An inquiry-based approach of traditional ‘step-by-step’ experiments
L.
Szalay
*a and
Z.
Tóth
b
aEötvös Loránd University, Faculty of Science, Institute of Chemistry, Pázmány Péter sétány 1/A, H-1117 Budapest, Hungary. E-mail: luca@chem.elte.hu
bUniversity of Debrecen, Faculty of Science and Technology, Department of Inorganic and Analytical Chemistry, Egyetem tér 1., H-4032 Debrecen, Hungary
First published on 4th August 2016
Abstract
This is the start of a road map for the effective introduction of inquiry-based learning in chemistry. Advantages of inquiry-based approaches to the development of scientific literacy are widely discussed in the literature. However, unless chemistry educators take account of teachers' reservations and identified disadvantages such approaches will never have the place they deserve in the everyday teaching of chemistry. If circumstances do not allow for complicated and open-ended inquiry tasks, simpler and more structured inquiry-based tasks may be used. As a first step, teachers could be asked to modify and adapt established ‘step-by-step’ instructions to practical activities which require some stages to be designed by the students. If this happens only a few times in a school year the question arises about its effectiveness to develop experimental design skills and to reinforce knowledge and ideas taught in chemistry lessons. The present study describes the results of an empirical research project aimed to finding the answer. Modification of step-by-step practical activities as described requires limited time and effort, yet the results suggest that many students benefit from this approach.
Introduction
Possible advantages and disadvantages of inquiry-based science education (IBSE)
Inquiry-based science education, as interpreted by the National Research Council of the United States of America in the Inquiry and the National Science Education Standards (Olson and Loucks-Horsley, 2000), is a complex process. It uses activities that involve not just planning investigations and using tools to gather, analyse, and interpret data, but also to pose questions, collecting information related to the problem, proposing answers and communicating the result. The full process models the way scientists work. Therefore, it is a long road to get there and the best approach might be to take small steps one at a time.Advantages and disadvantages of inquiry-based approaches have been discussed widely in the literature. Researchers supporting IBSE argue that teaching strategies which actively engage students in the learning process (applying active thinking and drawing conclusions from data) are more likely to increase conceptual understanding (Minner et al., 2010) and develop higher order cognitive skills (Tomperi and Aksela, 2014) than strategies which rely on more passive techniques. They also increase motivation, at least among ‘curious’ and ‘socially motivated’ students (Hofstein and Kempa, 1985). It can reasonably be expected that applying IBSE could help to develop a better understanding of the nature of science, the importance of collaboration and communication in science and the difference between science and pseudoscience (Finlayson et al., 2015).
Kirschner et al. (2006), however, warn that minimally guided instructions might be less effective and less efficient than instructional approaches which place a strong emphasis on the guidance of students' learning. They add that there is evidence also that it may have negative results, with students developing misconceptions or incomplete or disorganized knowledge. Sweller (1988) emphasises that inquiry approaches can be less effective than traditional approaches such as direct instructions if the cognitive load placed on students is not properly managed. Another possible disadvantage is that IBSE is disliked by ‘achiever’ and ‘conscientious’ students (Bolte et al., 2013). Finally, there are always students who feel uncomfortable when they are asked to plan their experiments (Cheung, 2011). As an answer to the doubts described above, Hmelo-Silver et al. (2007) stress that, in addition to learning content, developing ‘softer skills’ such as epistemic practices, self-directed learning and collaboration are important learning outcomes. According to them, it is valuable to investigate in which circumstances guided inquiry approaches work, the kinds of desired outcomes for which they are effective, the kinds of valued practices they promote, and the kinds of support and scaffolding needed for different populations and learning goals. It is also worth citing Taber's (2011) critique of Kirschner et al.'s assertion that IBSE – generally identified as a constructivist pedagogy – ignores the “architecture of cognition”. He states that “constructivist pedagogy in science education has long put stress on the way the cognitive system constrains and facilitates learning (Osborne and Wittrock 1983), so for example, teachers need to see student learning as usually an incremental process, and so plan teaching in terms of suitable, regularly reinforced, learning quanta”. Taber also points out that constructivist approaches do not aim to set students the task of rediscovering “through unguided exploration … the cultural capital of modern science in a series of laboratory sessions” as this would be unrealistic. Hence, he disputes Kirschner et al.'s view on constructivism as a new way of describing discovery learning.
Research problem
The 2006 PISA analysis suggests that Hungarian students should develop a wider range of scientific skills such as devising scientific investigations and evaluating data gathered (PISA 2006). Since inquiry has been advocated as an effective pedagogical strategy for promoting deep conceptual understanding and more sophisticated scientific thinking (see above and also Criswell, 2012), one strategy that might prove to be effective is use of short inquiry laboratory activities (Cheung, 2011). We felt that introducing the theoretical and practical aspects of inquiry-based education (IBSE) into the pre-service training of chemistry teachers and at continuous professional development (CPD) courses of in-service chemistry teachers might help to address these challenges.As a result of several projects funded within the 7th Framework Programme of the European Commission, inquiry-based teaching and learning resources are freely available on the internet (e.g.McLoughlin et al., 2015). Modules and programmes for teachers were also developed in inquiry-based science education (e.g.Bolte et al., 2012). Successful implementations of the training modules, with positive feedback collected from the students (e.g.Dumitrescu et al., 2014), and as a result of those, better student achievement were reported (e.g.Savec and Devetak, 2013). However, according to our own experiences (Szalay, 2015) IBSE is viewed sceptically by some people, especially practicing teachers. They argue that the requirements of the curriculum do not allow sufficient time for unguided inquiry and only topics that are closely connected to the curriculum could be covered. This is enforced by Cheung (2011), who writes that some non-users may believe that guided-inquiry labs are not feasible in their chemistry classes due to constraints such as large class size, lack of time and the need to prepare students for public examinations. He adds that although the benefits of inquiry teaching are well documented, few teachers use it in schools. After the CPD courses some teachers saw value in IBSE, but others were unconvinced. This mirrors the outcomes of similar courses in other countries (e.g.Bernard et al., 2015).
An earlier investigation (Kertész and Szalay, 2009) confirmed that in Hungary, as in several other countries, science teachers have to work under significant constraints in terms of preparation time, teaching time, laboratory assistance (technical support), external support and funding. These circumstances must be taken into consideration when designing inquiry activities for students. If not, many teachers will not use the activities in their own teaching practice.
