Disciplinary literacy instructions on writing scientific explanations: a case study from a chemistry classroom in an all-girls school†
Gde Buana Sandila
Putra
and
Kok-Sing
Tang
*
National Institute of Education, Nanyang Technological University, Singapore. E-mail: koksing.tang@nie.edu.sg; kok-sing.tang@curtin.edu.au
First published on 21st April 2016
Abstract
This paper is a case study that reports on findings from a design-based research with the purpose of helping secondary school chemistry students in an all-girls school develop the ability to construct scientific explanations – an important literacy skill in learning science. A series of lessons on the topic of chemical bonding was designed to explicitly teach the three-part structure often found in the genre of scientific explanations and provide opportunities for students to apply the structure. The lesson series was observed and the students' worksheets and test papers were collected and analysed. The analysis of the structure of the students' written scientific explanations was done through genre analysis. Most of the students were found to be able to write well-structured scientific explanations addressing the topic of chemical bonding but only a fraction of them could re-contextualise the explanation structure.
Science is one of the most challenging disciplines to learn. Not only do students need to learn the content knowledge of science but also its peculiar language, linguistic features, and practices (Lemke, 1990; Wellington and Osborne, 2001; Fang, 2005). One of the most prominent linguistic practices in science is structuring logical explanations of natural phenomena (Halliday and Martin, 1993), and as suggested by various researchers (e.g., Moje et al., 1997; Osborne and Patterson, 2011), the genre of scientific explanations is crucial, which students need to learn in order to be able to participate in the discipline of science. Although the genre of scientific explanations is central to the science discipline, explicit teaching of it is rarely observed in science classrooms (McNeill et al., 2004) and ought to be taught (Osborne et al., 2004). The present study, therefore, attempts to address this gap in the current teaching practices by introducing an explicit form of disciplinary literacy instructions to improve students' written scientific explanations. In particular, the present study is a case study to illustrate how a chemistry teacher in an all-girls school taught scientific explanations explicitly in the topic of chemical bonding and its outcome towards students' writing skill. The findings of this study will be discussed in light of developing effective disciplinary literacy instructions and consequently helping students acquire the skills to participate in the science discipline.
Theoretical and analytical frameworks
The theoretical frameworks we adopt to position ourselves in the present study are drawn from the notions of disciplinary literacy, philosophy of science on scientific explanations, and genre studies. The notion of disciplinary literacy provides a perspective on how classroom teaching could look like to nurture scientifically literate students. Philosophy of science sheds some light on what constitutes scientific explanations. Lastly, genre studies provide a lens to view and analyse scientific explanations as a unique genre in science which has its own writing convention.Disciplinary literacy
Disciplinary literacy refers to the ability to use specialised language and practices valued and used in a given discipline to navigate and participate in the discipline. Thus, disciplinary literacy is a pedagogy that aims to nurture students to be literate in the discipline they are pursuing. The main idea of disciplinary literacy pedagogy is to have students to be apprentice disciplinarians and practise what disciplinarians do. Moje (2007) reviewed the various views towards disciplinary literacy pedagogy and noted four distinct patterns in the way disciplinary literacy is conceived and taught:(1) disciplinary literacy pedagogy as teaching cognitive literacy processes,
(2) disciplinary literacy pedagogy as teaching epistemological processes of the disciplines,
(3) disciplinary literacy pedagogy as teaching linguistic processes of the disciplines, and
(4) disciplinary literacy pedagogy as teaching linguistic and discursive navigation across cultural boundaries.
In the present study, we adopt the third view, disciplinary literacy pedagogy as teaching linguistic processes of the discipline, as a lens to guide us in our instructional design. This approach seeks to bring awareness of text features and structures to students, and argues that linguistic features and structures of texts of a particular discipline can and should be made explicit to students with teachers' guidance so that students will be more familiar with the texts in the discipline. The term ‘explicit’ here is meant to emphasise the need for linguistic features and structures of texts to be pointed out and discussed with students, rather than just exposing them to disciplinary texts. Specifically in our study, we focus on teaching a rhetorical genre that is commonly encountered by students of science – scientific explanations.
