Open Access Article
Open Access ArticleUnderstanding ionic bonding – a scan across the Croatian education system
R.
Vladušić
*a,
R. B.
Bucat
b and
M.
Ožić
a
aFaculty of Science, University of Split, Croatia. E-mail: vladusic@pmfst.hr; mia.ozic@pmfst.hr
bSchool of Chemistry and Biochemistry, The University of Western Australia, Nedlands, W.A., Australia. E-mail: bob.bucat@uwa.edu.au
First published on 13th April 2016
Abstract
A study was conducted on the understandings of the accepted model of ionic substances that are held by participants at all levels of the chemical education system in Croatia, including secondary school students, university students, and chemistry teachers. We follow the research of Taber who found that a diagram of a layer of a sodium chloride crystal can be perceived by students as due to electrostatic attractions, consistent with the currently accepted view, or in an alternative molecular framework in which ionic bonding is seen as the process of electron transfer from one atom to another. A Croatian translation of the instrument used by Taber was administered to 650 secondary school students, 264 tertiary undergraduate students, and 86 teachers of chemistry at the secondary level. It was found that significant percentages of the participants, including teachers, interpreted the diagram with conceptions consistent with the molecular framework. Significant numbers of participants at all levels showed evidence of beliefs consistent with Taber's categories of a history conjecture (ionic bonds exist only between the partners in electron transfer), a valency conjecture (the number of ionic bonds that an ion can form is dependent on the electron configuration of the parent atom and is related to the number of electrons gained or removed to form a “stable octet”), and a “just forces” conjecture (ionic bonds involve something more than forces of attraction). The existence of alternative conceptions of ionic bonding at all levels of the education system, including among the teachers, is a situation of considerable concern. Obviously the pedagogical content knowledge of teachers cannot be based on an inadequate level of content knowledge. Remedial action seems to be important. As a first step, we speculate on the possible sources of the alternative conceptions. This includes an analysis of the chemical validity of the subject matter that is commonly presented in textbooks.
Introduction
Chemistry is one of the most important branches of science (Özmen, 2004) and has been regarded as a highly abstract, complex and difficult subject for middle school and college students (Nakhleh, 1992; Johnstone, 2000). Chemical bonding is one of its most important topics and is a key concept in school, college and university level chemistry curricula (Coll and Treagust, 2001; Nicoll, 2001; Taber and Coll, 2002).Understanding chemical bonding is fundamental and essential for the understanding of almost every topic in chemistry (Gómez and Martín, 2003), from reactivity in organic chemistry to spectroscopy in analytical chemistry (Nicoll, 2001; Pabuçcu and Geban, 2012) because it is concerned with combinations of particles, and the nature of bonding between particles can be used to rationalise the chemical and the physical properties of substances (Rompayom et al., 2011).
Students have difficulties in the area of bonding because of the topic's abstract nature (Ben-Zvi et al., 1988). Understanding has to be developed through diverse models, varying from simple analogical to sophisticated abstract models possessing mathematical complexity (Fensham, 1975, as cited in Coll and Treagust, 2003) with a wide range of symbolic representations (Taber and Coll, 2002). Also, chemical bonding is an area far from the student's daily experience (Birk and Kurtz, 1999; Tan and Treagust, 1999) where everyday words are used with different meanings.
Such problems render chemical bonding a topic that students commonly find problematic and a wide range of misconceptions, or alternative conceptions, have been identified (Tan and Treagust, 1999; Coll and Taylor, 2002; Taber, 2002a; Özmen, 2004).
The subject matter of interest
We cannot presume that all readers have exactly the same perception of the chemistry relevant to this article as the authors. Accordingly, we consider it worthwhile to define the subject matter of interest insofar as it is appropriate to the secondary level of schooling.Bonds are forces of attraction that hold chemical species together in substances. These forces are the result of attractions between species with opposite (positive and negative) electrical charges, such as ions, atomic nuclei, and electrons. With the advancement of chemistry and the synthesis of new materials, as well as better understanding of natural substances, the nature of bonding in substances has become less easily defined. With increasing knowledge of macromolecular chemistry, host–guest molecules and “non-covalent” interactions, the nature of bonds has become more problematic. Indeed the definition of the concept of molecule presents challenges. Nevertheless, it is common at the school level of education to introduce students to various models of bonding that are used to rationalise the particular properties of substances, which are categorised according to commonality of properties.
Bearing in mind that the categorisation of a substance is not possible a priori to knowledge of its properties, summaries of the accepted models of the four categories of substances commonly recognised are those listed below.
Metallic substances
The metal atoms are in an orderly lattice. Delocalised valence electrons of the metal atoms are pooled into a common “sea” of mobile electrons. Metallic bonding that binds the structure is the electrostatic attraction between the positive ions and the “sea” of negative electrons.Covalent network substances
Atoms are in a three-dimensional ordered arrangement within the crystal. Each atom is bound to other atoms by covalent bonds. A covalent bond between two atoms is due to the attraction of both of the nuclei of the two atoms bound to one or more shared pairs of electrons in the internuclear region. A crystal can be regarded as a single giant molecule.Covalent molecular substances
Each molecular substance consists of identical molecules in which atoms of the elements of which it is composed are joined by covalent bonds. The covalent bonds are relatively strong compared to forces of attraction between the molecules.Ionic substances
Cations and anions are in a regular ordered array in the crystal. The structure is held together by ionic bonding: electrostatic forces of attraction between each ion to all of the nearest-neighbouring oppositely charged ions. In this way, ionic bonding is the net cooperative effect acting over the entire crystal.This paper describes an investigation of perceptions of ionic bonding by school students, pre-service teachers and teachers. We therefore outline here more fully the scientifically accepted features of the model of bonding in crystals that are categorised as ionic substances – at a level commonly regarded as appropriate for secondary school students:
• Each substance is composed of both positively charged ions (cations) and negatively charged ions (anions) arranged in a three-dimensional ordered array.
• The relative numbers of cations and anions are in proportions consistent with its composition (as indicated by its chemical formula).
• The composition (and chemical formula) of a substance is determined by the charge on the cations and the charge on the anions – so that the relative numbers of each are such that the crystal has zero charge.
• Each cation is surrounded by a fixed number of nearest-neighbouring anions to which it is equally attracted. This is called the coordination number of the cation.
• Each anion is, in turn, surrounded by a fixed number of nearest-neighbouring cations to which it is equally attracted. This is called the coordination number of the anion.
• The whole set of electrostatic attractions between the cations and anions dispersed throughout the crystal of the ionic substance, which hold the crystal in place, are together referred to as ionic bonding.
• The number of oppositely-charged nearest neighbours of an ion (its coordination number) cannot be deduced from its charge (sometimes called the valency).
Review of relevant research
Nicoll (2001) has reported on chemical bonding misconceptions held by undergraduate chemistry students, and perceived five sub-categories of misconception: (i) polarity (some students didn't associate the concept of polarity with electronegativity); (ii) bond confusion (some appeared to confuse the definitions of ionic and covalent bonding, stating, for instance that ionic bonding is sharing of electrons, consistent with the findings of Boo (1998) and Sheehan et al., (2011)); (iii) general bonding (some provided incorrect explanations of why bonding occurs); (iv) wrong bond (hydrogen bonding is not differentiated from ionic and covalent bonds) and (v) micro bonding (for example, student Casey's submicroscopic picture of a molecule includes touching electrons).Kind (2014) investigated aspects of chemistry content knowledge held by 265 UK-based pre-service teachers in five chemistry concept areas. She found that well qualified, academically able novice teachers hold some significant misconceptions of basic chemical concepts likely to constrain development of pedagogical content knowledge (PCK) that promotes scientifically appropriate learning in their students. These include: ‘energy is released when bonds break’; ‘carbon is responsible for bond formation’; ‘hydrogen and oxygen are produced when water boils’; ‘covalent bonds are weaker/stronger than ionic bonds’ and mass/density confusion.