Criswell (2012) also warns that too many pre-service and in-service teachers have expressed frustration when they use inquiry-based activities with their students. These relate to the belief that IBSE might lead to a less robust comprehension of science concepts than when they are taught by more traditional teaching strategies such as direct instructions. Criswell states that to allow inquiry to achieve its goals, the teacher must manage the cognitive load experienced by students while they engage in inquiry activities. One way to tackle this is to reduce the degree of freedom for the learner in a problem-solving task.
For example, by modifying step-by-step practical activities in a way that requires some stages to be designed by the students, practitioners can introduce the idea of inquiry-based processes, as the first step towards meeting the complex requirements of the (open ended) IBSE criteria.
Furthermore, in our view, teachers may find it easier to manage the situation if an inquiry-based activity originates from an established and widely used practical laboratory activity with step-by-step instructions. Such activities could be adapted, at least in part, to create inquiry-based tasks, by asking a small team of students to design one or two steps of an experiment and reviewing the results obtained through the team discussion. In the model we suggest, these adapted activities must be strongly related to the curriculum.
As early as in 1986, Allen et al. described how to convert a verification experiment to a guided inquiry format. According to them, an experiment for adaptation must be relatively simple, require uncomplicated apparatus and apply well-established concepts. Data collected should lend itself to ‘discovery’-type analysis, where the conclusions can be tested.
The adapted experiment should be part of the introduction to a topic rather than a verification experiment (the principal concepts should not be ‘taught’ before the activity). The detailed procedural steps should be reduced significantly so that the students must ‘think’ about how to collect the required data and their analysis. A step or procedure should be included toward the end of the experiment that allows students to verify their analysis and conclusions about the principal concept. A short discussion ‘thought’ question should be included in the activity. The latter criterion is reinforced by the literature, since student interaction has an important impact on learning (e.g.Criswell, 2012). It is also important to ensure that students have the necessary knowledge and skills (both theoretical and practical) before starting the inquiry step of the experiment (Bruck and Towns, 2009).
In our view, correct design of even a relatively simple step of an experiment provides evidence that a student is thinking in a correct scientific way. The question arises about the effectiveness of doing just a few partially inquiry-based activities on the ability of a student to develop the skill of experimental design. Also important is the effectiveness of such activities in helping students gain a deep understanding of the disciplinary content knowledge to which the activity had been linked. Finally, the relationship between any significant effect of the approach described and student's gender and prior knowledge is of interest.
Research method
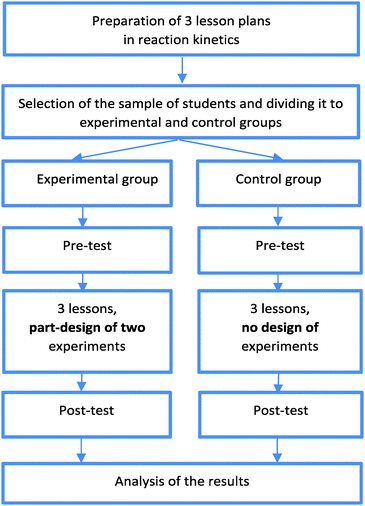
An empirical research to address the questions raised above was organised in the school year 2014/2015 in Hungary. It was part of a national project that aimed to develop teaching materials to help the initial and in-service teacher training. It included a brief pilot consisting of three chemistry lessons about reaction kinetics. Test and control groups were studied. Pre- and post-tests were used to identify and assess the possible effects of an inquiry-based approach to traditional practical laboratory activities on the development of students' skills to design experiments and develop other relevant knowledge and skills in chemistry.Sample
The research was organised in 12 schools involving 15 teachers and 660 students (those who completed the pre- and post-tests). Teachers asked permission of their school principals to participate and to involve their students. Teachers explained to the students that their test results would not count in their school chemistry assessment. This ethical precaution was appropriate as the research activity was considered to be a useful learning opportunity for the students (Taber, 2014).Each student (all were 14–15 year-olds) had two 45 minutes chemistry lessons per week in the school year 2014/2015. There were 31 groups of students, divided randomly into 15 experimental groups and 16 control groups. The class group size varied between 14 and 39 students. Some teachers participated with only one group, whereas others with as many as four or five groups. If a teacher had more than one group of students participating in the research and half of the groups were already chosen to be ‘experimental’, the other half of the groups automatically became ‘control’ groups. (It would have been difficult to apply the matched pair design, since the groups of students were different in so many parameters.) The number of students in the experimental groups was 335 (50.8%) and in the control group it was 325 (49.2%). All groups had a gender mix. The gender ratio (boys/girls) in the experimental group was 141/194 and in the control group it was 121/204 (the difference is not significant, Pearson Chi-square p = 0.421).
Instruments
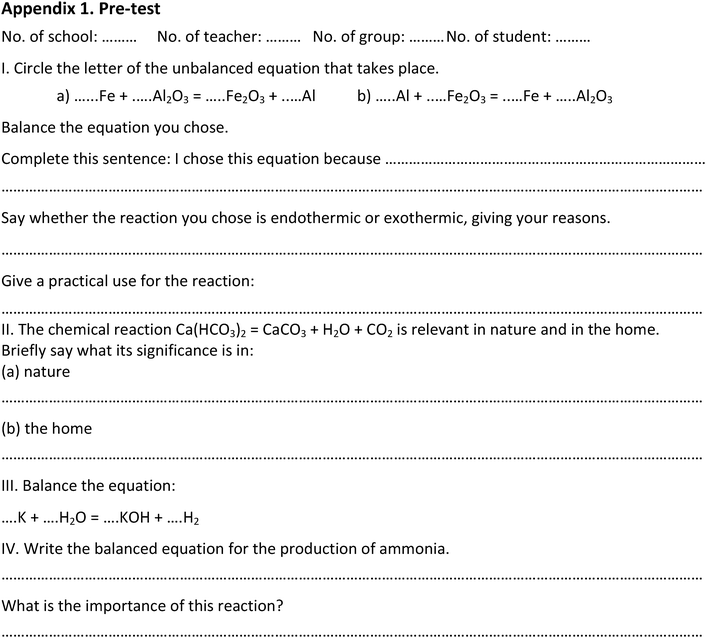
The pre-test consisted of 1 item to assess the ability to design an experiment, 15 items to assess disciplinary content knowledge (DCK), 1 item to assess the ability to find reliable information about chemical problems and 7 items (5-point Likert scale) to assess the student's attitude toward chemistry and the learning environment at chemistry lessons. Students were asked also to give their gender and their marks in math, physics, chemistry, and biology that they got in the previous school year.
The item measuring the ability to design an experiment in the pre-test was as follows (translated from Hungarian to English):
Choose one of the three conditions necessary for a chemical reaction to occur (see previous task). Design an experiment to provide evidence that this condition is required for the reactions to take place.