Scientific explanations
Scientific explanations are a rhetorical genre that is central to the discipline of science. According to the Common Core Standards (Council of Chief State School Officers, 2010) and the Next Generation Science Standards (National Research Council, 2012), one of the key goals for science education at the secondary level is to make students construct scientific explanations. The importance of scientific explanations is further reflected by numerous research studies devoted to explore this important genre in the science discipline (e.g., Moje et al., 1997; Taber and Watts, 2000; Rottman and Keil, 2011; Wang, 2014). In the chemistry discipline, for example, scientific explanations have been examined in terms of learners' explanations (Taber and Watts, 2000; Stefani and Tsaparlis, 2009) and teleology (Talanquer, 2007) to shed light on the kinds of explanations used in chemistry classrooms.However, despite the many research studies done on scientific explanations, there is little consensus about the nature of scientific explanations and the conceptualisation of scientific explanations has not been clearly articulated for science education. Braaten and Windschitl (2011) attempted to unify the various views on scientific explanations by reviewing various models of scientific explanations from philosophy of science and conceptualising scientific explanations in science education. They reviewed that there are five major scientific explanation models: the “Covering Law” model, statistical-probabilistic model, causal model, pragmatic model, and unification model. In addition, they described three common uses of scientific explanations in science education: explanation as explication, causation, and justification.
We adopt the lenses of Covering Law, causal, and unification models to view scientific explanations in this study. The Covering Law or the deductive-nomological model refers to the view of scientific explanations as a result of deduction from laws or law-like statements (nomology) arisen from natural observations of regularities to generate deductive or logical arguments to explain a phenomenon. This model encourages students to think of laws or facts to support their explanations but it often leads to overgeneralisation and causes scientifically unsound explanations. Braaten and Windschitl (2011, p. 645) gave an example of a student in their classroom responding to “why do you think that water vapour is starting to rise from the beaker of water?” with “because it's on the hot plate heating up, and that's what happens right before it boils.” The student appealed to a natural phenomenon or fact that she observed daily to construct her explanation. Although her explanation was logically deduced from a natural observation, it was not scientifically supported or sound.
The causal model, hence, is necessary to supplement the Covering Law model. Salmon (1978) argued that the power of scientific explanations is enhanced when explanations involve ideas from scientific theories to account for phenomena. The causal model strengthens the Covering Law model by resolving the causal gap between the laws or law-like statements and the phenomena by providing theories and underlying mechanisms of the phenomena. The inclusion of ideas from scientific theories in explanations will generate logical and scientifically sound explanations.
The unification model asserts that explanations are enhanced when explanations can unify seemingly disconnected phenomena into a coherent relationship, providing holistic understanding of phenomena (Friedman, 1974). Friedman (1974) suggested that well-established scientific theories afford the ability to explain phenomena across a range of observations and help us unify phenomena and their explanations. The unification model, thus, encourages the use of well-established scientific theories or laws as the basis of scientific explanations. This model complements well with both Covering Law and causal models. Not only are laws or law-like statements and linking of ideas from scientific theories required in scientific explanations, the laws must also be well-established. Using these three models as criteria or foundations, we view scientific explanations in this study as coherent logical deductions from well-established scientific theories and laws to make sense of natural phenomena.
The word ‘explanation’ is ambiguously used in science classrooms. Commonly it can be used as explication, causation, and justification (Braaten and Windschitl, 2011). Explanation as explication is when explanation is used in its “common sense” meaning – to get further elaboration or definition of phenomena. The use of the word in this sense is rarely a concern in science education research. However, the other two uses of the word are widely researched.
Explanation as causation focuses on causal and mechanistic accounts of phenomena (Hammer et al., 2008), on the other hand, explanation as justification focuses on argumentation – defending or making sense of scientific claims. Both views on explanations are interconnected and related epistemically in terms of using logic and evidence, and often treated as one. However, the distinction between the two is necessary (Osborne and Patterson, 2011). Osborne and Patterson (2011) argued that explanations and argumentation serve different purpose. Explanations promote creation of new knowledge to account for new phenomena while argumentation promotes exploration of the justification of belief and a dialectic between construction and critique (Ford, 2008). Therefore, they should not be treated as a single practice as the pedagogical practices required to teach students are different.
Numerous research studies have been done to address explanation as justification (e.g.Sandoval and Reiser, 2004; Sandoval and Millwood, 2005; McNeill et al., 2006; McNeill and Krajcik, 2008) and the typical classroom instruction to construct explanation as justification consists of “three components: claim, evidence, and reasoning” (McNeill et al., 2006; McNeill and Krajcik, 2008). However, little has been done to address the pedagogical requirement to teach explanation as causation. Thus, in the present study we focus on explanation as causation and address its pedagogical requirement.