Boo (1998) identifies and describes 48 Grade 12 students' understandings about chemical bonds and energetics. He reported that the majority of students were unable to predict the overall energy change of bond formation because of their misconceptions about the nature of a chemical bond. For a large number of those students, the chemical bond was seen as a physical entity. Boo (1998) speculated that the notion that bond making requires energy input may be the result of extrapolating views about events in the macroscopic world into the sub-microscopic world: in the macroscopic world, energy is needed to make things; therefore, in the sub-microscopic world, energy is also needed to make bonds.
Boo (1998) reported on ionic bonding misconceptions, too. He found that there are students who seemed to find it difficult to conceive of the electrostatic nature of the ionic bond. Some seemed to be fixated on the idea that a bond must necessarily involve a pair of electrons between two atoms. Also, some students have the misconception that the result of attraction between two oppositely charged ions formed is the neutralization or cancellation of charges, leading to the formation of a neutral molecule.
Even for students who seemed to have some scientifically acceptable views of ionic bonding, other alternative conceptions were identified in consideration of the reactions that occur upon mixing of aqueous solutions of sodium chloride and lead(II) nitrate, or of putting magnesium into dilute hydrochloric acid solution. For example, a small minority of students believed that the ionic bond is broken during the dissolving process and the breaking of the ionic bond was conceptualized as the reversal of the process by which it was imagined to have formed; that is that when the ionic bond in sodium chloride is broken, the positive charge on the sodium ions is neutralized by the gain of electrons from the chloride ions.
Boo (1998) also found that some students believe that ionic bonds are not affected during dissolution in water, and that only weaker bonds between ionic “molecules” (which in many cases are seen as van der Waals bonds) are broken. For example, 15 of 48 students believed that in dilute hydrochloric solution, there are ionic bonds between hydrogen ions and chloride ions. They also believed that in aqueous sodium chloride solution there are ionic bonds between sodium ions and chloride ions. In other words, in the aqueous state, sodium chloride exists as discrete pairs of Na+ and Cl− units.
The existence of the alternative picture of discrete ion pairs or ionic molecules has been confirmed by many researchers. For example, Barker (1995) found that students' responses suggested that the product of the reaction between magnesium and dilute hydrochloric acid, apart from hydrogen gas, would be magnesium chloride molecules in solution.
Similarly, Hilbing (as cited in Barke et al., 2009) asked a group of German gymnasium students in grade 10 about ionic bonding and how they describe the solid salt after evaporation of water from salt solution. Most of the participants answered with “salt particles” or “NaCl particles” or drew pictures implying such particles.
Barker and Millar, (2000) found that some students seem to imagine that ionic compounds exist as discrete molecules as in covalent compounds and therefore think of ionic bonds as uni-directional and subject to the same ‘rules of behaviour’ as covalent bonds. Besides finding that many students did not appreciate that ionic bonding is three-dimensional, Butts and Smith (1987) report that some of them consider sodium chloride to be molecular, suggesting that covalent bonds hold sodium and chlorine atoms together. While some students think that the molecules in the solid are held together by covalent bonds, some others see ionic bonds in sodium chloride connecting molecules, creating the crystal structure.
Consistent with what has been reported so far, Coll and Treagust (2003) found that secondary school learners' most prevalent alternative conception of ionic bonding concerned perceptions of molecularity in the lattice. Although their dominant mental model of ionic bonding is of attraction of oppositely charged species, the formation of ions was seen to occur through electron transfer, which is in turn driven by the octet rule of full-shell stability.
The octet rule has grown as a key explanatory principle in the topic of chemical bonding. Unfortunately, many students consider that a reason why bonding occurs is because of the “motivation” of atoms to achieve full outer electron shells. That idea, or maybe even the octet framework (Taber, 1998) as a mental conceptualisation, could be so strongly developed that some A level students considered a sodium cation to be a more stable species than a sodium atom because it has filled outer shell. Some students even thought that the sodium Na7− anion would be stable because of its octet structure (Taber, 2000a).
Molecular and electrostatic frameworks of ionic bonding
For many students at completion of formal schooling, ionic bonding is perceived as electron transfer (Taber, 2002a). Taber speculates that the strength of this conviction must reflect the way the topic is presented by textbooks, if not by teachers.In order to make better sense of these and related aspects of students' understandings of ionic bonding, Taber (1997) describes the tendency of students to think of ion-pairs as a “molecular framework”. He suggests three categories of ways of thinking, referred to as a valency conjecture, a history conjecture and a “just forces” conjecture, which are different from the propositions of curricular science, referred to as the “electrostatic framework”. These are summarised in Table 1.
| Molecular framework | Electrostatic framework | |
|---|---|---|
| Status | Alternative framework | Curricular science |
| Role of molecules | Ion-pairs are implied to act as molecules of an ionic substance. | Ionic structures do not contain ion pairs – there are no discrete ion-pairs in the lattice. |
| Focus | The electron transfer event through which ions may be formed. | The force between adjacent oppositely charged ions in the lattice. |
| Valency conjecture | Atomic electronic configuration determines the number of ionic bonds formed (e.g. a sodium atom can only donate one electron, so it can only form an ionic bond to one chlorine atom). | The number of bonds formed depends on the co-ordination number, not the valency or ionic charge (e.g. the co-ordination is 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in NaCI). 6 in NaCI). |
| History conjecture | Bonds are only formed between atoms that donate/accept electrons (e.g. in sodium chloride a chloride ion is bonded to the specific sodium ion that donated an electron to that particular anion, and vice versa). | Electrostatic forces depend on charge magnitudes and separations, not prior configurations of the system (e.g. in sodium chloride a chloride ion is bonded to six neighbouring sodium ions). |
| ‘Just forces’ conjecture | Ions interact with the counter ions around them, but for those not ionically bonded these interactions are just forces (e.g. in sodium chloride, a chloride ion is bonded to one sodium ion, and attracted to further five sodium ions, but just by forces – not bonds). | A chemical bond is just the result of electrostatic forces – ionic bonds are nothing more than this (e.g. the forces between a chloride ion and each of the neighbouring sodium ions are equal). |
Taber (1997) administered a diagnostic instrument called “The Truth about bonding” (TTAIB) to 370 students and confirmed that the thinking of many of them is consistent with conjectures of the alternative molecular framework.
Motivation for the research and research question
According to Magnusson et al., (1999), knowledge of students' understandings of science is one of five discrete components of pedagogical content knowledge. Moreover, recognition and awareness of students' learning difficulties, which include alternative conceptions, are necessary steps prior to thoughtful and carefully planned teaching and explanation of content. The teacher's job is not usually to move students from a state of ignorance to a state of knowledge, but more often to shift student’s thinking away from the existing ways of understanding the world to new ways (Taber, 2009). To be able to do that, teachers need, above all, to have adequate subject matter knowledge. Experience as a teacher of pre-service chemistry teachers suggested to one of the authors (R. V.) that some students think about chemical bonding with beliefs alternative to the assumptions of the scientifically accepted model. It seemed to be necessary to understand these alternative conceptions in order to plan successful teaching/learning interventions with these pre-service chemistry teachers.Although there is much information in the research literature about students' alternative conceptions of chemical bonding, data on how chemistry teachers perceive the concepts of bonding are rare. It was decided to organize a wide research study on the understanding of ionic bonding in an effort to recognize alternative conceptions in samples of high school students, undergraduate chemistry faculty students, graduate pre-service chemistry teachers, and chemistry teachers in Croatia. We hoped that the picture of understandings at each level of education could point to the most problematic issues in the whole system, expose possible relationships, and lead us to the sources of the alternative beliefs.
The research question that guided this research was: “To what extent do the understandings about bonding in ionic substances of Croatian high school students, faculty students and school teachers compare with the accepted electrostatic model?”