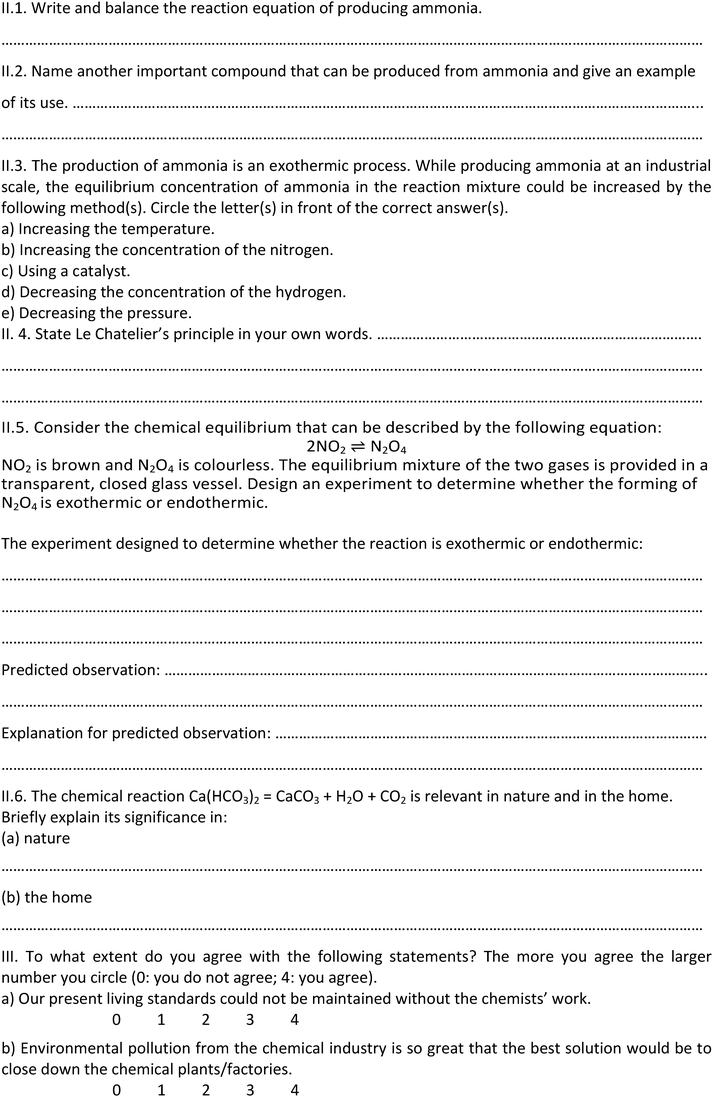
The post-test consisted of 2 items assessing the ability to design an experiment, 12 items assessing disciplinary content knowledge (DCK) and 7 items (5-point Likert scale) assessing a student’s attitude towards chemistry and the learning environment at chemistry lessons.
The 2 items measuring the ability to design an experiment in the post-test were as follows (translated from Hungarian to English):
Task 1: The reaction taking place between bromine water and methanoic acid can be described by the following equation:
| Br2 + HCOOH → 2HBr + CO2 |
Bromine water is yellow, but the other reactant and the products are colourless. Choose a factor that influences the rate of reaction. Design an experiment to prove that the factor you chose does influence the rate of reaction.
Task 2: Consider the chemical equilibrium that can be described by the following equation:
| 2NO2 ⇌ N2O4 |
The other tasks measured factual knowledge, understanding and their application (Bloom taxonomy). The participating teachers were asked to mark students' answers given to the questions of the pre-test and the post-test. They recorded these marks in an Excel spreadsheet following the instructions provided (see Appendix 3).
Three chemistry lesson plans
Three lesson plans on reaction kinetics were provided for each teacher participating in the research. The topics of the lessons were:Lesson 1: Rate of reaction
Lesson 2: Chemical equilibrium
Lesson 3: Factors that affect the chemical equilibrium
Teachers were asked to organise the three lessons following one another as part of the normal teaching process, but using the lesson plans provided. Experimental (intervention) and control groups followed the same lesson plans, apart from the two tasks described below (see the student sheets and teacher guides that were the attachments of the Lesson plan 1 and Lesson plan 3, translated into English in Appendix 4 and Appendix 5). In the experimental groups the students were asked to design some aspects of the experiments, whereas the control groups got step-by-step descriptions for each experiment.
The two tasks that were given to the experimental groups to design experiments were part of Lesson 1 and Lesson 3 (their most important features are given below, translated from Hungarian to English).
Lesson 1: Students were asked to
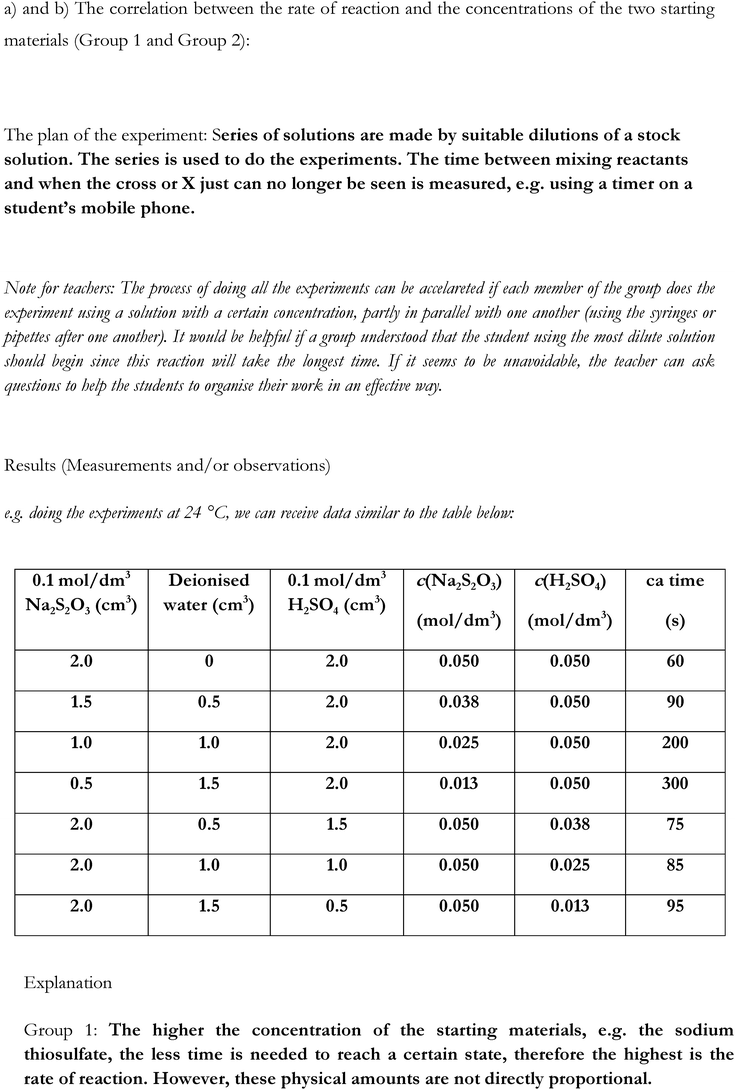
• perform an experiment following step-by-step instructions to form colloidal S by mixing Na2S2O3 and H2SO4solutions of known concentration (providing them with the knowledge needed for the next part of the activity)
• design an experiment using materials and equipment provided to investigate the effect of the following factors on the rate of reaction:
• Group 1 and Group 2: concentrations of the Na2S2O3/H2SO4solutions.
