT, suggests that more work remains to be done in terms of the course structure.
7 Conclusions and implications for teaching
Perhaps more than showing that a particular exercise structure results in improved learning, this study demonstrates the benefits of a multifaceted approach to both exercises and lecturing in subjects such as thermodynamics where both quantitative and qualitative understanding is essential for mastery and challenging for students to achieve. In terms of the lectures, the results of this study support the recent findings of Georgiou and Sharma (2015) that active learning improves both student experiences and actual learning in thermodynamics. The fact that their lectures employed interactive lecture demonstrations in contrast to the methods adopted in this study seems to indicate that many of the benefits of active learning are not specific to any particular approach but rather stem from the broader framework of principles and activities that are endemic to all of the active learning approaches.With regard to the kinds of problems and exercises instructors wish to incorporate into their courses, it is important to construct these so that both quantitative and qualitative components are present. In particular, the qualitative components should be designed so that they challenge alternative student conceptions. The progressive nature of the tasks is also important and one should move from less challenging exercises which cover the lower levels of the Revised Bloom's taxonomy, to more difficult problems that tap into the upper categories of the taxonomy and require higher order cognitive skills from the students. Simultaneously, the time reserved for the exercises should be scaled according to their difficulty.
The different types of tasks given to the students should cover a wide range of activities. These could include everything from solving equations, writing explanations and justifying conclusions to drawing molecular level depictions of the system and plotting or sketching graphs and diagrams. Particularly the easiest exercises tapping to the lowest categories of the Revised Bloom's taxonomy are ideal for incorporating introductory examples into the exercises before the topics are covered in the actual lectures. To make the activating exercises in the lectures as useful as possible, it is recommended that the students are at least somewhat familiar with the material beforehand. A third important component is the availability of assistance and support from both peers and instructors when engaging with course tasks. In truth, it is difficult to imagine a situation where there could be too much of such support available for the students, especially in light of the fact that many students enter physical chemistry courses such as Thermodynamics with negative preconceptions and low expectations of success, created in part by a loud minority of older students who have not managed to pass the course in the past. We think that it behooves the lecturer to address these attitudes at the earliest possibility before they turn into self-fulfilling prophesies. Finally, since timely feedback on performance is also important for the development of problem-solving skills, personal feedback should be available at least for some part of the exercises on a regular basis even though it requires a lot of resources on the part of the course-organizer. In the future, a similar kind of mixed method action research approach will be used to develop the course structure of a quantum chemistry course in the University of Helsinki, incorporating many of the lessons learned in this study.
Appendices
A Course content
The names of the modules covered in each lecture of the course in the autumn of 2014 are listed below. The names have been translated from Finnish into English. In 2012 and 2013 the same topics were covered, but of course especially between 2012 and 2013 the actual material changed greatly and in 2012 there were some topics that were left out from the 2013 and 2014 lectures. In addition to the items listed below the course started with an introductory module where the course structure, tasks and learning goals ware laid out and discussed with the students. The results and the correct solutions of the first partial exam were also discussed during one of the lectures at the middle of the course and the students were given the possibility of seeing their marked exams. A similar feedback session was also held after the course for the second partial exam.(1) The thermodynamical system
(2) Essential mathematical tools
(3) Zeroth law and the Boltzmann distribution
(4) Pressure and the ideal gas law
(5) Ideal gas law as a limiting law
(6) Equations of state for real gases
(7) Condensation and critical quantities
• First law of thermodynamics
(1) Work, heat and internal energy
(2) Internal energy and the first law
(3) Enthalpy and heat capacity
(4) Joule and Joule–Thomson experiments
(5) Examples of the first law and the gas laws
(6) A review on enthalpy, internal energy, work and heat
(7) Standard enthalpy of formation
(8) Temperature dependence of reaction enthalpies and the measurement of ΔU and ΔH
(9) Discussion questions on the first law
• Second and third laws of thermodynamics
(1) Spontaneous processes and the second law
(2) Entropy
(3) The statistical view of entropy
(4) Entropy change in practical processes
(5) Third law entropies
(6) Application of standard entropies
(1) Gibbs and Helmholtz energies
(2) Maxwell relations
(3) Applications of Maxwell relations
(4) Standard reaction Gibbs energies
(5) Temperature and pressure dependence of the Gibbs energy
• Physical transformations of pure substances
(1) Gibbs phase rule