Methodology
Diagnostic instrument
A translated version of the diagnostic tool “The truth about ionic bonding” (TTAIB) developed by Taber (2002b) was used for this study (see Appendix 1). It consisted of 20 statements that identify ways of thinking, which are consistent with either of Taber's molecular or electrostatic frameworks of ionic bonding. Respondents were asked to decide whether each statement is true or false. In this study we additionally sought to evaluate students' confidence levels in their judgements of the validity of the statements. As well as a judgement of the validity of each of the 20 statements, students were asked to select one of the following: “I am sure”, “I'm not sure” and “just guessing”.Two authors of this article (R.V. and M.O.) independently translated TTAIB into Croatian. After analysis of the translated statements and discussion with the author from an English speaking area (R.B.B.), it was agreed that the final version of the translation, despite the differences of expression caused by different language discourses, corresponds as closely as possible to Taber's original. We refer to the modified diagnostic instrument as “+TTAIB” (see Appendix 2).
Before administration of the +TTAIB diagnostic instrument, its content was compared with the high school curriculum to establish compliance. Of greatest concern with respect to the validity of the instrument was the precision of wording of each of the statements to achieve sufficient chemical accuracy in succinct sentences. Some of the statements are not precisely formulated technically, but as the author of the instrument stated, writing clear and unambiguous items, which are also succinct and readily comprehended, can be challenging (Taber et al., 2012). For example, four items (2, 4, 10 and 11) were open to the criticism that in the molecular framework, reference is made to electron transfer between ions, and so strictly these items are not correctly worded. The wording of statement 2, for example, is: “A sodium ion is only bonded to the chloride ion it donated its electron to”. This implies that each sodium ion (rather than each sodium atom) is the donor of an electron. In the context of defining the molecular framework of ionic bonding, a more precise statement is: “The sodium ion is only bonded to the chloride ion which arose from the chlorine atom which received an electron from the sodium atom”. We agree with Taber et al. (2012) that the complexity of this kind of expression could be a greater obstacle to understanding the meaning of the idea than the statement used.
There are other issues of validity of diagnosis related to the wording of the statements. For example, statement 13 is: “There is a bond between the ions in each molecule, but no bonds between the molecules”. The first part of this statement indicates the existence of molecules (of sodium chloride) formed from ions, and the second part implies that molecules in the layers of a sodium chloride crystal are not connected by bonds. If a participant indicates that the statement is incorrect, we are left in ignorance as to whether they disagreed with the first part, the second part, or with the whole statement.
Participants and research context
Since we wanted to find out what thinking about ionic bonding could be observed at all levels within the Croatian educational system, we administered +TTAIB to high school students, students of the Faculties of Science, and secondary school teachers of chemistry. The number of participants in the study was 650 high school students, 264 faculty students, and 86 chemistry teachers. For context, we provide basic information about each group of participants involved in this research.High school students from different parts of Croatia, geographically divided into 4 regions, participated in the research. However, an ideal representation with respect to the number of high school students who live in each region could not be achieved. The instrument was applied to more students in the first year of high school – the year in which the topic of chemical bonding is presented in the high school curriculum – than in higher grades.
Both undergraduate and graduate faculty students were involved in this research. The majority of the 180 undergraduates, 89 at the first year level, 66 at second year, and 25 at third year, were enrolled in programs of chemistry or chemistry and biology. Of the 84 graduate participants, all of whom were intending to be chemistry teachers, 43 were in their first year and 41 in their second year.
There is much information in the scientific literature about students' alternative conceptions of chemical bonding, but no reports of investigations into teachers' understandings in this field can be found. Research findings in relation to this could have the potential to significantly affect the process of teaching about chemical bonding and the learning of scientific concepts. We decided, therefore, to administer +TTAIB to a sample of teachers of chemistry in Croatia. Eighty six responses, from all four regions, were received.
Data collection and analysis
Data collection was varied according to participant groups. The high school students and faculty students were provided with two sheets of paper: one was +TTAIB and the other a short guide on how to complete it, with empty tables for responses and self-confidence judgements. The +TTAIB survey was conducted anonymously, during regular classes, in the presence of the teacher. This process generally lasted about 20 minutes.The printed version of +TTAIB could not be distributed in all regions of the Republic of Croatia since most of the teachers were physically inaccessible to the researchers. So a web questionnaire was created via Google Docs. Emails were sent to all chemistry teachers (about 650 of them) with a link to the web questionnaire. Judgements about the accuracy of the instrument statements, as well as about the confidence in their responses, were indicated by mouse clicks. The time for completing a web survey was not restricted. Eighty six teachers responded online.
Limitations of the study
There are several limitations in the certainty of the findings. We have already, in the Methodology section, discussed issues inherent in the diagnostic instrument: (a) some statements were not technically accurate and (b) some statements involved more than one idea, so that the reasoning behind responses was indeterminate.The diagnostic instrument was administered to students in a limited time and under controlled classroom conditions. Consequently students responded in a more constrained situation than did teachers who responded online when and where it suited them. This may have affected the validity of findings in comparability of responses.
As is the case with any paper-and-pencil diagnostic instrument, we know only the responses that participants give, while we are limited in our ability to interpret the thinking processes that lead to those responses. Therefore our findings are strictly observations about student responses, rather than about students' thought processes. On the other hand, the art of the instrument design is to give the researchers more confidence in some level of interpretation about students' understandings.
Ethical considerations
The research was organized at the Faculty of Science, University of Split, as a part of the wider investigation in the aim of PhD thesis construction. Everything was performed in compliance with the relevant laws and institutional guidelines. The approval for the research was provided by Ethics Committee of Faculty of Science, University of Split, Croatia (classification mark 641-01/13-01/00009) and by Ministry of Science, Education and Sport (classification mark: 602-01/14-01/00326). Special attention was paid to ethical considerations about investigating with children. The surveys were filled anonymously, only by participants who agreed to be involved in the research.Results
We present the results in sub-sections corresponding with the various conceptions identified by Taber (1997).Consistency with the electrostatic framework of ionic bonding
Responses to seven statements in the diagnostic instrument provide evidence as to whether participants had an electrostatic conception of ionic bonding. Of these, six (1, 7, 8, 9, 15, 19) are statements consistent with the electrostatic model, while statement 20 is directly contrary to this model. Table 2 shows the percentages of participants in each category who correctly judged the validity of these seven statements; that is, who indicated agreement in the cases of statements 1, 7, 8, 9, 15, and 19, and disagreement with statement 20. The table also shows the percentages of these respondents who admitted to guessing.| Statement | Teachers n = 86 | Faculty students n = 264 | High school students n = 650 |
|---|---|---|---|
| a In the second and third columns, the percentages do not correspond with integral numbers of the stated sample populations. Some students did not respond to all statements (variable from statement to statement), and the values listed are the percentage correct of those who did respond. For example, although 264 faculty students participated, only 263 responded to statement 1, and of these 236 (89.7%) correctly assessed the validity of the statement. | |||
| 1. A positive ion will be attracted to any negative ion. | 72.1 (3.2) | 89.7 (2.1) | 83.8 (7.7) |
| 7. An ionic bond is the attraction between a positive ion and a negative ion. | 80.2 (0) | 69.1 (1.1) | 71.1 (8.2) |
| 8. A positive ion can be bonded to any neighbouring negative ion, if it is close enough. | 84.9 (4.1) | 65.4 (9.3) | 64.9 (14.5) |
| 9. A negative ion will be attracted to any positive ion. | 79.1 (1.5) | 65.8 (6.4) | 61.6 (9.8) |
| 15. The reason a bond is formed between chloride ions and sodium ions is because they have opposite charges. | 83.7 (2.8) | 71.6 (2.6) | 72.6 (9.6) |
| 19. A negative ion can be bonded to any neighbouring positive ion, if it is close enough. | 81.4 (5.7) | 71.5 (4.7) | 67.4 (14.7) |
| 20. There are no molecules shown in the diagram. | 91.9 (6.3) | 68.1 (3.4) | 57.4 (13.2) |
The data in Table 2 show that a majority of the respondents at each level of education agreed with statements 1, 7, 8, 9, 15, and 19 which are consistent with the electrostatic model of ionic bonding, and disagreed with statement 20. Nevertheless, substantial numbers of students, and even teachers, appear to have alternative conceptions. For example, nearly 30% of faculty students and high school students disagreed with the fundamental tenet expressed in statement 15, as also did 16% of teachers. Furthermore, 43% of school students and 32% of university students, and even 8% of chemistry teachers, agreed with statement 20.