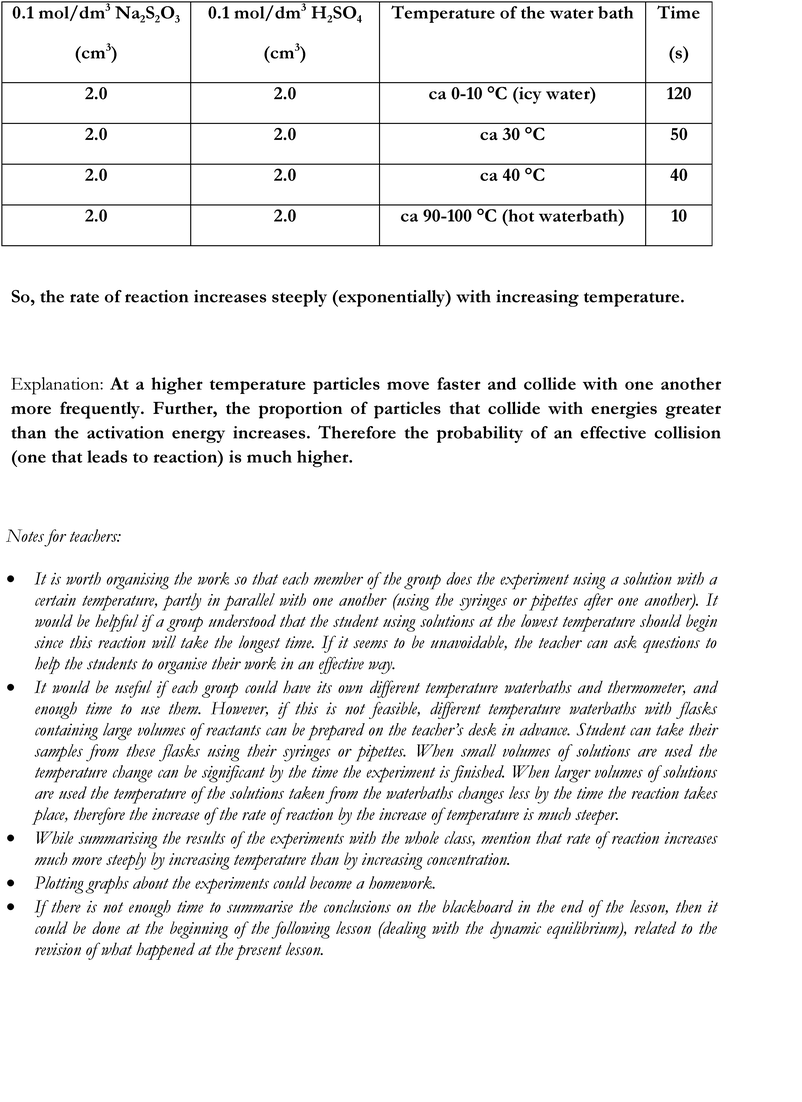
• Group 3: temperature of the starting materials.
Lesson 3: The students were asked to
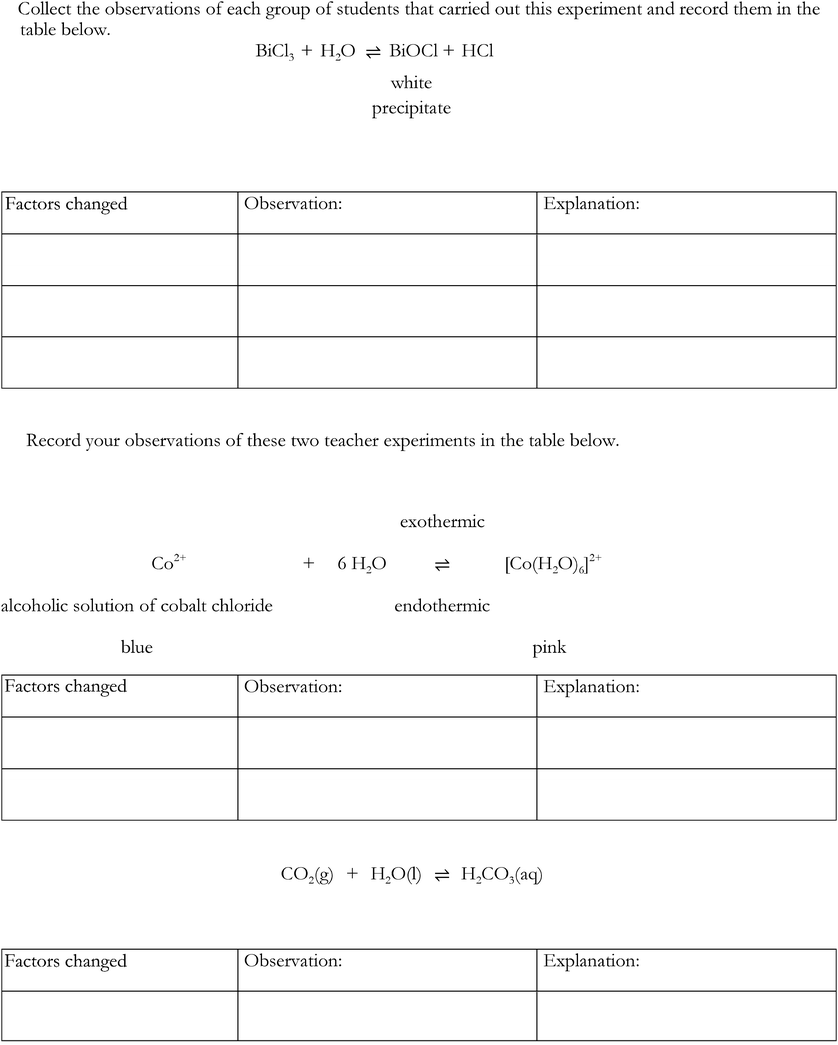
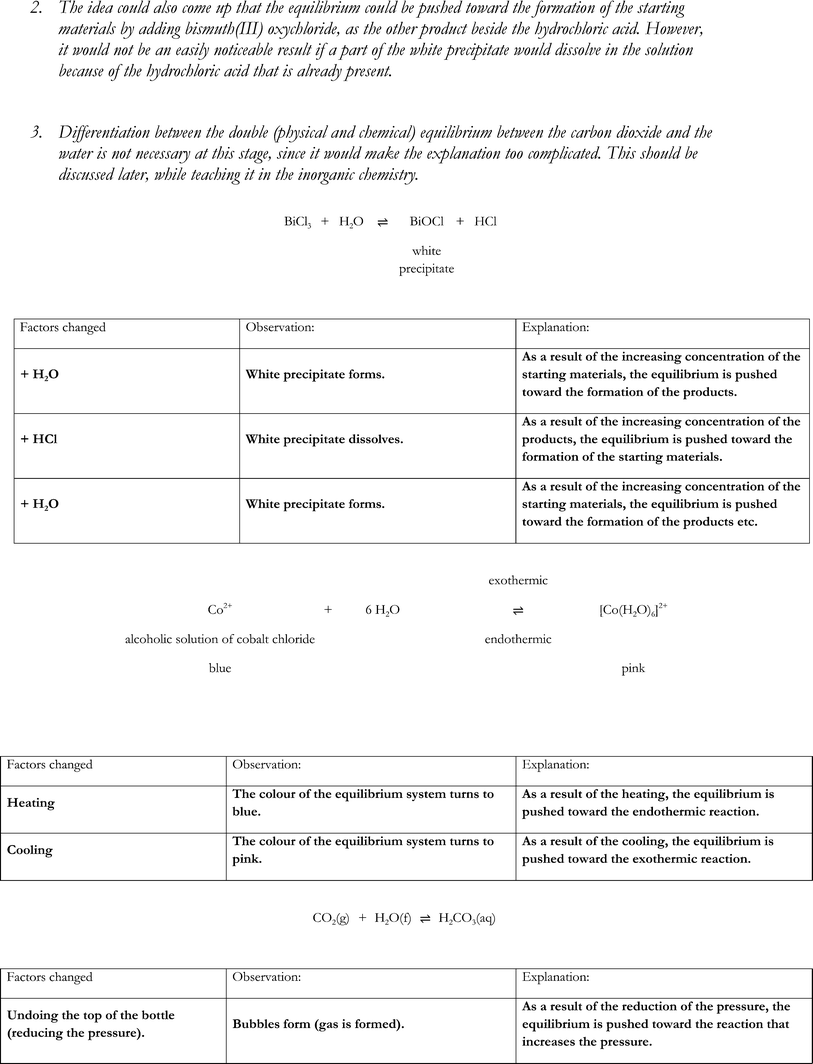
• add distilled water drop-by-drop to BiCl3solution until they identify a change (formation of white precipitate, BiOCl) and had to balance the given equation BiCl3 + H2O ⇌ ![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[O with combining low line]](https://www.rsc.org/images/entities/i_char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif) + HCl (providing them with the knowledge needed for the next part of the activity)
+ HCl (providing them with the knowledge needed for the next part of the activity)
• design experiments using materials and equipment provided to collect evidence to support the idea that in a chemical equilibrium, an increase in concentration drives the reaction to the opposite side:
• adding products favours reactants
• adding reactants favours products.
Research design
The research model could be seen in Fig. 1.Research questions (RQ)
(1) Is there any significant change in the experimental group students' ability to design experiments? If yes, is there a correlation between this change and students' gender and/or previous chemical knowledge (measured by the pre-test)? (RQ1)(2) Do students in the experimental groups achieve significantly different scores in the post-test than the students of the control groups, considering the tasks measuring disciplinary content knowledge (DCK)? If yes, is there a correlation between the change of DCK measured and students' gender and/or previous chemical knowledge (measured by the pre-test)? (RQ2)
Results and discussion
Reliability
Statistical analysis of data was accomplished by the SPSS Statistics software. Table 1 shows Cronbach's alpha values of the DCK tasks of the tests. There were several reasons why reliability is examined only for the DCK task among the three distinct sections of the tests, and not for the design tasks and the attitude questions. There was only one experiment design task in the pre-test and two design tasks in the post-test (the correlation of the post-test design tasks will be discussed later). Students' answers to the attitude questions were analysed, but – as it could be expected – this very brief intervention did not cause changes that are worth discussing here. Cronbach's alpha values in Table 1 are a rather low (omitting some of the tasks did not improve them). The