(2) Basic properties of phase diagrams
(3) Typical phase diagrams
(4) Prediction of phase boundaries
• Simple mixtures
(1) Partial molar quantities
(2) Gibbs and Duhem equation
(3) Ideal and ideally dilute solutions
(4) Activity and the activity coefficient
(5) Colligative properties
(6) Non-ideality of electrolyte solutions
(7) Two component phase diagrams
• Material equilibrium
(1) Chemical potential and reaction equilibrium
(2) Microscopic view on equilibrium
(3) Pressure dependence of equilibrium
(4) Temperature dependence of equilibrium
B Examples of course tasks
Below are given several examples of both ex-tempore exercises and the problems of the course translated from Finnish into English. The classification of each task in terms of Bloom's taxonomy is indicated in red letters inside parentheses. The letter k refers to the knowledge dimension and the letter c to the cognitive process dimension in the taxonomy. In the knowledge dimension the different subcategories have been numbered as (1) factual, (2) conceptual, (3) procedural, (4) metacognitive. In the cognitive process category the subcategories have been numbered as (1) remember, (2) understand, (3) apply, (4) analyze, (5) evaluate, and (6) create. So, for example, (k2c4) would correspond to knowledge category: conceptual knowledge, cognitive process category: analyze. A similar classification of chemistry Matriculation Examination problems has been performed by Tikkanen and Aksela (2012) and more detailed information about the classification process can be found therein. A slightly modified pictorial representation of the revised Bloom's taxonomy from an original depiction by Rex Heer is shown in Fig. 5.¶Example exercise I. A plastic syringe is equipped with a frictionless piston of mass M and area AM as shown in the figure on the right. The lower end of the piston has been closed and the piston contains argon gas. In state A the syringe has been placed in a container with a water and ice mixture and has been allowed to reach thermal equilibrium with the mixture. In state B the syringe has been moved to a container with boiling water and has been again allowed to reach thermal equilibrium.
(a) Name and draw the forces affecting the piston in the states A and B. Pay special attention to the relative sizes of the forces. What is the pressure inside the piston in A and B? (k2c4)
(b) Using the ideal gas equation derive an expression for the distance of the piston from the bottom of the syringe as a function of temperature. What happens to the pressure, volume and temperature inside the syringe when moving from state A to state B? (k3c3)
(c) How will the results of (a) and (b) change if the syringe contains nitrogen gas instead of argon? (k2c4)
(d) Draw or describe what is happening on the molecular level in the syringe when moving from state A to state B. (k2c4)
(This ex-tempore exercise is based on the articles by Kautz et al. (2005) and Madden et al. (2011).)
Example exercise II. We are investigating what happens when 0.5 g of metallic magnesium is dropped in a HCl solution in a closed diathermic container with air inside when either A the pressure is kept constant or B the volume is kept constant. At the beginning of the reaction the pressure inside the container is the same as in the outside laboratory i.e. 1 bar. The temperature of the laboratory is 298 K. Only expansion work occurs in the processes.
(a) Make a sketch of a possible experimental setup in both A and B and write the chemical equation for the reaction. (k3c3)
(b) In which case A or B is more heat released to the environment and why? (k2c4)
(c) Is ΔH = q in either case? (k1c2)
(d) Using the formation enthalpies in Atkins' book, calculate the standard reaction enthalpy ΔrH°. (k2c3)
(e) Calculate q, w, ΔU and ΔH of process A. You can assume that the following relation holds ΔrH° = ΔH. (k2c3)
(f) How does the work done on the surroundings change if we look at process B instead of A? (k2c4)
(This ex-tempore exercise is based on two articles by Nilsson and Niedderer (2012, 2014).)
Example problem 1. The following data has been obtained for the density of a certain hydrocarbon at different pressures at 273.15 K:
| p/kPa 25.33 50.66 75.99 101.3 |
| ρ/kg m−3 0.29123 0.58410 0.87855 1.1747 |
(a) Determine the molar mass of the hydrocarbon in two different ways from the above data. At least one of your chosen methods must account for the fact that the measurements have been performed on a real gas. What is the hydrocarbon in question? (3 points) (k3c4)
(b) Calculate the compression factor for the gas at the given pressures. What can you say about the intermolecular interactions in the gas based on you results? (2 points) (k2c3)
(c) Compare the molar masses you obtained in (a) with each other. How would the experimental setup have to be changed so that the less accurate of the two methods you chose for (a) would give better results? (1 point) (k3c5)
(This task was worth 6 points. It has been heavily modified from an original problem in Levine's Physical Chemistry (Levine, 2008).)
Example problem 2. EDTA (ethylenediaminetetraaceticacid) is one of the most typical complexating ligands used in industrial applications. We want to determine the standard formation enthalpy of EDTA when its enthalpy of combustion is known from the experiments. Write down the combustion reaction and find the standard combustion enthalpy from the literature. Using this value calculate the standard formation enthalpy of EDTA and compare it with literature values. Can you explain the possible difference in the results? To find the standard combustion enthalpy you can use for example the NIST Chemistry Webbook webpage. Remember to cite your sources! (k3c4).