Clearly the conceptions of many students and some teachers with respect to the representation of a sodium chloride crystal structure are not consistent with the electrostatic model of ionic bonding.
In all cases except for statement 1, fewer teachers made incorrect judgements than students at either level, as we might expect. However, more teachers disagreed with statement 1 than students. Fewer teachers agreed with statement 1 than with any of the other correct statements, while more students agreed with this statement than with any other. We can only speculate as to whether the cause of this “anomaly” is one of chemical understanding, or whether statement 1 is misinterpreted by some respondents. It is conceivable that some teachers, with a greater knowledge base than the students, read more into this statement than was intended. For example, while statement 1 is intended to focus on the non-directionality of ionic bonding (that is, that ionic bonding from a positive ion is not limited to just one of the surrounding negative ions), some teachers may have judged the statement to be incorrect on the grounds that some of the negative ions in a crystal are too far away for significant bonding, or that some may be “shielded” by other ions.
Across all statements, significantly higher percentages of school students admitted to guessing, so the uncertainty in the values in the third data column particularly is considerable.
Consistency with Taber's molecular framework of ionic bonding
We now turn our attention to the consideration of participants' conceptions that are consistent with Taber's molecular framework. Agreement with 14 of the statements in +TTAIB (2, 3, 4, 5, 6, 10, 11, 12, 13, 14, 16, 17, 18, and 20) can indicate alternative conceptions of ionic bonding consistent with Taber's molecular framework. The findings in relation to each of Taber's proposed conjectures are presented and discussed sequentially.Five statements in +TTAIB (2, 4, 10, 11, and 18) are designed to identify the existence of conceptions consistent with the history conjecture. The results are displayed in Table 3. In every case, the desirable response is disagreement with the statement. The lower the percentage correct (the statement adjudged to be false), the higher is the number of participants with conceptions consistent with the history conjecture of a molecular framework of ionic bonding.
| Statement | Teachers n = 86 | Faculty students n = 264 | High school students n = 650 |
|---|---|---|---|
| 2. A sodium ion is only bonded to the chloride ion it donated its electron to. | 69.8 (1.7) | 60.2 (6.9) | 54.9 (13.4) |
| 4. The reason a bond is formed between chloride ions and sodium ions is because an electron has been transferred between them. | 32.6 (0) | 31.9 (6.0) | 27.4 (19.8) |
| 10. It is not possible to know where the ionic bonds are, unless you know which chloride ions accepted electrons from which sodium ions. | 52.3 (8.9) | 47.5 (20.8) | 38.1 (28.0) |
| 11. A chloride ion is only bonded to the sodium ion it accepted an electron from. | 67.4 (5.2) | 48.9 (5.4) | 40.1 (20.1) |
| 18. An ionic bond is when one atom donates an electron to another atom, so that they both have full outer shells. | 31.4 (3.7) | 35.0 (9.8) | 31.7 (17.6) |
The results show that alternative conceptions consistent with the history conjecture of a molecular framework are common. A majority of high school students, faculty students, and teachers believe that the transfer of electrons from a sodium atom to a chlorine atom is the origin of a bond in sodium chloride (statement 4). Similarly large percentages of respondents agreed with statement 18, even though this statement gives two opportunities for disagreement: that an ionic bond is associated with an electron transfer and that attainment of a full outer shell is a motivation for bond formation.
More participants disagreed with statements 2, 10 and 11 than with statements 4 and 18. Nonetheless, it seems to be of considerable significance that substantial percentages of respondents, including teachers, agreed with statements 2, 10, and 11, all of which explicitly link location of bonds to a particular pairs of sodium ions and chloride ions that result from electron transfer.
In the +TTAIB survey, four statements (3, 12, 14 and 17) are designed to identify conceptions that are consistent with Taber's valency conjecture. In every case, agreement with the statement indicates that a respondent conceives of ionic bonding in a way consistent with the valency conjecture, rather than with the electrostatic model of the curriculum. The findings are shown in Table 4.
| Statement | Teachers n = 86 | Faculty students n = 264 | High school students n = 650 |
|---|---|---|---|
| 3. A sodium atom can only form one ionic bond, because it only has one electron in its outer shell to donate. | 46.5 (5.0) | 31.4 (3.6) | 20.7 (23.3) |
| 12. A chlorine atom can only form one ionic bond, because it can only accept one more electron into its outer shell. | 47.7 (2.4) | 33.3 (10.2) | 26.7 (21.6) |
| 14. A negative ion can only be attracted to one positive ion. | 79.1 (2.9) | 73.4 (6.2) | 57.3 (15.7) |
| 17. A positive ion can only be attracted to one negative ion. | 79.1 (4.4) | 67.0 (6.8) | 60.2 (17.4) |
These findings show that conceptions consistent with the valency conjecture of Taber's molecular framework are prevalent among students at both levels, as well as among teachers. Statements 14 and 17 refer to unspecified positive and negative ions, with no reason given for the propositions that ions are attracted to only one counter ion. In these cases, 30–40% of students and 21% of teachers agreed with the statements. Even worse is that the vast majority of students, and even most teachers, judged statements 3 and 12 to be correct. The implication that full outer shells provide driving forces for bond formation has been noted in other educational systems (Taber 2002a, Taber et al., 2012).
It is interesting, and open to speculation, that many fewer respondents disagreed with statements 3 and 12 than with statements 14 and 17. Statements 3 and 12 contain a clause that corresponds with the propositions of 14 and 17 as well as a rationale (such as “because it only has one electron in its outer shell to donate”) that explicitly targets the valency conjecture. So statements 3 and 12 have an extra opportunity for disagreement that is present in statements 14 and 17. And yet fewer disagreed. It is instructive to try to put ourselves “in the minds” of the respondents to try to understand this difference. It is possible that some respondents would have disagreed with statements 3 and 12 on the grounds that they specify that the particles are atoms, rather than ions. If this distracter were eliminated (by referring to ions, rather than atoms), the difference would be even greater.
Three statements in the +TTAIB diagnostic instrument, 5, 13 and 16, are designed to expose the existence of this kind of thinking. The results are provided in Table 5. In every case, the correct judgement of statement validity is demonstrated by disagreement with the statement.
| Statement | Teachers n = 86 | Faculty students n = 264 | High school students n = 650 |
|---|---|---|---|
| 5. In the diagram a chloride ion is attracted to one sodium ion by a bond and is attracted to up to three other sodium ions just by forces. | 62.8 (1.8) | 51.1 (12.2) | 49.0 (16.7) |
| 13. There is a bond between the ions in each molecule, but no bonds between the molecules. | 89.5 (3.9) | 71.1 (10.7) | 65.5 (16.5) |
| 16. In the diagram a sodium ion is attracted to one chloride ion by a bond and is attracted to three other chloride ions just by forces. | 58.1 (2.0) | 44.3 (12.0) | 40.1 (22.0) |
Once again, there is reason for concern. The responses to statements 5 and 16 show that more than half of students and nearly 40% of chemistry teachers interpret the sodium chloride diagram to mean that each ion is connected by a bond to only one of the adjacent counter ions, and “just by forces” with other three, even though there is nothing in the diagram which can provoke that conclusion. The responses to statement 13 indicate that significant numbers of teachers and students visualise molecules in the sodium chloride diagram.
| Statement | Teachers n = 86 | Faculty students n = 264 | High school students n = 650 |
|---|---|---|---|
| 6. In the diagram each molecule of sodium chloride contains one sodium ion and one chloride ion. | 72.1 (1.6) | 51.1 (6.7) | 39.1 (14.2) |
| 13. There is a bond between the ions in each molecule, but no bonds between the molecules. | 89.5 (3.9) | 71.1 (10.7) | 65.5 (16.5) |
| 20. There are no molecules shown in the diagram. | 91.9 (6.3) | 68.1 (3.4) | 57.4 (13.2) |
Correct statement 20 is a very direct proposition that requires no interpretation and does not depend on inference. On one hand it is heartening that 92% of teachers agree with it, and yet there is cause for concern that 8% chose to disagree with it. And very considerable percentages of students at both levels disagreed with statement 20. It is difficult to argue other than that these respondents conceive of molecules when they inspect the diagram of sodium chloride. Significant percentages of participants in each category agreed with statements 6 and 13, both of which presume the existence of molecules.