Genre studies
Genre studies focus on text structures which are valued in disciplinary literacy pedagogy. There are two branches of genre studies that inform the present study, namely Systemic Functional Linguistics (SFL) (Halliday and Matthiessen, 2004) and English for Specific Purposes (ESP) (Swales, 1990). Both SFL and ESP assert that the organisation of language within a culture is influenced by its social purpose and context. In this sense, each discipline, thus, has its own unique way of organising language and has various genres which are dependent on the communicative purposes. Each of these genres has a unique writing convention to achieve the intended communication. For example, the genre of scientific argumentation has the purpose of convincing audience to the claim presented and thus it has to be presented in a specific way to achieve that purpose, which is by presenting claim, evidence, and reasoning (Mcneill and Krajcik, 2008). The teaching of language organisation and genre in the discipline, therefore, becomes a crucial classroom teaching practice as it allows students to recognise and be familiar with the unique language used in the discipline, and subsequently acquire the skills to participate in the discipline.Based on research studies on the genre of scientific explanations (e.g., Unsworth, 2001; Osborne and Patterson, 2011) and philosophy of science on scientific explanations, we generate a three-part structure of scientific explanations called the PRO (Premise–Reasoning–Outcome) structure (see Table 1; also Tang, 2015). This structure consists of three rhetorical moves or steps: (1) stating the ‘Premise’, (2) inferring the ‘Reasoning’, and (3) stating the ‘Outcome’. The element of ‘Premise’ refers to well-established scientific facts, laws, models, or theories that students can use to ground their explanations. As in the Covering Law and unification models, scientific explanations need to be deduced from some laws or well-established facts that can support the observed phenomena and, hence, a law or theory such as Kinetic Theory of Matters needs to be stated when writing scientific explanations. Particularly in chemistry, the use of models is important to establish scientific explanations (Taber and Watts, 2000; Coll and Taylor, 2002; Chittleborough and Treagust, 2007). ‘Reasoning’ refers to the logical gap between a theory and an observed phenomenon. A scientific theory or fact often does not explain a phenomenon directly. For example, the fact that strong ionic bonding is present in sodium chloride does not explain that it cannot conduct electricity in the solid state. There is a logical gap between the scientific fact and the observed phenomenon – that because of the strong ionic bonding, the ions are held in a fixed position, unable to move to conduct electricity. The element of ‘Reasoning’ is, thus, essential to establish a cause–effect relationship that the causal model suggests. The element of ‘Outcome’ simply refers to the observed phenomenon to be explained. In writing a scientific explanation, it is imperative to state the phenomenon to be explained as it is the end point of the logical deduction from the law. This PRO structure becomes the basis of the teaching of the scientific explanation genre and our analysis, in which we compared students' written scientific explanations to this move structure.
During classroom implementation, we used the term “Principle” instead of “Premise” because it was felt that many students were not familiar with the word “Premise”.
Methodology
Research design
This study utilised a case study approach (Yin, 2013) to investigate how a class of secondary students learnt to construct scientific explanations in chemistry. As our aim was to understand how the PRO structure was taught in a classroom context, case study is a suitable methodology as it provides a holistic and in-depth examination of a teaching issue within a bounded system consisting of the teacher, students, curriculum, and school environment.Research context
The intervention study was carried out in a secondary 3 (9th grade) chemistry classroom in a government-aided all-girls school in Singapore, where the medium of instruction is English language. The students' academic ability was above average, based on their performance in the Primary School Leaving Examination (PSLE), a national examination taken by every 6th grade student in Singapore. The school was recruited because the school management was interested in participating in the project.
In this study there were 28 female secondary 3 chemistry students in Kathryn's class. Out of three classes taught by Kathryn, this class was selected because it was the only class that took pure chemistry subject, compared to the other two classes that took a combined science subject with a chemistry component. At the end of secondary 4 (10th grade), they had to sit for the Singapore-Cambridge General Certificate of Education (Ordinary Level) examinations. According to Kathryn, these students were of higher learning ability than another pure chemistry class taught by another teacher.
The lesson series was designed to complete in 8 periods of 30 minutes in the span of 2 weeks, as summarised in Table 2. The topic of chemical bonding was chosen because it required the students to write many complex explanations. As such, one of the aims of the lesson design was to teach the students how to write scientific explanations explicitly by using the PRO structure and writing scaffolds. A worksheet was designed to complement the lesson series and integrated into the students' printed notes, consisting of writing scaffolds, and content information.