Example problem 3. The functioning of a car engine can be modeled with the so-called Otto-cycle. A simplified version of the cycle contains the following stages:
| A → B | Reversible adiabatic compression. |
| B → C | Isochoric and reversible pressure increase due to combustion of fuel |
| C → D | Reversible adiabatic expansion. |
| D → A | Isochoric and reversible pressure decrease. |
Modelling air with a diatomic ideal gas, let us look at the Otto-cycle in this case when the following information is known:
 | (1) |
(a) What are the values of CV,m and Cp,m for a diatomic ideal gas when molecular vibrations can be neglected? Justify your answer briefly. (0.5 points) (k2c2)
(b) Sketch the described Otto-cycle in (V,p)-coordinate system and calculate the values of V, T, and p at A, B, C, and D. (1.5 points) (k3c4)
(c) What can be said about q, w, ΔU, ΔH, ΔS, ΔSsur and ΔStot for the whole cycle just based on the figure you drew? (1 point) (k2c4)
(d) Calculate q, w, ΔU, ΔH, ΔS, ΔSsur, and ΔStot for the processes A → B, B → C, C → D and D → A. Calculate the corresponding values for the whole cycle. (3 points) (k3c4)
(This task was worth 6 points. It has been modified from an original problem in Atkins' Physical Chemistry (Atkins and de Paula, 2010).)
C The conceptual thermodynamics test
Below is the Conceptual thermodynamics test used in this research and the short introduction provided at the beginning of the test. All the questions have been translated from Finnish into English and some of them are based partly on the instruments used to assess student alternative conceptions in the literature (Meltzer, 2004). In addition to the introductory text in the CTT, the Course instructor took some time in the lectures to explain why student responses to the CTT were valuable: Besides providing data for the research, the answers also gave the lecturer an idea of which parts of the course material were challenging to the students and which parts were already well understood, making it possible to prepare the lectures accordingly. It also provided the students with a review of which skills in the General chemistry courses were relevant in the context of thermodynamics.The questions in Group I were added in 2014 and their results have thus not been included in the actual study when comparing the years 2013 and 2014. These questions are based on the items in the Conceptual Survey in Thermodynamics by Wattanakasiwich et al. (2013). The reason for their inclusion in 2014 was to provide the lecturer with a sense of how familiar the students were with phase transition processes on the basis of the first year courses. Thus the CTT in this study practically consisted of a total of 29 questions organized into five groups.
Group I. (1) Cup A contains 100 g of water and cup B contains 300 g of water. The water in both cups was initially at room temperature. Then the water in cup A was heated until its temperature becomes 75 °C and the water in cup B was heated until its temperature becomes 50 °C. When the water in both cups cooled down to room temperature which cup has had more heat transferred from it?
(a) Cup A had more heat transferred out.
(b) Cup B had more heat transferred out.
(c) Both cups had the same amount of heat transferred.
(d) Not enough information is given to determine the answer.
(e) I don't know.
(2) Lauri thinks that in order to make tea one has to use boiling water. He tells his friends that “I could not make tea if I was camping on a high mountain because water doesnt boil at high altitudes.” Which statement do you strongly agree with
(a) Markus says, “Yes it does, because water boils below 100 °C due to the decreased pressure.”
(b) Minna says, “Lauri is incorrect because water always boils at the same temperature.”
(c) Mika says, “The boiling point of the water decreases but the water itself is still at 100 °C.”
(d) Matti says, “I agree with Lauri. The water never gets to its boiling point”
(e) I don't know.
(3) A sample of 100 g of ice at 0 °C and another of 100 g of liquid water at 0 °C are put into a freezer which has a temperature below 0 °C. After waiting until the temperatures of the samples equal the freezer temperature what can you say about the heat released by the water and the ice?
(a) The ice has released more heat.
(b) The water has released more heat.
(c) Both have released equal amounts of heat because the final temperature is the same.
(d) There is no solution because ice doesn't contain heat.
(e) There is no solution because water can't exist at 0 °C.
(f) I don't know.
Group II. One mole of argon gas is slowly compressed to half of its original volume at constant temperature in the system shown in the figure to the right.
(1) What happens to the pressure of the gas?
(a) The pressure doesn't change.
(b) The pressure halves.
(c) The pressure doubles.
(d) I don't know.
(2) What can you say of the work done in the process?
(a) The system does work on the surroundings.
(b) The surroundings do work on the system.