There are apparent inconsistencies among the response data. For example, 28% of teachers agreed with statement 6, even though responses to statement 20 show that 92% of them do not believe that there are molecules in the sodium chloride structure.
Reliability of responses
The data obtained in this research are worthless if the participants have responded to statements randomly. In most research, an assumption is made that the responses have been made carefully and conscientiously so that the responses reliably provide insights into the participants' conceptions. In this research we have an inbuilt design feature, along with large numbers of participants, to assess the degree of reliability of responses.There are some pairs of statements in the diagnostic instrument which we should expect every participant to respond to identically. For example, one would expect a participant to give identical responses, whether agreement or disagreement, to statements 14 and 17 in Table 4. The number of participants who gave identical responses (whether both are judged to be correct, or both incorrect) is an indicator of the stability of their understandings, and a measure of the confidence that we can have that the data are reliable.
The full set of paired statements used in this way includes statements 7 and 15 (Table 2), 2 and 11 (Table 3), 14 and 17 (Table 4), as well as 5 and 16 (Table 5). The percentages of participants who responded to both statements in each pair are shown in Tables 7, 8, 9 and 10.
| Teachers n = 86 | Faculty students n = 264 | School students n = 649 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Both correct | 62 | 72.1 | 144 | 54.5 | 365 | 56.2 |
| Both incorrect | 7 | 8.1 | 37 | 14.0 | 82 | 12.6 |
| Total | 69 | 80.2 | 181 | 68.6 | 447 | 68.9 |
| Teachers n = 86 | Faculty students n = 264 | School students n = 650 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Both correct | 54 | 62.8 | 106 | 40.2 | 184 | 28.3 |
| Both incorrect | 22 | 25.6 | 82 | 31.1 | 216 | 33.2 |
| Total | 76 | 88.4 | 188 | 71.2 | 400 | 61.5 |
| Teachers n = 86 | Faculty students n = 264 | School students n = 649 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Both correct | 65 | 75.5 | 155 | 58.7 | 272 | 41.9 |
| Both incorrect | 15 | 17.4 | 48 | 18.2 | 159 | 24.5 |
| Total | 80 | 92.9 | 203 | 76.9 | 431 | 66.4 |
| Teachers n = 86 | Faculty students n = 264 | School students n = 649 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Both correct | 46 | 53.5 | 89 | 33.7 | 184 | 28.4 |
| Both incorrect | 28 | 32.6 | 101 | 38.3 | 255 | 39.3 |
| Total | 74 | 86.0 | 190 | 72.0 | 439 | 67.6 |
Consistency of students' responses to matched pairs of items has been used in the past to evaluate the “goodness” of the items. One can imagine, for example, that ambiguously worded items may be interpreted differently by various students, so that their answers are not an indication of their science knowledge. On the other hand, inconsistent responses to matched pairs can be a function of the respondents, rather than of the items: for example, a long duration of a test, or the conditions under which it is held, may cause some respondents to answer carelessly. Furthermore, for some time it has been recognised (Taber, 2000b, 2001) that students may at some point be in a state of fluidity between two or more competing explanatory conceptions. In this unstable cognitive situation it is conceivable that small differences between the matched pairs, or the circumstances at the time of responding to each, could give rise to responses based on different conceptual frameworks. Regardless of the reason, different responses to paired statements lessen the reliability of the data as a basis for making conclusions about participants' understandings.
There are sufficient inconsistent participant responses to the statements in each pair such that, along with knowledge of the percentages who admitted to guessing, we can say that the data in Tables 2–6 have some uncertainty. Nevertheless, these data give us sufficient confidence that the majority of students responded to the statements in consistent, stable, and non-random ways. We can be confident of the existence of the misconceptions identified among many participants.
This is especially so if we note that the percentages of participants who gave consistent responses to different statements in each pair were largely independent of the percentages of correct and incorrect responses. For example:
• The percentages of correct responses to the paired statements 7 and 15, and 14 and 17, in Table 2, were much higher than in the case of the pair 5 and 16 in Table 5, and yet the stability of responses was similar.
• Although there was a very considerable difference between the percentages of correct responses to the paired statements 2 and 11 in Table 3, the majority of students showed stable response patterns. The same can be said of responses to statements 5 and 16 in Table 5.
Discussion
The most significant general conclusion to be drawn from the findings is that the model of ionic bonding regarded as scientifically acceptable for inclusion in the school curriculum is not well understood by considerable numbers of students at both the secondary and tertiary levels, and even by a significant number of teachers. Perhaps the most telling single finding is that from Table 2 that 8% of teachers disagreed with statement 20 that “there are no molecules shown in the diagram” of sodium chloride. Nearly 70% of teachers demonstrated conceptions consistent with Taber's molecular framework history conjecture that ionic bonds are only located between those pairs of ions that result from an electron transfer act (statements 4 and 18 in Table 3). More than half of the teachers show inadequate conceptions of simultaneous bonding from each ion to several neighbouring counter ions. They are seemingly confused in this sense by notions of valency, agreeing with, for example, statement 3 (Table 4) that “A sodium atom can only form one ionic bond, because it only has one electron in its outer shell to donate.” Approximately 40% of teachers showed beliefs consistent with Taber's “just forces” conjecture through their agreement with statements 5 and 16 (Table 5). These teachers seemingly do not regard all electrostatic forces of attraction between oppositely charged ions as ionic bonding: rather, toward one near-neighbouring counter ion there is a special kind of connection called an ionic bond that is different from the forces of attraction to the others. Visualisation of molecules in the diagram of the sodium chloride structure is exhibited not only by the 8% who disagreed that no molecules were shown in the diagram, but also by the 28% who agreed with statement 6 (Table 6) that “In the diagram each molecule of sodium chloride contains one sodium ion and one chloride ion.”If we accept that teachers are the most influential factors in student’s learning, there is reason for concern about the quality of the teaching environment that school students experience in Croatia. Even when one has mastery of the subject matter, the challenge for any teacher is to develop a store of pedagogical content knowledge (Shulman, 1986; Magnusson et al., 1999; Bucat, 2004), which will allow planning of strategies, techniques, challenges, language, and exercises that can help the students acquire meaningful understanding. Good quality pedagogical content knowledge cannot be derived from poor quality subject matter knowledge.
At least it is true that, except for statements 1 (Table 2) and 18 (Table 3), more teachers correctly assessed the validity of the diagnostic statements than students of either category.
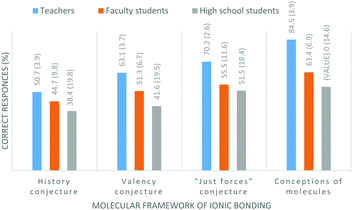
Fig. 1 displays, for each group of participants, the percentages of correct judgements over all of the statements related to each of the molecular framework conjectures.