| Period | Activity |
|---|---|
| 1 | Students watching video on the conductivity of salt in solid and molten states |
| 1 | Students having discussions on the video |
| 2 | Teacher lecturing about bonding in ionic compounds |
| 2 | Teacher teaching writing explanations using the PRO structure |
| 2 | Students attempting questions using the PRO structure and jumbled-up explanation sequences |
| 3 | Teacher wrapping up sub-topic ionic compounds |
| 4 | Students discussing about simple covalent compounds |
| 5 | Teacher lecturing about simple and giant covalent compounds |
| 6 | Students attempting questions using the PRO structure and jumbled-up explanation sequences |
| 7 | Teacher wrapping up sub-topic bonding in covalent compounds |
| 8 | Teacher lecturing about bonding in metals |
| 8 | Teacher wrapping up the topic |
Although certain methods of teaching were discussed, Kathryn made the final decision on which strategies she would adopt and how she would enact the lesson series. The students were required to write scientific explanations throughout the lesson series with the writing scaffolds gradually removed. At the end of the term, the students sat for a term test which covered various topics that they had learnt, including chemical bonding. In the term test paper, we asked Kathryn to include five explanation questions that would assess the students' ability to write scientific explanations for our research purpose.
Research questions
This study aimed to understand the teaching of scientific explanations through an explicit disciplinary literacy instruction and its outcome. Thus, the research questions being explored were:(1) How did Kathryn teach the genre of scientific explanation through an explicit disciplinary literacy instruction?
(2) What were some of the outcomes of such teaching?
Research data and analytical methods
Prior to the data collection, this study was reviewed and approved by the university's institutional review board. The participants were briefed about the study and given written copies of the summary of the study and their rights as participants. Signed written consents from the teacher and the students' parents/guardians, and written assents from the students were obtained to allow us to video record the classroom activities and collect the students' written artefacts. All names in this paper are pseudonyms to preserve anonymity.The data sources used in this paper include videos of classroom observations, worksheets, and test papers. A total of 4 hours of video records were collected in the lesson series for analysis. The videos were viewed as a whole to see the progression of the teaching of scientific explanations (see Table 2). Furthermore, the videos were then segmented using the Transana software into discrete units according to discernible boundaries in order to facilitate coding and annotation. Video segments where Kathryn taught and facilitated the writing of scientific explanations to her students were identified for more in-depth analysis. These segments were transcribed and analysed. In particular, we used discourse analysis to analyse in detail how Kathryn taught her students how to construct scientific explanations explicitly, specifically using the PRO structure, and how she utilised writing scaffolds to achieve the learning objective.
To explore the student’s learning outcome from Kathryn's teaching, we collected the students' artefacts in the forms of notes, worksheets, and test papers. The artefacts were analysed using genre analysis (Swales, 1990; Bhatia, 1993) to examine the rhetorical move structure in the students' scientific explanations. We identified the three elements essential to scientific explanations: Premise, Reasoning and Outcome in the students' written scientific explanations. We put more focus on analysing scientific explanations written by the students in the term test paper because unlike their notes and worksheets, they could not refer to any study materials while constructing the explanations. The analysis in this study focused on the structure of scientific explanations written by students. Although we did consider the content of the students' explanations to guide us to identify the Premise, Reasoning, and Outcome, we did not assess the content accuracy of the explanations. What we were particularly interested in was whether the students knew how and were able to construct a sound scientific explanation. The analysis was done by two researchers independently with an inter-rater agreement of 82.6%. Any disagreement was discussed and resolved. The content of the explanations was separately and independently assessed by Kathryn as part of the school assessment. Kathryn's grading focused on the understanding of concepts rather than the structure of the explanation.
Analyses and findings
RQ 1 – Kathryn's teaching of explanations
We analysed the video records of Kathryn teaching in the chemistry class and noted how she attempted to incorporate some elements of explicit disciplinary literacy teaching of constructing scientific explanations.| Excerpt 1 | |
|---|---|
| Speaker | Utterance |
| Teacher | This is the PRO framework. What does PRO stand for? Professional, eh? Alright, so if you want to be very professional in writing, in crafting your answer, we need to look at number one, the Principle. |
| Principle simply means what you know about this particular bond. For example, the structure and the bonding. You can state for me what kind of bonding is it, how is it being held together. The forces of attraction. Alright? | |
| R is the Reason, alright? So for anything that happens, for anything that you observe, there must be a reason. Why is it when solid, it didn't light up, but when molten, it lights up. Alright? And Observation is what you see, alright? | |
In an attempt to introduce the PRO structure, Kathryn began by telling the students why they needed to know about the PRO structure, which was to write an explanation professionally. She continued by unpacking what each of the P, R, and O stood for, and what they were. In the extract above we could see that Kathryn attempted to unpack the PRO as general as possible, in that the Premise (she used the term Principle instead) was something that the students knew, Reasoning was the reason that could account for the observable outcome – a justification connecting the Premise and the Outcome, and Outcome (she used the term Observation instead) was something that the students could observe in the experiment. Kathryn's move to tell students why they needed to know the PRO structure at the start ensured that students knew why they needed to have such a structure. However, Kathryn did not elaborate how to “write (what) or answer (to what kind of question) professionally”. The general unpacking of the term PRO with some exemplars was to let the students know what was required of them and allowed them to generalise the structure and apply the structure to topics other than chemical bonding. However, in the subsequent lesson, in an attempt to simplify and contextualise the PRO structure, Kathryn used the PRO structure in a more specific manner, confined to the topic of chemical bonding as shown in Excerpt 2.