(c) No work is done.
(d) I don't know.
(3) What can you say about the heat exchanged in the process?
(a) There is no heat exchange.
(b) Heat is transferred from the system to the surroundings.
(c) Heat is transferred from the surroundings to the system.
(d) I don't know.
(4) What happens to the kinetic energy of the gas?
(a) It increases.
(b) It decreases.
(c) It doesn't change.
(d) I don't know.
Group III. A sample of ideal gas is taken through the cyclic process shown on the right. In the subprocess c → a the temperature of the gas stays constant. You can assume the processes are happening slowly so that the system is always at equilibrium.
What can you say about the values of the following properties for the cyclic process?
| >0 | =0 | <0 | I don't know. | |
|---|---|---|---|---|
| (a) Temperature change | ||||
| (b) Work done on the system | ||||
| (c) Heat transferred into the system | ||||
| (d) Internal energy change | ||||
| (e) Enthalpy change | ||||
| (f) Gibbs free energy change |
Group IV. (1) What can you say about the values of Gibbs free energy, enthalpy and entropy changes for a process where one litre of water freezes at 1 atm and 273 K?
| >0 | =0 | <0 | More information required | I don't know. | |
|---|---|---|---|---|---|
| ΔG | |||||
| ΔH | |||||
| ΔS |
(2) What can you say about the values of Gibbs free energy, enthalpy and entropy changes if the freezing occurred at the same pressure but at 276 K?
| >0 | =0 | <0 | More information required | I don't know. | |
|---|---|---|---|---|---|
| ΔG | |||||
| ΔH | |||||
| ΔS |
(3) What can you say about the signs of Gibbs free energy, enthalpy and entropy changes if the freezing occurred at the same pressure but at 270 K?
| >0 | =0 | <0 | More information required | I don't know. | |
|---|---|---|---|---|---|
| ΔG | |||||
| ΔH | |||||
| ΔS |
Group V. The dissociation reaction of phosphorus pentachloride into chlorine and phosphorus trichloride is
| PCl5(g) ⇌ PCl3(g) + Cl2(g) | (2) |
• ΔfH°[PCl5(g)] = −374.9 kJ mol−1
• ΔfH°[PCl3(g)] = −287.0 kJ mol−1.
(1) The dissociation of phosphorus pentachloride is an
(a) exothermic reaction.
(b) endothermic reaction.
(c) I don't know.
(2) Where does the equilibrium of the reaction shift if temperature is lowered at constant pressure?
(a) It shifts towards the products.
(b) It shifts towards the reactants.
(c) There is no shift.
(d) I don't know.
(3) Where does the equilibrium of the reaction shift if volume is decreased at constant temperature?
(a) It shifts towards the products.
(b) It shifts towards the reactants.
(c) There is no shift.
(d) I don't know.
(4) Where does the equilibrium of the reaction shift if part of the pentachloride is removed at constant volume?
(a) It shifts towards the products.
(b) It shifts towards the reactants.
(c) There is no shift.
(d) I don't know.
(5) Where does the equilibrium of the reaction shift if helium gas is added at constant temperature and pressure?
(a) It shifts towards the products.
(b) It shifts towards the reactants.
(c) There is no shift.
(d) I don't know.
(6) Where does the equilibrium of the reaction shift if helium gas is added at constant temperature and volume?
(a) It shifts towards the products.
(b) It shifts towards the reactants.
(c) There is no shift.
(d) I don't know.
Group VI.
| Are the following statements true or false? | True | False | I don't know |
|---|---|---|---|
| 1. The transformation of a tidy room into a disordered one is | |||
| an example of the second law of thermodynamics in action. | |||
| 2. The first law is a statement about the conservation of energy | |||
| 3. The entropy of the system increases in spontaneous processes | |||
| 4. The reaction Gibbs energy decreases in spontaneous processes in constant temperature and pressure. |
Acknowledgements
I would like to thank Prof. Sari Lindblom-Ylänne for many suggestions and comments, and for instruction in the use of the SPSS program. I thank Dr Juha Taina for conducting half of the interviews in this study and for invaluable suggestions, Dr Anne-Maria Ernwall-Hytönen for conducting the other half of the interviews in this study, and Prof. Maija Aksela for her insightful comments on the manuscript. I also thank the supervisor of my PhD work Prof. Lauri Halonen for the possibility to lecture Thermodynamics at the University of Helsinki and the LASKEMO doctoral school and the Jenny and Antti Wihuri Foundation for the funding of my PhD work. Finally, I am grateful to Pau Rodríguez Ruiz for loving support.References
- Al-Balushi S. M., (2009), Factors influencing pre-service science teachers' imagination at the microscopic level in chemistry, Int. J. Sci. Math. Educ., 7, 1089–1110.