Bearing in mind that incorrect responses suggest conceptions consistent with Taber's conjectures, the data in Fig. 1 tell us that all of the molecular framework conjectures are more prevalent among high school students than faculty students, and least prevalent among teachers. The apparent differences between the percentages of teachers and students displaying the various conjectures are exacerbated if we take into account the relatively large numbers of students who guessed.
Although disappointingly large percentages of teachers' answers (49%, 37%, and 30%, respectively) indicated conceptions consistent with the history, valency, and “just forces” conjectures, at least the number of answers that indicated conceptions of molecules in ionic compounds was only 15% (compared with 37% and 47% for the students). The authors recognise, but cannot explain, an apparent contradiction between the relatively large percentages of teachers who displayed evidence of the history, valency, and “just forces” conjectures, relative to the much smaller percentage of answers that suggest conceptions of molecules.
The summary data portrayed in Fig. 1 also tell us that the history conjecture is the most prevalent amongst all three categories of participants. For all categories of participants, the prevalence of conjectures is in the order history > valency > “just forces” > perception of molecules.
In summary, a significant number of teachers have demonstrated inadequate understanding of the accepted model of ionic substances. Furthermore, the understanding of ionic bonding by many faculty students leaves much to be desired, and students who go on into pre-service teacher training courses are derived largely from this sector. Perhaps then, in the cycles of school students who become pre-service teacher trainees who become teachers, who teach students, at least in the topic of ionic bonding the poor understandings of so many school students are not surprising.
We have exposed a serious systemic situation that applies to one topic in the chemistry curricula that deserves attention. We also wonder if similar situations may exist in respect of any other topics, although of course we have no evidence concerning that.
Speculation on the origin of misconceptions
The most obvious sources of students' understandings, whether good or poor, are the teachers and the textbooks. We consider the possibility of misunderstandings arising from these sources.There is an obvious source of this misconception in textbooks. Almost without exception, the section on ionic bonding is introduced via a discussion of the formation of a positive ion and a negative ion by electron transfer – usually between a sodium atom and a chlorine atom. Fig. 2, copied from one of the three official textbooks, supposedly portrays the formation of an ionic bond.
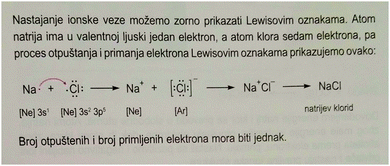 | ||
| Fig. 2 Portrayal of ionic bond formation in an authorised textbook – one of several with very similar presentations (Habuš et al., 2015). Translation of the text: “We can clearly show the formation of ionic bond with Lewis symbols. An atom of sodium has one electron in its valence shell, and an atom of chlorine has seven electrons, so the process of release and acceptance of electrons is represented with Lewis symbols as follows. …The number of released electrons and the number of received electrons must be equal.” [With permission of Profil Klett, Zagreb.] | ||
The issues related to this portrayal are many. Firstly, consideration of the structure of a sample of sodium chloride is concerned with the arrangements in space of the ions, and what holds the structure together, and how its properties (high melting point, brittleness, lack of electrical conductivity) can be accounted for, and is not related to how the ions might be formed. In fact, any sample is much more likely to have been formed by crystallisation from an aqueous solution, in which the ions already exist, than from an electron transfer reaction. Electron transfer reactions are the subject matter of the topic of oxidation–reduction processes, not of the topic of the structure of ionic substances.
Furthermore, the process that portrayed transfer of electrons to a chlorine atom is quite unreal: the students may have been shown the reaction between sodium and the substance chlorine consisting of diatomic Cl2 molecules, but reaction with chlorine atoms is beyond the experiences of most of us. We support Taber's proposal (2002a) that the explanation of ionic bonding is more meaningful with an example of salt formation by neutralization, followed by evaporation of solvents. In that case, the ions already exist in the solution, so, there is no need to explain their hypothetical origin from atoms. From the introduction to the topic, the focus should be on the interactions between ions.
In the textbook discussion, ionic bond formation is linked directly with transfer of electrons (consistent with Taber's molecular framework of ionic bonding), and because it concerns only one sodium ion and one chlorine ion, there is an implication that the resulting bonding is highly localised along the axis between these two ions (consistent with the history conjecture). The text does not go on to consider the network arrangement of a multitude of ions and the operation of electrostatic forces of attraction to all neighbouring counter ions, successively extending throughout the lattice in a cooperative fashion. So the text does in fact portray the formation of something like an ion pair, rather than a three-dimensional arrangement of many ions.
The textbook portrayal of the formation of a sodium ion-chloride ion pair, using Lewis symbols, resembles the way that covalent bond formation is usually represented. Covalent bonds are directional between nuclei. Perhaps it is unfortunate that the Croatian textbook suggests comparability between ionic bond formation and covalent bond formation, rather than making a clear distinction between them.
The charges on simple ions have some dependence on the electron configurations of the atoms from which they are derived. The charges of cations and anions determine the stoichiometry of a crystal and, therefore, its formula – but not the coordination number of the cations and anions.
In their advanced textbook, Keeler and Wothers (2008) express the issue well:
Sometimes you will see reference made to an “ionic bond” between two ions: such terminology is best avoided. The reason for this is that in an ionic solid each ion interacts with many other ions, both with the same and the opposite charge to itself. The energy which binds the structure does not come from single interactions between ions (the so-called ionic bonds), but comes as a result of the interactions between all the ions in the sample.
| Na(s) + 1/2Cl2(g) → NaCl(s) |
Unless this distinction has been made, the representation in Fig. 2 could well lead to a conception that the product of reaction is an ion pair or molecule, or many of them.
An obvious (untested) potential source of the conception of molecules in ionic compounds is in the use of “formula units” for purposes of quantitative calculations. For example the formula unit for the most common example of ionic compound, sodium chloride, is NaCl. It is rare to see a textbook making explicit the distinction between the meanings of, say, H2O (absolute numbers of each type of atom in each molecule) and NaCl (relative numbers of each type of ion in the aggregate of many ions). If a student thinks about the valency of ions in ionic compounds in a similar way to the meaning of valency of atoms in molecules, it would not be surprising that the formula unit is considered to represent a discrete structural unit. In fact this possibility is enhanced in Croatia because the Croatian word for “unit” (jedinka) means something which exists as an independent entity, so it is in conflict with the meaning of the term formula unit considered as specified numbers of ions. We have no evidence if this Croatian language characteristic is a significant factor, so cannot make any statement about the generalisability of this finding to other countries.
The concept of the “formula unit” is unnecessary: quantitative stoichiometric calculations can be based on the amount (in moles) of Na+ ions (or of Cl− ions) in the sample of interest. We question whether the potential for the misconception arising from the use of the “formula unit” can be offset by any benefit that can be gained by its use. While we recommend that teachers do not use the “formula unit”, if it is used, it is vitally important to help students to understand that the formula unit of an ionic compound does not imply the existence of molecules. This may be a challenging task.
Recommendations for curriculum, textbook and instruction
We recommend careful consideration of the way that the topic of ionic bonding is presented, in light of the research findings from this study and the work of others discussed earlier. Although we base our recommendations on experimental evidence in the Croatian context, we believe that some of these may be universally applicable. Listed below are several important issues about the model of ionic bonding that must be taken into consideration. Some of these are standard practice currently.• Unlike Fig. 2, which can imply the formation of a single ion pair, useful imagery of an ionic substance should include very many cations and anions distributed in an ordered way among each other in the crystal, without reference to a hypothetical way in which the ions might be imagined to form. This issue has been discussed earlier in some detail in speculation on the origin of identified misconceptions, in the sub-section entitled “Regarding history conjecture”. If, in the instructions on ionic bonding, the starting point is the existence of ions (rather than their hypothetical formation), the problem of students using the electron configuration of atoms to wrongly deduce the number of bonds from each ion should be avoided.