| Excerpt 2 | |
|---|---|
| Speaker | Utterance |
| Teacher | Alright, so basically the three things we have mentioned will be the principle, the reason, and your observation, alright, known as the PRO. |
| So basically principle is what you know about the compound in terms of structure and bonding. What do you know, alright? | |
| And when we talk about reasons, the reasons will be the things we see on the micro level, okay, are things we see at the micro level, in terms of the forces of attraction, or in terms of the free mobile ions. Okay? | |
| And of course, the last one is actually the observation. So observation would be, stating, alright, what you observe about its physical properties. | |
In Excerpt 2, which occurred the day after the lesson in Excerpt 1, Kathryn reminded the students on the PRO structure. Kathryn contextualised the PRO structure to the content of the topic. Specifically, she equated the term Premise to something about the structure and bonding, Reasoning to something on the (sub)microscopic level (e.g., forces of attraction and free mobile ions), and Outcome to something about physical properties. We see this as an attempt to familiarise the students with the explanations required specifically for this topic of chemical bonding. By limiting the PRO structure to specific concepts taught in the topic, we anticipate that the students may only apply this PRO structure to explain phenomena related to the topic of chemical bonding.
This activity acted as a scaffold to the students. All the necessary points for the explanation were given to them and they just had to arrange them accordingly. We see this as an essential part of the teaching of explanations. Although Kathryn had elaborated the three elements of scientific explanations, the students needed to have some hands-on practices to apply the PRO structure and this activity provided the opportunity for them to specifically construct an explanation without having to recall the details on the content.
| Excerpt 3 | |
|---|---|
| Speaker | Utterance |
| Teacher | Okay, alright, very quickly, can you alert me which one is the principle? Jessica? Which statement? Can you read out for us? Which one do you think is the principle? That means tell me something about the structure and the bonding. |
| Jessica | No mobile ions |
| Teacher | There are no mobile ions? |
| Jessica | No, no, ions can move freely. |
| Teacher | “Ions can move freely”. Alright, what is this for? Maybe we start from behind, maybe it is easier? If we start from observation, can you tell me which one is an observation? |
| Jessica | Can conduct electricity |
| Teacher | Can conduct electricity in molten state. This is an observation, yah? Anymore observation from here? No? What about the main reason for conducting electricity? |
| Jessica | Ions can conduct electricity |
| Teacher | Ah, ions can conduct electricity, so this is a reason, what else? Or there's nothing else? |
| Jessica | Move freely |
| Teacher | Ions can move freely, any other reasons? You see, my main idea is I want to conduct electricity, so what is the reason? The reason is because there is free mobile ions, alright? What about the rest of them, when I talk about “strong electrostatic forces of attraction”, “oppositely charged ions”, what are they? In principle, what they are, the structure, the bonding itself. So the bonding is the “strong electrostatic forces of attraction”, alright, and “oppositely charged ions are held less tightly together”. Okay, so let's take a look and see whether or not we have it in the correct sequence. They say that in the molten state, what happens is, the strong electrostatic forces of attraction have be overcome by the strong heating, so oppositely charged ions are now free, okay, less tightly held together. And because of that, they can move freely and because they can move freely, therefore they can conduct electricity. |
Kathryn's decision to restart the discussion and to start from identifying the Outcome is worth a second look. While an explanation is typically deductive, in Excerpt 3 she took an inductive approach to get her students to reach a full explanation that had all the elements of an explanation. She started from the phenomenon being discussed (Outcome) and then probed for the Reasoning and then the Premise. She did this while still maintaining her usual teaching style of engaging students through IRF triadic pattern. The only difference was that she explicitly labelled the stage of her explanation (e.g., “so this is a reason”, “in principle…”). When Kathryn finally made a summary at the end, she reformulated her chain of thoughts and constructed the explanation deductively, from the Premise to finally reach the Outcome.