- Alexopoulou E. and Driver R., (1996), Small-group discussion in physics: peer interaction modes in pairs and fours, J. Res. Sci. Teach., 33, 1099–1114.
- Airasian P. W., Cruikshank K. A., Mayer R. E., Pintrich P. R., Raths J. and Wittrock M. C., (2001), in Anderson L. W. and Krathwohl D. R. (ed.), A taxonomy for learning, teaching and assessing: a revision of Bloom's taxonomy of educational objectives, New York: Longman.
- Arjoon J. A., Xu X. and Lewis J. E., (2013), Understanding the state of the art for measurement in chemistry education research: examining the psychometric evidence, J. Chem. Educ., 90, 536–545.
- Atkins P. and de Paula J., (2010), Atkins' Physical Chemistry, 9th edn, Oxford, New York: Oxford University Press.
- Azizoğlu N., Alkan M. and Geban Ö., (2006), Undergraduate pre-service teachers' understandings and misconceptions of phase equilibrium, J. Chem. Educ., 83, 947–953.
- Bain K., Moon A., Mack M. R. and Towns M. H., (2014), A review of research on the teaching and learning of thermodynamics at the university level, Chem. Educ. Res. Pract., 15, 320–335.
- Banerjee A. C., (1995), Teaching chemical equilibrium and thermodynamics in undergraduate general chemistry classes, J. Chem. Educ., 72, 879–881.
- Becker N. and Towns M., (2012), Students' understanding of mathematical expressions in physical chemistry contexts: an analysis using Sherin's symbolic forms, Chem. Educ. Res. Pract., 13, 209–220.
- Becker N., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R., (2013), Reasoning using particulate nature of matter: an example of a sociochemical norm in a university-level physical chemistry class, Chem. Educ. Res. Pract., 14, 81–94.
- Biggs J., (1996), Enhancing teaching through constructive alignment, High. Educ., 32, 347–364.
- Bloom B. S., (1956), Taxonomy of educational objectives, Ann Arbor, Michigan: Edwards Bros.
- Bodner G. M. and Domin D. S., (2000), Mental models: the role of representations in problem solving in chemistry, Univ. Chem. Educ., 4, 24–30.
- Boudreaux A. and Campbell C., (2012), Student understanding of liquid-vapor phase equilibrium, J. Chem. Educ., 89, 707–714.
- Bransford J. D., Brown A. L. and Cocking R. R., (ed.) (2000), How people learn: brain, mind, experience and school (expanded ed.), Washington DC: National Academy Press.
- Bucy B. R., Thompson J. R. and Mountcastle D. B., (2007), Student (mis)application of partial differentiation to material properties, AIP Conf. Proc., 883, 157–160.
- Bunce D. M., Flens E. A. and Neiles K. Y., (2010), How long can students pay attention in class? A study of student attention decline using clickers, J. Chem. Educ., 87, 1438–1443.
- Carson E. M. and Watson J. R., (1999), Undergraduate students' understanding of enthalpy change, Univ. Chem. Educ., 3, 46–51.
- Carson E. M. and Watson J. R., (2002), Undergraduate students' understandings of entropy and gibbs free energy, Univ. Chem. Educ., 6, 4–12.
- Christensen W. M., Meltzer D. E. and Ogilvie C. A., (2009), Student ideas regarding entropy and the second law of thermodynamics in an introductory physics course, Am. J. Phys., 77, 907–917.
- Cohen J., (1988), Statistical power analysis for the behavioral sciences, Hillside, New Jersey: Lawrence Erlbaum associates.
- Crouch C. H. and Mazur E., (2001), Peer instruction: ten years of experience and results, Am. J. Phys., 69, 970–977.
- de Raadt M., (2012), Student created cheat-sheets in examinations: impact on student outcomes. Proceedings of the Fourteenth Australian Computing Education Conference (ACE2012), CRPIT123, 71–76.
- Dobson J. L., (2008), The use of formative online quizzes to enhance class preparation and scores on summative exams, Adv. Physiol. Educ., 32, 297–302.
- Dreyfus B. W., Geller B. D., Meltzer D. E. and Sawtelle V., (2015), Resource letter TTSM-1: teaching thermodynamics and statistical mechanics in introductory physics, chemistry, and biology, Am. J. Phys., 83, 5–21.
- Elo S. and Kyngäs H., (2008), The qualitative content analysis process, J. Adv. Nurs., 62, 107–115.
- Engel T. and Reid P., (2014), Physical Chemistry, 3rd edn, Harlow, UK: Pearson Education Limited.