reason for these low, but just about acceptable, values might be that the items of the pre-test and post-test varied hugely in the cognitive domain of Bloom's taxonomy. However, in our view, it was necessary to cover a wide range of DCK that is different to the ability to design experiments to gain some understanding of how the intervention influenced the students' other relevant knowledge and skills.| Experimental group | Control group | |
|---|---|---|
| Pre-test/DCK tasks | 0.643 | 0.609 |
| Post-test/DCK tasks | 0.638 | 0.487 |
Results according to types of tasks
The summarised results of the pre-tests and post-tests in the experimental and control groups for the different types of tasks are given in Table 2.| Tasks/group | M Pre-test (%) | SDPre-test (%) | M Post-test (%) | SDPost-test (%) | Δ (%) | p |
|---|---|---|---|---|---|---|
| a +: significant difference (p < 0.05). | ||||||
| All tasks/experimental | 26.8 | 16.4 | 30.0 | 16.0 | +3.2 | + |
| All tasks/control | 26.4 | 15.4 | 25.0 | 12.5 | −1.4 | − |
| p | − | + | ||||
| Design task/experimental | 6.6 | 19.6 | 23.2 | 26.9 | +16.6 | + |
| Design task/control | 7.2 | 21.5 | 13.4 | 21.3 | +6.2 | + |
| p | − | + | ||||
| DCK task/experimental | 30.2 | 6.6 | 31.6 | 16.2 | +1.4 | − |
| DCK task/control | 29.6 | 16.8 | 27.7 | 13.5 | −1.9 | − |
| p | − | + | ||||
There were no statistically significant differences in the achievement of the experimental and the control groups according to the types of tasks in the pre-test. Looking at the results of all tasks in the post-test, a small, but significant effect of the intervention is shown by the data in the experimental group, whereas no significant change could be detected in the control group. The increase of achievement in the experimental group is most likely due to the significant increase in the ability to design experiments. It is interesting that just doing step-by-step experiments (the control group) also seemed to help to develop experiment designing skills. No significant change was detected in the results of the DCK tasks in either the experimental or the control group after the intervention. However, there is small, but statistically significant difference between the achievement in the DCK tasks of the experimental and the control group in the post-test, favouring the students' achievement of the experimental group. High standard deviations show that the sample was very heterogeneous according to their knowledge and skills as measured by the tests.