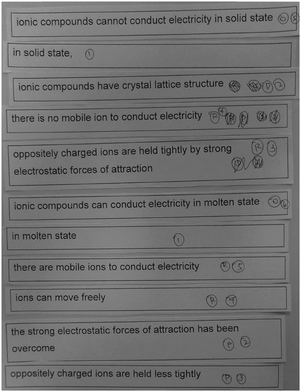
Kathryn also had her students compare their answers to each other. In Excerpt 4 below, Kathryn projected one of her students', Cecilia's explanation (see Fig. 2) onto the screen and asked other students to comment and compare their own explanations with Cecilia's. Melissa was nominated to share her thoughts. She commented that her own explanation was not as detailed as Cecilia's. Kathryn in turn invited the class to help Melissa fill up the missing piece in her explanation. Later Kathryn pointed out the missing piece and elaborated what made a complete explanation.
| Excerpt 4 | |
|---|---|
| Speaker | Utterance |
| Teacher | Can I give you a very quick one minute? You read through again what she has written, talk to your friend, and compare with yours… |
| (Students discussing) | |
| Teacher | Are we quite ready? Melissa, can we start with you? Is there anything wrong with the answers first of all? |
| Melissa | (silent) |
| Teacher | …It's okay if you pick out a mistake, we just learn from there. If there's any you just say what it is, just point out. |
| Melissa | (inaudible) |
| Teacher | …I ask you to compare your answer to her answer, is there any difference? |
| Melissa | About the same. |
| Teacher | About the same? So that means there is a little bit not the same. So what is not the same? |
| Melissa | Her answer is more detailed than mine |
| Teacher | Her answer is more detailed than yours. That means something is missing from your answer. What is missing from your answer? |
| Melissa | The molten state thing. |
| Teacher | What about the molten state thing?… Can you read out what you have for the molten state? |
| Melissa | Potassium chloride in molten state, however, have free moving ions as they are not held together by electrostatic forces of attraction. |
| Teacher | Alright, so what do you think she has missed out girls? She says that the molten state, the ions are free to move about to conduct electricity, is that what you mention? |
| Melissa | No, they are free moving |
| Teacher | They are free moving, to conduct electricity, yeah? So if you compare hers to Cecilia's answer, what do you think is missing? |
| Student | The potassium chloride |
| Teacher | Good. One very obvious thing that is missing is this, “potassium chloride”… What else is missing from Melissa's answer? Let me repeat her answer again. She say, “in the mobile state, there are free mobile ions to conduct electricity”. Is this statement correct? Yes, but did she explain to you why is it that now these ions become mobile? No. So this is the meaning, the explanation is not there. So if you look at Cecilia's answer, can you tell me what is the explanation for free moving ions or free mobile ions? Yes, Valencia? |
| Valencia | The forces of attraction has been overcome. |
| Teacher | Very good! The forces of attraction has been overcome, so this is an important link between the two. So when you talk about molten state, you need to explain to me what do you mean by molten state. It means to say the forces of attraction has been overcome, and because of that, now, my ions can move freely. No longer is it held in a fixed position... talk about the principle, in this answer, which one is the principle that she has mentioned? What you know about it? That it's a? Crystal lattice structure. |
Kathryn attempted to engage her students to critique each other's explanations in order to arrive at a well-written explanation. We see this as an important feature of disciplinary literacy teaching as immersing students in the practice of the discipline is one of the key ideas of disciplinary literacy teaching. The students were given opportunity to be like apprentice scientists, critiquing each other's work just like scientists do. The critique session was still predominantly guided by Kathryn but considering that this was the first time the students were tasked to do so, they could be unsure of what to do and, therefore, needed guidance from Kathryn.
In summary, there were three features in Kathryn's teaching repertoire that we consider to be important in explicit disciplinary literacy teaching of scientific explanation: (1) introducing and unpacking the structure of scientific explanations, (2) providing support for students to construct scientific explanations, and (3) immersing students in the practice of discussion and critique.
RQ 2 – students' scientific explanations
Five questions were included in the students' term test paper to assess how well they could explain each of the following:(a) Why does copper have a high melting point of 1036 °C?
(b) Why is aluminium malleable and ductile?
(c) Why is iron a good conductor of electricity?
(d) Why does hot air rise?
(e) Why do liquids take the shape of their containers but not solids?
Questions (a) and (c) are covered during the lessons on chemical bonding taught by Kathryn, questions (b) and (e) are related to chemical bonding while question (d) is remotely related to the topic of chemical bonding. The students' answers to the five questions were analysed for their structure (Swales, 1990; Bhatia, 1993). Fig. 3 exemplifies how the analysis was done.