- Erbe B., (2007), Reducing test anxiety while increasing learning: the cheat sheet, Coll. Teach., 55, 96–97.
- Gabel D. L., Samuel K. V. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 64, 695–697.
- Georgiou, H. and Sharma, M. D., (2015), Does using active learning in thermodynamics improve students' conceptual understanding and learning experiences, Eur. J. Phys., 36, 015020.
- Greenbowe T. J. and Meltzer D. E., (2003), Student learning of thermochemical concepts in the context of solution calorimetry, Int. J. Sci. Educ., 25, 779–800.
- Hadfield L. C. and Wieman C. E., (2010), Student interpretations of equations related to the first law of thermodynamics, J. Chem. Educ., 87, 750–755.
- Hadwin A. and Oshige M., (2011), Self-regulation, coregulation and socially shared regulation: exploring perspectives of social in self-regulated learning theory, Teach. Coll. Rec., 113, 240–264.
- Hahn K. E. and Polik W. F., (2004), Factors influencing success in physical chemistry, J. Chem. Educ., 81, 567–572.
- Heller P., Keith R. and Anderson S., (1992), Teaching problem solving through cooperative grouping part 1: group versus individual problem solving, Am. J. Phys., 60, 627–636.
- Hernández G. E., Criswell B. A., Kirk N. J., Sauder D. G. and Rushton G. T., (2014), Pushing for particulate level models of adiabatic and isothermal processes in upper-level chemistry courses: a qualitative study, Chem. Educ. Res. Pract., 15, 354–365.
- Hewstone M., Stroebe W. and Jonas K., (ed.) (2010), Introduction to Social Psychology, 4th edn, Malden, Massachusetts: Blackwell Publishing Ltd.
- Jasien P. G. and Oberem G. E., (2002), Understanding of elementary concepts in heat and temperature among college students and k-12 teachers, J. Chem. Educ., 79, 889–895.
- Johnson M., (2001), Facilitating high quality student practice in introductory physics, Phys. Educ. Res. Am. J. Phys. Suppl., 69, 1–11.
- Johnson D. W. and Johnson R. T., (1999), Making cooperative learning work, Theor. Pract., 38, 67–73.
- Johnson B. C. and Kiviniemi M. T., (2009), The effect of online chapter quizzes on exam performance in an undergraduate social psychology course, Teach. Psychol., 36, 33–37.
- Johnson B. R., Onwuegbuzie A. J. and Turner L. A., (2007), Toward a definition of mixed methods research, J. Mix. Methods Res., 1, 112–133.
- Kautz C. H., Heron P. R. L., Loverude M. E. and McDermott L. C., (2005), Student understanding of the ideal gas law, part I: a macroscopic perspective, Am. J. Phys., 73, 1055–1063.
- Kautz C. H., Heron P. R. L., Shaffer P. S. and McDermott L. C., (2005), Student understanding of the ideal gas law, part II: a microscopic perspective, Am. J. Phys., 73, 1064–1071.
- Kim E. and Pak S., (2002), Students do not overcome conceptual difficulties after solving 1000 traditional problems, Am. J. Phys., 70, 759–765.
- Knight J. K. and Wood W. B., (2005), Teaching more by lecturing less, Cell Biol. Educ., 4, 298–310.
- Lasry N., Mazur E. and Watkins J., (2008), Peer instruction: from harvard to the two-year college, Am. J. Phys., 76, 1066–1069.
- Lawson D. and Henderson B. B., (2015), The costs of texting in the classroom, Coll. Teach., 63, 119–124.
- Levine I. N., (2008), Physical Chemistry, 6th edn, New York: McGraw-Hill Higher Education.
- Lindblom-Ylänne S. and Nevgi A., (ed.) (2009), Yliopisto-opettajan käsikirja, Helsinki: WSOY.
- Loverude M. E., Kautz C. H. and Heron P. R. L., (2002), Student understanding of the first law of thermodynamics: relating work to the adiabatic compression of an ideal gas, Am. J. Phys., 70, 137–148.
- Lyon D. C. and Lagowski J. J., (2008), Effectiveness of facilitating small-group learning in large lecture classes, J. Chem. Educ., 85, 1571–1576.
- Madden S. P., Jones L. L. and Rahm J., (2011), The role of multiple representations in the understanding of ideal gas problems, Chem. Educ. Res. Pract., 12, 283–293.
- Maskiewicz A. C. and Lineback J. E., (2013), Misconceptions are “so yesterday!”, CBE Life Sci. Educ., 12, 352–356.