Results according to gender
Table 3 shows the average results of the pre-tests and the post-tests according to gender in the various types of tasks in the experimental and the control group.| Gender/tasks | Groups | M pre-test (%) | M post-test (%) | Δ (%) | p |
|---|---|---|---|---|---|
| a +: significant difference (p < 0.05). | |||||
| Boys/all tasks | Experimental | 27.1 | 29.8 | +2.7 | + |
| Control | 27.7 | 25.1 | −2.6 | + | |
| p | − | + | |||
| Girls/all tasks | Experimental | 26.6 | 30.2 | +3.6 | + |
| Control | 25.6 | 25.0 | −0.6 | − | |
| p | − | + | |||
| Boys/design tasks | Experimental | 7.3 | 24.0 | +16.7 | + |
| Control | 9.1 | 16.5 | +7.4 | + | |
| p | − | + | |||
| Girls/design tasks | Experimental | 6.0 | 22.6 | +16.6 | + |
| Control | 6.1 | 11.6 | +5.5 | + | |
| p | − | + | |||
| Boys/DCK tasks | Experimental | 30.3 | 31.1 | +0.8 | − |
| Control | 30.9 | 27.0 | −3.9 | + | |
| p | − | + | |||
| Girls/DCK tasks | Experimental | 30.1 | 32.0 | +1.9 | − |
| Control | 28.8 | 28.1 | −0.7 | − | |
| p | − | + | |||
There were no statistically significant differences between the achievement of the experimental and the control groups of students according to gender on the pre-test. Both boys' and girls' achievements increased significantly in the post-test (‘all tasks’) in the experimental group as a result of the intervention. When the results are grouped according to the types of tasks, it is clear that this is probably due to the significant increase in the ability to design experiments of both boys and girls in the experimental group. The interesting effect of just doing step-by-step experiments (control group) also appeared to help develop experiment designing skills of both boys and girls. There was a small decrease in the achievement of students of the control group, which appears to be slightly more significant in the boys' case. This is mainly because boys in the control group achieved significantly lower scores in the DCK tasks in the post-test than in the pre-test.
Results according to achievements in the pre-test
Table 4 shows the average results of the pre-tests and the post-tests according to the achievement in the pre-test and the types of tasks.| Achievement in pre-test/tasks | Groups | M pre-test (%) | M post-test (%) | Δ (%) | p |
|---|---|---|---|---|---|
| a +: significant difference (p < 0.05). | |||||
| Lowest/all tasks | Experimental | 9.65 | 20.2 | +10.5 | + |
| Control | 10.4 | 18.9 | +8.5 | + | |
| p | − | − | |||
| Medium/all tasks | Experimental | 25.3 | 28.4 | +3.1 | + |
| Control | 24.7 | 24.7 | 0 | − | |
| p | − | + | |||
| Highest/all tasks | Experimental | 45.5 | 41.5 | −4.0 | + |
| Control | 44.1 | 31.5 | −12.6 | + | |
| p | − | + | |||
| Lowest/design tasks | Experimental | 0.0 | 10.0 | +10.0 | + |
| Control | 0.3 | 6.6 | +6.3 | + | |
| p | − | − | |||
| Medium/design tasks | Experimental | 1.2 | 20.7 | +19.5 | + |
| Control | 4.6 | 11.2 | +6.6 | + | |
| p | + | + | |||
| Highest/design tasks | Experimental | 18.5 | 38.8 | +20.3 | + |
| Control | 16.7 | 22.5 | +5.8 | − | |
| p | − | + | |||
| Lowest/DCK tasks | Experimental | 11.3 | 22.6 | +11.3 | + |
| Control | 12.0 | 21.7 | +9.7 | + | |
| p | − | − | |||
| Medium/DCK tasks | Experimental | 29.3 | 30.1 | +0.8 | − |
| Control | 28.0 | 27.8 | −0.2 | − | |
| p | − | − | |||
| Highest/DCK tasks | Experimental | 50.0 | 42.1 | −7.9 | + |
| Control | 48.7 | 33.5 | −15.2 | + | |
| p | − | + | |||
There was only one case when a statistically significant difference was measured between the average scores of the experimental and the control groups of students according to their achievement in the pre-test (medium achievement group), but in that case the result of the control group was significantly better in the design task of the pre-test than the result of their counterparts in the experimental group. Nevertheless, the achievement of the experimental group was still significantly better in the post-test than that of the control group. The data listed under ‘all tasks’ show that the results of the intervention depended on the achievement in the pre-test in both the experimental and in the control groups. The results of the lowest achievement groups were significantly better in the post-test than in the pre-test in both the experimental and control groups as a result of the intervention. However, there is a difference in the increase of the experimental and control groups, favouring the experimental group. Among the medium achievement groups, only the experimental group's results were significantly better in the post-test than in the pre-test. It is a warning sign that the groups of students who got the highest scores in the pre-test had significantly poorer results in both the experimental and the control groups in the post-test than in the pre-test. The decrease of the scores of the control group was significantly higher than that of the experimental group.
Looking at the data grouped by the types of tasks, there was a statistically significant increase in the scores of all achievement groups in the ability to design experiments in the experimental groups. Doing step-by-step experiments also seemed to increase the ability of designing experiments in the control groups, although this change was not statistically significant in the highest achievement control group.
Very interesting results can be seen when it comes to the DCK tasks. The average scores of the lowest achievement groups are significantly higher in both the experimental and the control groups after the intervention. No significant changes were detected in the medium achievement groups (either in the experimental or the control groups). However, worryingly the data show that the group of students who made the highest achievement in the pre-test obtained significantly lower average scores in the post-test than in the pre-test, in both the experimental and the control groups. It is also interesting that this decrease was less in the experimental group than in the control group and the scores of the experimental group were still significantly higher in the post-test than that of the control group.