In Fig. 3, rhetorical move 1 (stating the Premise) was identified by locating clauses that showed facts about the object in questions. For instance, for question (a) which was about copper metal, clauses that were about the structure and bonding of copper metal were identified as the Premise. Move 2 (inferring the Reasoning) was identified by locating clauses that were not ‘obvious’ facts and had to be inferred from the Premise – answering the ‘so what?’, such as clauses about energy requirement in question (a). Move 3 (stating the Outcome) was identified by locating clauses that showed the phenomena and were similar, if not the same, to the question, such as clauses about the high melting point in question (a). The same method of analysis was applied to questions (b), (c), (d), and (e). The students' written scientific explanations were then labelled as ‘PRO present’ if all three moves were found, and ‘PRO absent’ if any of the moves was found to be missing. The results of the analysis were then tabulated in Table 3.
| Question | Percentage of P | Percentage of R | Percentage of O | Percentage of PRO |
|---|---|---|---|---|
| Note: n = 27. | ||||
| (a) | 100 | 92 | 81 | 81 |
| (b) | 85 | 67 | 70 | 41 |
| (c) | 74 | 96 | 92 | 70 |
| (d) | 92 | 33 | 77 | 22 |
| (e) | 92 | 92 | 70 | 56 |
The high percentages of the PRO structure and its elements found in (a) and (c) suggest that most students have understood the structure and applied their knowledge about these two questions during the test. However, in (b), (d), and (e), only low to moderate percentages of the PRO structure were found, suggesting otherwise.
Triangulating this result with the analysis of classroom videos, we posit that the high percentage in (a) and (c) was due to the highly contextualised disciplinary literacy teaching. As Kathryn equated Premise to the structure, Reasoning to the (sub)microscopic level, and Outcome to physical properties during the instruction, the students could have inferred that the PRO structure taught in the lessons was the structure of the scientific explanation on Chemical Bonding and could only be applied to explain phenomena related to Chemical Bonding.
Interestingly, although question (b) is related to Chemical Bonding while (e) is not, moderate percentage of the PRO structure was found in students' answers to both questions. Upon closer analysis of the nature of the explanation required, although question (e) seems to be unrelated to Chemical Bonding, the requirement for the explanation is similar to that for questions on Chemical Bonding. In question (e), the Premise can be about the structure of liquids and solids; the Reasoning can be about the ability of particles to move about (submicroscopic level); and Outcome is about the physical properties of liquids and solids. Some of the students could have recognised the connection between question (e) and the contextualised PRO structure and thus applied it in their answer to question (e). The reason why only a moderate percentage of the PRO structure was found in questions (b) and (e) could be due to lack of writing practice for such questions.
The low percentage of the PRO structure found in the students' answers to question (d) suggests that most students could not apply their knowledge of the PRO structure to an entirely new context, especially the Reasoning element. We posit that the highly contextualised instruction impeded the students' application of the PRO structure to a new context. Students could have failed in generalising and re-contextualising the PRO structure to the structure of written scientific explanations.
Discussions and teaching implication
Kathryn's teaching of writing scientific explanations presented above offered a glimpse of how explicit disciplinary literacy teaching of scientific explanations could be realised. The teaching featured explicit instructions on how the students could construct scientific explanations using the PRO structure, scaffolding activity of rearranging paper strips containing fragments of a full explanation, and the whole-class discussion on the students' explanations. Typically science teachers teach scientific explanations implicitly by modelling the required explanation through a series of triadic dialogues without discussing how the explanation is constructed or structured like how Kathryn did it. The issue with teaching scientific explanations implicitly is that it may only help students learn how to explain one particular phenomenon being discussed, most probably through memorisation. Students may not be able to see the structure or pattern behind all the explanations modelled by their teacher. The teaching of the PRO structure may help ease students' task in figuring out what is required of them to construct scientific explanations.The findings on the students' written explanations suggest that not all of the students managed to produce explanations with the PRO structure in new contexts. Given that it was Kathryn's first attempt at integrating explicit disciplinary literacy teaching in a short 4 hour lesson series, there may be insufficient time for the students to adapt to Kathryn's teaching and to internalise the PRO structure in their writing of scientific explanations. Furthermore, Kathryn's teaching style of contextualising the PRO structure may limit the generalisability of the structure as the students were only taught how to use it for one specific topic. Despite that, the contextualisation had its merits as reflected by the high percentage of the presence of PRO in questions (a) and (c). What we could learn from this is that teachers should balance between contextualised and general ways of teaching the PRO structure in order to make students construct explanations in the specific topic and generalise the structure to new contexts, especially when the PRO structure is introduced for the first time.