- Mazur E., (1997), Peer Instruction: A User's Manual, New Jersey: Prentice Hall.
- Mellema S., (2001), A physics lecture for the 21st century, Phys. Educ., 36, 306–311.
- Meltzer D. E., (2004), Investigation of students' reasoning regarding heat, work, and the first law of thermodynamics in an introductory calculus-based general physics course, Am. J. Phys., 72, 1432–1446.
- Meltzer D. E., (2006), Investigation of student learning in thermodynamics and implications for instruction in chemistry and engineering, Physics Education Research Conference Proceedings, Syracuse, NY.
- Meltzer D. and Mannivannan K., (2002), Transforming the lecture-hall environment: the fully interactive physics lecture, Am. J. Phys., 70, 639–654.
- Miller R. L., Streveler R. A., Nelson M. A., Geist M. R. and Olds B. M., (2005), Concept inventories meet cognitive psychology: using beta testing as a mechanism for identifying engineering student misconceptions, Proceedings of the American Society for Engineering Education Annual Conference (electronic), Portland, OR.
- Miller R. L., Streveler R. A., Olds B. M., Chi M. T. H., Nelson M. A. and Geist M. R., (2006), Misconceptions about rate processes: preliminary evidence for the importance of emergent conceptual schemas in thermal and transport sciences, Proceedings of the American Society for Engineering Education Annual Conference (electronic), Chicago, IL.
- Miller C. J., McNear J. and Metz M. J., (2013), A comparison of traditional and engaging lecture methods in a large, professional-level course, Adv. Physiol. Educ., 37, 347–355.
- Moravec M., Williams A., Aguilar-Roca N. and O'Dowd D. K., (2010), Learn before lecture: a strategy that improves learning outcomes in a large introductory biology class, CBE Life Sci. Educ., 9, 473–481.
- Nicoll G. and Francisco J. S., (2001), An investigation of the factors influencing student performance in physical chemistry, J. Chem. Educ., 78, 99–102.
- Nilsson T. and Niedderer H., (2012), An analytical tool to determine undergraduate students' use of volume and pressure when describing expansion work and technical work, Chem. Educ. Res. Pract., 13, 348–356.
- Nilsson T. and Niedderer H., (2014), Undergraduate students' conceptions of enthalpy, enthalpy change and related concepts, Chem. Educ. Res. Pract., 15, 336–353.
- Nottis K. E. K., Prince M. J. and Vigeant M. A., (2010), Building an understanding of heat transfer concepts in undergraduate chemical engineering courses, U.S. China. Educ. Rev., 7(2), 1–9.
- Novick S. and Nussbaum J., (1981), Pupils' understanding of the particulate nature of matter: a cross-age study, Sci. Educ., 65, 187–196.
- Patton M. Q., (2001), Qualitative Research & Evaluation Methods, 3rd edn, Thousand Oaks, California: Sage Publications Inc.
- Phillips D. C., (ed.) (2000), Constructivism in Education: Opinions and Second Opinions on Controversial Issues. Ninety-Ninth Yearbook of the National Society for the Study of Education, Chicago: University of Chicago Press.
- Podolefsky N. S., Perkins K. K. and Adams W. K., (2010), Factors promoting engaged exploration with computer simulations, Phys. Rev. Spec. Top. - Phys. Educ. Res., 6, 020117.
- Prince M., (2004), Does active learning work? A review of the research, J. Eng. Educ., 93, 223–231.
- Räisänen M., Postareff L. and Lindblom-Ylänne S., (2016), University students' self- and co-regulation of learning and processes of understanding: a person-oriented approach, Learn. Individ. Differ., 47, 281–288.
- Ralle B. and Eilks I., (ed.) (2002), Participatory action research within chemical education, in Research in chemical education – What does this mean? Aachen, Germany: Shaker, pp. 87–98.
- Scott L. U. and Heller P., (1991), Team work works! Strategies for integrating women and minorities into the physical sciences, Sci. Teach., 58, 24–28.
- Smith D. K., (2006), Use of the mid-lecture break in chemistry teaching: a survey and some suggestions, J. Chem. Educ., 83, 1621–1624.
- Smith T. I., Christensen W. M. and Thompson J. R., (2009a), Addressing student difficulties with concepts related to entropy, heat engines, and the carnot cycle, AIP Conf. Proc., 1179, 277–281.
- Smith M. K., Wood W. B., Adams W. K., Wieman C., Knight J. K., Guild N. and Su T. T., (2009b), Why peer discussion improves student performance on in-class concept questions, Science, 323, 122–124.
- Smith M. K., Wood W. B., Krauter K. and Knight J. K., (2011), Combining peer discussion with instructor explanation increases student learning from in-class conceptual questions, CBE Life Sci. Educ., 10, 55–63.