• The stoichiometry of an ionic compound corresponds with the relative numbers of cations and anions that result in zero net charge on the crystal. So, for example, in sodium chloride, consisting of Na+ ions and Cl− ions, there must be an equal number of cations and anions, and the formula is NaCl. Correspondingly, in magnesium chloride there are twice as many Cl− ions as Mg2+ ions, and the formula is MgCl2.
• Formulae of ionic compounds must be interpreted differently from formulae of molecular covalent compounds. For example, while the formula CH4 tells us the absolute number of atoms of the elements in each molecule of methane, the formula MgCl2 indicates only the relative numbers (that is, the ratio) of Mg2+ ions and Cl− ions distributed among each other. This important distinction cannot be learned without explicit instructions.
• The formula CH4 can tell us the number of single bonds by which each C atom is bonded to H atoms in CH4 molecules. This is called the valency (or covalency) of C atoms in this situation. By contrast with covalent molecular substances, the formulae NaCl and MgCl2 tell us nothing about the number of nearest-neighbouring counter ions to which there are the strongest electrostatic attractions: this is the experimentally determined coordination number. This can be demonstrated by reference to various 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salts (all of which have equal numbers of cations and anions). In a NaCl crystal, each Na+ ion is surrounded by six octahedrally distributed Cl− ions, and each Cl− ion is surrounded by six Na+ ions. However, in zinc sulfide (zinc blende or sphalerite, ZnS) each ion is surrounded by four counter ions occupying positions at the corners of a tetrahedron around each ion. And in cesium chloride, CsCl, each ion has eight nearest-neighbouring counter ions: the electrostatic forces of attraction are directed to counter ions at the corners of a cube from each ion at the centre of the cube. The textbook introduction to this topic shown in Fig. 2 does not begin to approach these central ideas. But if we do not explicitly make these distinctions, we shouldn't be surprised if students presume that what applies to molecular substances also applies to ionic substances. Why should they think otherwise? A considerable challenge for student learning (and, therefore, for instructors) is recognising that a crystal in which each ion is surrounded by six counter ions has 1
1 salts (all of which have equal numbers of cations and anions). In a NaCl crystal, each Na+ ion is surrounded by six octahedrally distributed Cl− ions, and each Cl− ion is surrounded by six Na+ ions. However, in zinc sulfide (zinc blende or sphalerite, ZnS) each ion is surrounded by four counter ions occupying positions at the corners of a tetrahedron around each ion. And in cesium chloride, CsCl, each ion has eight nearest-neighbouring counter ions: the electrostatic forces of attraction are directed to counter ions at the corners of a cube from each ion at the centre of the cube. The textbook introduction to this topic shown in Fig. 2 does not begin to approach these central ideas. But if we do not explicitly make these distinctions, we shouldn't be surprised if students presume that what applies to molecular substances also applies to ionic substances. Why should they think otherwise? A considerable challenge for student learning (and, therefore, for instructors) is recognising that a crystal in which each ion is surrounded by six counter ions has 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. At very least we would recommend that teachers have a model of the crystal structure of sodium chloride to demonstrate this important point.
1 stoichiometry. At very least we would recommend that teachers have a model of the crystal structure of sodium chloride to demonstrate this important point.
• Each cation is equally attracted to each of its nearest-neighbouring anions, all of which are, in turn, equally attracted to each of its nearest-neighbouring cations. In this way, that which holds the crystal together, referred to as “ionic bonding”, operates cooperatively in all directions throughout the crystal.
• In Croatia, the concept of valency of an element as the combining power of its atoms with other atoms in chemical compounds is introduced in the 7th grade of elementary school – the first year of teaching chemistry as a separate subject. The discussion is entirely in the context of molecular compounds, in which valency can be taken to mean the number of bonds to each atom of the element in question. Generally, the concept of valency of ions in ionic compounds is not introduced at this level, and there is no explicit instruction that such a definition does not apply to ionic compounds. The concept of ionic valency is difficult to be precisely defined in terms of some sense of combining power. Indeed, the IUPAC “Gold Book” (McNaught and Wilkinson, 1997) does not list ionic valency among its definitions of terms used in chemistry. If we define ionic valency as the charge on ions in order to determine stoichiometry, then it is pointless to introduce the term at all; to do so would be to replace a rather simple concept with a more abstruse one.
• It is not useful to consider individual “ionic bonds”. Rather, we should think of the overall phenomenon of “ionic bonding”.
In common with many studies in science education, the prime concern of the study reported here is to provide a basis for the development of the pedagogical content knowledge of teachers – both individually and as a profession. The data, insofar as they apply to participants of all levels of education in Chemistry in Croatia, are new knowledge. Consideration of these data has led to speculation about the origins of the common misconceptions – not entirely unique to this study. In turn, the possible origins of misconceptions suggest recommendations for curricula, textbooks and instructions related to understanding the structures of substances that are modelled as ionic compounds. Teachers may debate our speculations on the origins of the misconceptions, as well as our consequent recommendations. They might carry out their own classroom-based action research, or they might become aware of future research studies motivated by this and other related studies. All of these aspects can give rise to a better knowledge base for improvement of teaching and learning; that is, to more highly developed pedagogical content knowledge. The challenge still remains, of course, for the teacher to decide on how best to design their teaching in light of their acquired subject-specific pedagogical content knowledge.
Appendix 1: TTAIB diagnostic instrument (Taber, 2002b)
Ionic bonding – true or false?
The statements below refer to the diagram of the structure of sodium chloride. The diagram shows part of a slice through the three dimensional crystal structure.Please read each statement carefully, and decide whether it is correct or not.
1. A positive ion will be attracted to any negative ion.
2. A sodium ion is only bonded to the chloride ion it donated its electron to.
3. A sodium atom can only form one ionic bond, because it only has one electron in its outer shell to donate.
4. The reason a bond is formed between chloride ions and sodium ions is because an electron has been transferred between them.
5. In the diagram a chloride ion is attracted to one sodium ion by a bond and is attracted to other sodium ions just by forces.
6. In the diagram each molecule of sodium chloride contains one sodium ion and one chloride ion.
7. An ionic bond is the attraction between a positive ion and a negative ion.
8. A positive ion can be bonded to any neighbouring negative ions, if it is close enough.
9. A negative ion can be attracted to any positive ion.
10. It is not possible to point to where the ionic bonds are, unless you know which chloride ions accepted electrons from which sodium ions.
11. A chloride ion is only bonded to the sodium ion it accepted an electron from.
12. A chlorine atom can only form one ionic bond, because it can only accept one more electron into its outer shell.
13. There is a bond between the ions in each molecule, but no bonds between the molecules.
14. A negative ion can only be attracted to one positive ion.
15. The reason a bond is formed between chloride ions and sodium ions is because they have opposite charges.
16. In the diagram a sodium ion is attracted to one chloride ion by a bond and is attracted to other chloride ions just by forces.
17. A positive ion can only be attracted to one negative ion.
18. An ionic bond is when one atom donates an electron to another atom, so that they both have full outer shells.
19. A negative ion can be bonded to any neighbouring positive ions if it is close enough.
20. There are no molecules shown in the diagram.
Appendix 2: the +TTAIB diagnostic instrument
Izjave se odnose na shematski prikaz strukture natrijevog klorida. Shema prikazuje dio jednog sloja kristala natrijeva klorida.Pažljivo pročitajte tvrdnje (izjave) i prosudite njihovu ispravnost.
1. Pozitivno nabijeni ion će biti privučen bilo kojem negativno nabijenom ionu.
2. Natrijev ion je vezan samo s onim ionom klora kojem je donirao elektron.
3. Atom natrija može formirati samo jednu ionsku vezu, jer u svojoj vanjskoj ljusci ima samo jedan elektron kojeg može donirati.
4. Izmjena elektrona između iona natrija i iona klora razlog je zbog kojeg dolazi do formiranja veze među njima.
5. Prema shemi, kloridni je ion privučen jednom ionu natrija tvoreći vezu, dok je s ostalim ionima natrija povezan samo silama.