Correlations among the design tasks and the DCK tasks
We calculated correlations among the results of the design tasks and the result of the tasks measuring the DCK related to solve the design tasks. In the pre-test (see Appendix 1) task V examined the students’ knowledge about conditions necessary for chemical reactions to occur. In task VI the students had to choose one of those conditions and design an experiment to provide evidence that this condition is required for the reactions to take place. There was no significant difference between the results of the control and the experimental group in task V or task VI. There were weak correlations (r = 0.21–0.25) between the results of task V and task VI in both the experimental and the control groups. However, as expected, there were significant differences in the results of task VI in both the experimental and the control groups between the students who could not name any conditions in task V and the ones who could name at least one condition.In the post-test (see Appendix 2) task I.2 examined the students' knowledge concerning factors that influence the rate of reaction. In task I.3 the students had to choose one factor and design an experiment to provide evidence that the factor influences the rate of reaction. For task I.2 there was no significant difference between the control and the experimental group. However, the experimental group's achievements were significantly better in task I.3 (‘design’). For the control group there is only a very weak correlation between the results of the task I.2 and I.3 (r = 0.14) and the correlation is stronger in the case of the experimental group (r = 0.29). The average scores in the case of the experimental group were 0.00 for the students who could not mention any factor and 0.94 for the ones who could mention at least one factor. The scores for the control group are 0.11 for the students who could not mention any factor and 0.58 for the ones who could mention at least one factor. The difference is greater for the experimental than the control group.
There was one more design task in the post-test (task II.5). The related DCK task (task II.3) examined the students' knowledge about factors influencing the equilibrium. The experimental group achieved better score in both tasks than the control group. A weak correlation (r = 0.25) was found in the case of the experimental group, but no significant correlation was found in the control group.
Results of the individual design tasks and their correlations
Students' average scores (M) and their standard deviations (SD) for the three design tasks are listed in Table 5. The relative SDs (i.e. the SD/mean values) are lower for the experimental group than for the control group after the intervention. This difference in relative SDs suggests that individual achievements in the experimental group are more similar than in the control group.| Test, task | Experimental group | Control group | p | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| a +: significant difference (p < 0.05). | |||||
| Pre-test, task VI | 0.20 | 0.59 | 0.22 | 0.65 | − |
| Post-test, task I.3 | 0.88 | 1.09 | 0.56 | 0.96 | + |
| Post-test, task II.5 | 0.51 | 0.90 | 0.25 | 0.60 | + |
The correlations among the design tasks are shown in Table 6.
These results could indicate that experimental group students who did better in the pre-test design tasks also did better in the post-test design tasks. These students might have benefited more from the IBSE intervention.
The distribution of the marks of the design tasks
The design tasks were marked according to the instructions given to the teachers (see Appendix 3). There were four response categories:• Mark 3: The two experiments and the predicted observations are given, together with the explanation.
• Mark 2: The two experiments and the predicted observations are given, together with the explanation, but observations and explanations are not clearly separated.
• Mark 1: Either the predicted observation or the explanation is not complete.
• Mark 0: In any other case.
The distributions of students' marks for the design tasks were compared by using cross-table analysis (chi squared test). The results are shown in Table 7. There was no significant difference for pre-test task VI between the distribution of the marks of the experimental group and the control group (p = 0.346).
| Test, task, group | Marks 3 (%) | Marks 2 (%) | Marks 1 (%) | Marks 0 (%) |
|---|---|---|---|---|
| Pre-test, task VI, experimental group | 1.8 | 3.9 | 6.6 | 87.8 |
| Pre-test, task VI, control group | 3.4 | 2.2 | 7.1 | 87.4 |
| Post-test, task I.3, experimental group | 13.7 | 12.2 | 22.1 | 51.9 |
| Post-test, task I.3, control group | 9.2 | 5.8 | 16.3 | 68.6 |
| Post-test, task II.5, experimental group | 6.9 | 5.6 | 18.8 | 68.7 |
| Post-test, task II.5, control group | 2.2 | 2.2 | 14.2 | 81.5 |
However, there are significant differences (p < 0.001) between the distributions of experimental and the control groups for both design tasks in the post-test. For task I.3 the proportion of marks 2 (two experiments and the predicted observations were given, together with the explanation, but observations and explanations were not clearly separated) are higher in the experimental group than in the control group. For post-test task II.5 especially the proportion of marks 2 and 3 are higher in the experimental group than in the control group.
Conclusions
Summary of the results
To answer RQ1 it could be stated that a positive change was measured in the ability to design experiments as a result of the short intervention in both the control groups and the experimental groups, which only in the case of the highest achievement control group did not prove to be significant. However, the changes in the experimental groups were significantly higher in the case of the medium and the highest achievement students than in the control groups. Medium and high achievement students’ of the experimental group seemed to gain more on an absolute scale, but lower achievement students gained more on a relative scale. There was also a significant increase in the ability to design experiments of both boys and girls in the experimental and the control group. Furthermore, the increase was significantly greater for the experimental group than for the control group.As for the RQ2: in the DCK tasks both the control and the experimental lowest achievement groups had significantly better results in the post-test than in the pre-test. No significant changes were detected in any of the medium achievement groups. However, both the control and the experimental highest achievement groups had significantly poorer results in the post-test than in the pre-test in the DCK tasks. The scores of the experimental group are better in each achievement group than the scores of their control counterparts, but the measured difference is only statistically significant in the case of the highest achievement groups. This is because the highest achievement control group scores decreased significantly more in the DCK tasks than their experimental counterparts. Especially the scores of the highest achievement boys are statistically less in the DCK tasks of the post-test than in the pre-test.
Implications
(1) It is worth modifying traditional practical laboratory activities to ones where experiments have to be partially designed by students even if it is considered to have more limited effect on the development of the investigation skills than the open-ended inquiry. It is a step towards the use of more comprehensive IBSE activities. It would be valuable even if it is used only a few times in the school year. These partly student-designed activities appear to• develop experimental design, one of the investigation skills needed for scientific literacy in each ability and gender group;
• motivate the lowest achievement group of students.
(2) Doing step-by-step experiments also develops experimental design, one of the investigation skills, but this has a lower effect than the experiments partly designed by the students.
(3) The lowest achievement group of students can be motivated by doing step-by-step experiments too. This also increases their disciplinary content knowledge.
(4) In the case of the highest achievement group of students, especially in the case of boys, the practical laboratory activity might have a negative effect on the disciplinary content knowledge gained in the lessons. This effect is more severe for students who only do step-by-step experiments. Therefore the highest achievement students, especially the highest achievement boys, should get special attention and further tasks to develop their knowledge and skills other than the designing experiment.