- Sözbilir M., (2002), Turkish chemistry undergraduate students' misunderstandings of Gibbs free energy, Univ. Chem. Educ., 6, 73–83.
- Sözbilir M., (2004), What makes physical chemistry difficult? Perceptions of Turkish chemistry undergraduates and lecturers, J. Chem. Educ., 81, 573–578.
- Sözbilir M. and Bennett J. M., (2007), A study of Turkish chemistry undergraduates' understandings of entropy, J. Chem. Educ., 84, 1204–1208.
- Sözbilir M., Pinarbaşi T. and Canpolat N., (2010), Prospective chemistry teachers' conceptions of chemical thermodynamics and kinetics, Eur. J. Math. Sci. Technol. Educ., 6, 111–120.
- Springer L., Stanne M. E. and Donovan S. S., (1999), Effects of small-group learning on undergraduates in science, mathematics engineering and technology: a meta-analysis, Rev. Educ. Res., 69, 21–51.
- Sreenivasulu B. and Subramaniam R., (2013), University students' understanding of chemical thermodynamics, Int. J. Sci. Educ., 35, 601–635.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15, 109–113.
- Thomas P. L. and Schwenz R. W., (1998), College physical chemistry students' conceptions of equilibrium and fundamental thermodynamics, J. Res. Sci. Teach., 35, 1151–1160.
- Thompson J. R., Bucy B. R. and Mountcastle D. B., (2006), Assessing student understanding of partial derivatives in thermodynamics, Proceedings of the 2005 Physics Education Research Conference of the American Institute of Physics.
- Tikkanen G. and Aksela M., (2012), Analysis of Finnish chemistry matriculation examination questions according to cognitive complexity, Nord. Stud. Sci. Educ., 8, 258–268.
- Towns M. H. and Grant E. R., (1997), “I believe I will go out of this class actually knowing something”: cooperative learning activities in physical chemistry, J. Res. Sci. Teach., 34, 819–835.
- Tripp D., (2005), Action research: a methodological introduction, Educ. Pesqui., 31, 443–466.
- Tsaparlis G., (2007), Teaching and learning physical chemistry: a review of educational research, in Advances in teaching physical chemistry, ACS symposium series, vol. 973, Washington, DC: American Chemical Society, pp. 75–112.
- Turányi T. and Tóth Z., (2013), Hungarian university students' misunderstandings in thermodynamics and chemical kinetics, Chem. Educ. Res. Pract., 14, 105–116.
- Turpen C. and Finkelstein N. D., (2009), Not all interactive engagement is the same: variations in physics professors' implementation of peer instruction, Phys. Rev. Spec. Top. Phys. Educ. Res., 5, 020101.
- van Roon P. H., van Sprang H. F. and Verdonk A. H., (1994), Work and ‘heat’: on a road towards thermodynamics, Int. J. Sci. Educ., 16, 131–144.
- Wattanakasiwich P., Taleab P., Sharma M. D. and Johnston I. D., (2013), Development and implementation of a conceptual survey in thermodynamics, Int. J. Innov. Sci. Math. Educ., 21, 29–53.
- Wieman C., Adams W., Loeblein P. and Perkins K., (2010), Teaching physics using PhET simulations, Phys. Teach., 48, 225–227.
- Wilson K. and Korn J. H., (2007), Attention during lectures: beyond ten minutes, Teach. Psychol., 34, 85–89.
- Womack S. T., (1997), What action research is: a review of the literature, ERIC Clearinghouse on Teacher Education. ERIC Document Reproduction Service, ED 414 255, Washington, DC.
- Wren D. and Barbera J., (2013), Gathering evidence for validity during the design, development, and qualitative evaluation of thermochemistry concept inventory items, J. Chem. Educ., 90, 1590–1601.
- Wren D. and Barbera J., (2014), Psychometric analysis of the thermochemistry concept inventory, Chem. Educ. Res. Pract., 15, 380–390.
- Zeni J., (1998), A guide to ethical issues and action research, Educ. Act. Res., 6, 9–19.
Footnotes |
| † Moodle, http://https://moodle.org/, accessed 20.8.2015. |
| ‡ IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. |
| § Mastering Chemistry, http://www.pearsonmylabandmastering.com/northamerica/masteringchemistry/, accessed 20.8.2015 |
| ¶ Rex Heer, 2009, http://www.celt.iastate.edu/teaching-resources/effective-practice/revised-blooms-taxonomy/, viewed 20.8.2015. |
| This journal is © The Royal Society of Chemistry 2016 |