6. Prema shemi, svaka molekula natrijevog klorida sadrži jedan natrijev i jedan kloridni ion.
7. Ionskom vezom nazivamo privlačenje između pozitivnih i negativnih iona.
8. Pozitivni ion može biti vezan s bilo kojim susjednim negativnim ionom, ukoliko je (su) isti dovoljno blizu.
9. Negativni ion može biti privučen bilo kojim pozitivnim ionom.
10. Nije moguće utvrditi gdje su uspostavljene ionske veze, osim ako znamo koji su natrijevi i kloridni ioni sudjelovali u izmjeni elektrona.
11. Kloridni ion je vezan samo s onim ionom natrija od kojeg je primio elektron.
12. Atom klora može formirati samo jednu ionsku vezu, jer u njegovu vanjsku ljusku stane još samo jedan elektron.
13. Postoji veza između iona u svakoj molekuli, ali nema veza između molekula.
14. Negativno nabijeni ion može biti privučen samo jednom pozitivnom ionu.
15. Razlog nastajanja veze između kloridnog i natrijevog iona je taj što su oni suprotnog naboja.
16. Prema shemi, natrijev je ion privučen jednom ionu klora tvoreći vezu, dok je s ostalim ionima klora povezan samo silama.
17. Pozitivno nabijeni ion može biti privučen samo jednom negativnom ionu.
18. Ionska veza je kad jedan atom donira elektron drugom atomu, tako da oba imaju popunjene vanjske ljuske.
19. Negativni ion može biti vezan s bilo kojim susjednim pozitivnim ionom, ukoliko je (su) isti dovoljno blizu.
20. Na shemi nema prikazanih molekula.
Acknowledgements
We would like to thank everybody who participated in this research: from high school students, faculty students to the chemistry teachers across whole Croatia. A special thank is due to the secondary and university teachers who conducted the diagnostic instrument in their schools and faculties.References
- Barke H.-D., Hazari A. and Yitbarek S., (2009), Misconceptions in chemistry: Addressing perceptions in chemical education, Berlin: Springer-Verlag Berlin and Heidelberg GmbH & Co. K.
- Barker V., (1995), A longitudinal study of 16–18 year olds' understanding of basic chemical ideas, D.Phil. thesis, Department of Educational Studies, University of York.
- Barker V. and Millar R., (2000), Students reasoning about basic chemical thermodynamics and chemical bonding: What changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Ben-Zvi R., Eylon B. and Silberstein J., (1988), Theories, principles and laws, Educ. Chem., 25(3), 89–92.
- Birk J. P. and Kurtz M. J., (1999), Effect of experience on retention and elimination of misconceptions about molecular structure and bonding, J. Chem. Educ., 76(1), 124–128.
- Boo H., (1998), Students understanding of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Bucat R., (2004), Pedagogical content knowledge as a way forward, Chem. Educ. Res. Pract., 5(3), 215–228.
- Butts B. and Smith R., (1987), HSC Chemistry Students' Understanding of the Structure and Properties of Molecular and Ionic Compounds, Res. Sci. Educ., 17, 192–201.
- Coll R. K. and Taylor N., (2002), Mental models in chemistry: senior chemistry students' mental models of chemical bonding, Chem. Educ. Res. Pract., 3(2), 175–184.
- Coll R. K. and Treagust D. F., (2001), Learners' mental models of chemical bonding, Res. Sci. Educ., 31, 357–382.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40(5), 464–486.
- Fensham P., (1975), Concept formation, in Daniels, D. J. (ed.), New movements in the study and teaching of chemistry, London: Temple Smith, pp. 199–217.
- Gómez P. J. S. and Martín F., (2003), Quantum versus ‘classical’ chemistry in university chemistry education: a case study of the role of history in thinking the curriculum, Chem. Educ. Res. Pract., 4(2), 131–148.
- Habuš A., Tomašić. V. and Liber S., (2015), Opća kemija 1, Udžbenik kemije za prvi razred gimnazije, 2nd edn, Zagreb: Profil Klett.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Lear., 7, 75–83.
- Johnstone A. H., (2000), Teaching of Chemistry—Logical or Psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Keeler J. and Wothers P., (2008), Chemical Structure and Reactivity: an integrated approach, New York: Oxford University Press.
- Kind V., (2014), A degree is not enough: a quantitative study of aspects of pre-service science teachers' chemistry content knowledge, Int. J. Sci. Educ., 36(8), 1313–1345.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N.G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Boston, MA: Kluwer, pp. 95–132.
- McNaught A. D. and Wilkinson A., (1997), Compendium of Chemical Terminology: IUPAC Recommendations, Blackwell Science, retrieved 24.8.2015. from http://goldbook.iupac.org/.
- Nakhleh M. B., (1992), Why Some Students Don't Learn Chemistry: Chemical Misconceptions, J. Chem. Educ., 69(3), 191–196.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Özmen H., (2004), Some Student Misconceptions in Chemistry: A Literature Review of Chemical Bonding, J. Sci. Educ. Tech., 13(2), 147–159.
- Pabuçcu A. and Geban O., (2012), Students' Conceptual Level of Understanding on Chemical Bonding, Int. Online J. Educ. Sci., 4(3), 563–580.
- Rompayom P., Tambunchong C., Wongyounoi S. and Dechsri P., (2011), Using Open-Ended Questions to Diagnose Student's Understanding of Inter- and Intramolecular Forces, US China Educ. Rev. B, 1, 12–23.
- Sheehan M., Childs P. E. and Hayes S., (2011), The Chemical Misconceptions of Pre-service Science Teachers at the University of Limerick: Do they change?, IOSTE-NWE: Contemporary Issues in Science and Technology Education, Reading, June 20–21.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Taber K. S., (1994), Misunderstanding the ionic bond, Educ. Chem., 31(4), 100–103.
- Taber K. S., (1997), Student understanding of ionic bonding: molecular versus electrostatic thinking? Sch. Sci. Rev., 78(285), 85–95.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Taber K. S., (2000a), Challenging Chemical Misconceptions in the Classroom? A paper presented at the British Educational Research Association Annual Conference, Cardiff University, September 7–10, 2000, retrieved 23.11.2015. from http://www.leeds.ac.uk/educol/documents/00001525.htm.
- Taber K. S., (2000b), Multiple frameworks?: Evidence of manifold conceptions in individual cognitive structure, Int. J. Sci. Educ., 22(4), 399–417.
- Taber K. S., (2001), Shifting sands: a case study of conceptual development as competition between alternative conceptions, Int. J. Sci. Educ., 23(7), 731–753.
- Taber K. S., (2002a), Chemical misconceptions – prevention, diagnosis and cure: theoretical background (Vol. I), London: Royal Society of Chemistry.
- Taber K. S., (2002b), Chemical misconceptions – prevention, diagnosis and cure: classroom resources (Vol. II), London: Royal Society of Chemistry.
- Taber K. S., (2009), Challenging Misconceptions in the Chemistry Classroom: Resources to Support Teachers, Educació Química EduQ número, 4, 13–20., retrieved 18.8.2015. from http://publicacions.iec.cat/repository/pdf/00000087/00000043.pdf.
- Taber K. S. and Coll R., (2002), Chemical Bonding, in Gilbert J. K. et al., (ed.) Chemical Education: Towards Research-based Practice, Dordrecht: Kluwer Academic Publishers, pp. 213–234.
- Taber K. S., Tsaparlis G. and Nakiboğlu, C., (2012), Student Conceptions of Ionic Bonding: Patterns of thinking across three European contexts, Int. J. Sci. Educ., 34(18), 2843–2873.
- Tan K. C. D., and Treagust D. F., (1999), Evaluating students' understanding of chemical bonding, Sch. Sci. Rev., 81(294), 75–83.
| This journal is © The Royal Society of Chemistry 2016 |