The results of our research lead us to fully support Cheung (2011) when he states that chemical educators worldwide should emphasise the implementation issues with inquiry practical activities rather than just the value of inquiry teaching or the limitations of ‘cookbook’ activities (step-by-step experiments). Also, we need to convince more teachers that it is still feasible to use guided inquiry practical activities in the chemistry curriculum, even though they need to face challenges such as large class size and lack of instructional time. However, we must not shrug our shoulders when chemistry teachers come up with all the difficulties that hinder the implementation of inquiry tasks in their teaching practice or with their negative experiences after trying to introduce inquiry. Instead we should raise their awareness of the advantages and disadvantages of guided and open ended inquiry. Given the constraints and circumstances under which teachers work, providing ready-made teaching materials is a considerable and significant help. Small steps, like turning a well-known laboratory practical into an experiment partly designed by the students, are also valuable good practice. Finding an interesting context can be the next step. This way we can hope that chemistry teachers will get positive experiences with inquiry and they also start applying more complex, open-ended inquiry tasks when circumstances allow it. All of this will help us develop further the road map for the effective introduction of inquiry-based learning in chemistry.
Further plans
Further analysis of the students' answers in the tests are necessary to find out about the details of skill development and various misunderstandings. The students' attitude toward chemistry and chemical industry and its correlation with their achievements in the tests will be the other subject of the next study.Acknowledgements
This work was partly supported by the Hungarian Research Fund (OTKA K-105262) and partly by the project called TÁMOP 4.1.2.B.2-13/1-2013-0007 “NATIONWIDE COORDINATION FOR THE RENEWAL OF TEACHER EDUCATION. The project was supported by the European Union and co-financed by the European Social Fund.References
- Allen J. B., Barker L. N. and Ramsden J. H., (1986), Guided Inquiry Laboratory, J. Chem. Educ., 63, 533–534.
- Bernard P., Maciejowska I., Krzeczkowska M. and Odrowz E., (2015), Influence of In-Service Teacher Training on Their Opinions about IBSE, Procedia – Social and Behavioral Sciences, 177, 88–99.
- Bolte C., Holbrook J. and Rauch F. (ed.), (2012), Inquiry-based Science Education in Europe: Reflections from the PROFILES Project, Berlin: Freie Universität Berlin, pp. 9–67. http://www.profiles-project.eu, last visited: 30.05.2016.
- Bolte C., Streller S. and Hofstein A., (2013), How to motivate students and raise their interest in chemistry education, in Eilks I. and Hofstein A. (ed.) Teaching Chemistry – A Studybook, Sense Publishers, pp. 67–95.
- Bruck L. B. and Towns M. H., (2009), Preparing Students to Benefit from Inquiry-Based Activities in the Chemistry Laboratory: Guidelines and Suggestions, J. Chem. Educ., 56(7), 820–822.
- Cheung D., (2011), Teacher Beliefs about Implementing Guided-Inquiry Laboratory Experiments for Secondary School Chemistry, J. Chem. Educ., 88, 1462–1468.
- Criswell B., (2012), Framing Inquiry in High School Chemistry: Helping Students See the Bigger Picture, J. Chem. Educ., 89, 199–205.
- Dumitrescu C., Olteanu R. L., Gorghiu L. M. and Gorghiu G., (2014), Learning Chemistry in the Frame of Integrated Science Modules – Romanian Students' Perception, Procedia – Social and Behavioral Sciences, 116, 2516–2520.
- Finlayson O., Maciejowska I. and Čtrnáctová H., (2015), Inquiry Based Chemistry Instruction, in Maciejowska I. and Byers B. (ed.) A Guidebook of Good Practice for the Pre-Service Training of Chemistry Teachers, Krakow: Faculty of Chemistry, Jagiellonian University, p. 119, http://www.ec2e2n.info/news/2015/<?pdb_no 1604?>1604<?pdb END?>_201510, last visited: 30.05.2016.
- Hmelo-Silver C. E., Duncan R. E. and Chinn C. A., (2007), Scaffolding and Achievement in Problem-Based and Inquiry Learning: A Response to Kirschner, Sweller, and Clark (2006), Educ. Psychol., 42(2), 99–107.
- Hofstein A. and Kempa R. F., (1985), Motivating strategies in science education: attempt of an analysis, Eur. J. Sci. Educ., 3, 221–229.
- Kertész J. and Szalay L., (2009), Summary of the work of the National Public Education Council's ad hoc committee for the investigation of science education, Hung. Chem. J., 64(4), 107–111. in Hungarian.
- Kirschner P. A., Sweller J. and Clark R. E., (2006), Why Minimal Guidance During Instruction Does Not Work: An Analysis of the Failure of Constructivist, Discovery, Problem-Based, Experiential, and Inquiry-Based Teaching, Educ. Psychol., 41(2), 75–86.
- McLoughlin E., et al., (2015), IBSE Teaching & Learning Units, Volume 2, Chemistry, ESTABLISH Project, http://www.establish-fp7.eu/sites/default/files/general/Chemistry.pdf, last visited: 30.05.2016.
- Minner D. D., et al., (2010), Inquiry_based Science Instruction – What Is It and Does It Matter? Results from a Research Synthesis Years 1984 to 2002, J. Res. Sci. Teach., 47(4), 474–496.
- Olson S. and Loucks-Horsley S., (2000), Inquiry and the National Science Education Standards, 29, Washington, D.C.: National Research Council, National Academy Press, http://www.nap.edu/openbook.php?record_id=9596, last visited: 30.05.2016.
- Osborne R. J. and Wittrock M. C., (1983), Learning science: a generative process, Sci. Educ., 67(4), 489–504.
- PISA, (2006), Science Competences for Tomorrow's World, Volume 1: Analysis, pp. 64–68.
- Savec V. F. and Devetak I., (2013), Evaluating the effectiveness of students' active learning in chemistry, Procedia – Social and Behavioral Sciences, 106, 1113–1121.
- Sweller J., (1988), Cognitive Load during Problem Solving: Effects on Learning, Cogn. Sci., 12, 257–285.
- Szalay L., (2015), Promoting Research-led Teaching of Chemistry, LUMAT, 3(3), 327–340, http://webcache.googleusercontent.com/search?q=cache:3bJ8pcKfnUsJ:luma.fi/file_download/596+&cd=1&hl=en&ct=clnk&gl=uk, last visited: 30.05.2016.
- Taber K. S., (2011), Inquiry teaching, constructivist instruction and effective pedagogy, Teach. Dev., 15(2), 257–264.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15(2), 109–113.
- Tomperi P. and Aksela M., (2014), In-service Teacher Training Project On Inquiry-Based Practical Chemistry, LUMAT, 2(2), 215–226, http://webcache.googleusercontent.com/search?q=cache:MnYxuxqTFE4J:www.luma.fi/file_download/429+&cd=1&hl=en&ct=clnk&gl=uk, last visited: 30.05.2016.
| This journal is © The Royal Society of Chemistry 2016 |