The teaching of PRO, like genre-based pedagogy, may draw criticism for being prescriptive and potentially inhibiting students' writing expressions (Hyland, 2007). However, the intention behind the teaching of PRO is to provide students with a framework to work with in constructing scientific explanations, not to dictate the way they write. Furthermore, providing the explanation structure to students can help those with low language proficiency to express their explanations.
The implication that we can draw from this case study on science, particularly chemistry teaching, is that literacy teaching could be integrated into the teaching of the content matter and should be carried out in classrooms. As chemistry teachers are more familiar with the specialised literacy practices of the discipline (such as writing a scientific explanation), they are more suitable for teaching the literacy practices in science as compared to English teachers who focus on teaching literacy use for a wider audience outside the discipline of science. Furthermore, in terms of teacher education, pre-service science teachers should be introduced to the various literacy practices of the discipline (e.g., writing explanations, translating texts into graphs, reading and interpreting chemistry texts) and the strategies for teaching them, in order to enable them to teach the literacy practices to their students in the future.
More work could be done in this study to further strengthen our findings. Comparing writing performance of students from a control group would shed some light on the benefits of explicit disciplinary literacy instructions. One of the main foci of analysis in this study is looking at the scientific explanation structure. We have not analysed the use of scientific vocabulary, subordinating conjunctions, or the syntax of the language that students use. Thus, looking at those parameters will be our research interest in the future. Furthermore, as this is a case study, we acknowledge that the findings may not be generalisable but offer snippets of how the teaching of scientific explanations could be carried out in the classroom and how students may write them.
Conclusion
The explicit disciplinary literacy instruction has its value in helping students acquire the skills to navigate in the discipline, in this case, understanding and applying the structure of the written scientific explanation. In this study, we provided a case study of disciplinary literacy teaching designed to help secondary school chemistry students develop the ability to write scientific explanations. The teacher observed in this case study exemplified some teaching repertoire that are important to disciplinary literacy teaching such as unpacking the structure of scientific explanations (through the PRO strategy), providing support for students to construct explanations, and immersing students in the practice of discussion and critique. From the term test, the high percentage of the PRO structure found in two questions on Chemical Bonding suggests that the students understood the structure of explanations for these two questions due to the teacher's contextualised way of unpacking the PRO structure within the context of Chemical Bonding. However, such a highly contextualised disciplinary literacy instruction may prove to be a double-edged sword. It may ease understanding in a particular context but at the same time impede generalisability and re-contextualisation.Overall, this paper provides insights into how explicit disciplinary literacy instructions could look like and offers a case for consideration in the teaching of chemistry. It also suggests the possibility and merits of having explicit disciplinary instructions in chemistry classroom.
References
- Bhatia V. K., (1993), Analysing genre: Language use in professional settings, New York: Routledge.
- Braaten M. and Windschitl M., (2011), Working toward a stronger conceptualization of scientific explanation for science education, Sci. Educ., 95(4), 639–669, DOI:http:// 10.1002/sce.20449.
- Chittleborough G. and Treagust D. F., (2007), The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level, Chem. Educ. Res. Pract., 8(3), 274, DOI: 10.1039/b6rp90035f.
- Coll R. K. and Taylor N., (2002), Mental models in chemistry: Senior chemistry students' mental models of chemical bonding, Chem. Educ. Res. Pract., 3(2), 175, DOI: 10.1039/b2rp90014a.
- Council of Chief State School Officers, (2010), Common Core State Standards.
- Fang Z., (2005), Scientific literacy: A systemic functional linguistics perspective, Sci. Educ., 89(2), 335–347.
- Ford M., (2008), Disciplinary authority and accountability in scientific practice and learning, Sci. Educ., 92(3), 404–423, DOI: http://10.1002/sce.20263.
- Friedman M., (1974), Explanation of scientific understanding, Journal of Philosophy., 71(1), 5–19.
- Halliday M. A. K. and Martin J. R., (1993), Writing science: Literacy and discursive power, Londo: The Falmer Press.
- Halliday M. A. K. and Matthiessen C. M., (2004), An introduction to functional grammar, Arnold Publishers.
- Hammer D., Russ R., Mikeska J. and Scherr R., (2008), Identifying inquiry and conceptualizing students' abilities, in Duschl R. A. and Grandy R. E. (ed.) Teaching scientific inquiry: Recommendations for research and implementation, Rotterdam: Sense Publishers, pp. 138–156.
- Hyland K., (2007), Genre pedagogy: Language, literacy and L2 writing instruction, Journal of Second Language Writing, 16(3), 148–164, DOI: http://10.1016/j.jslw.2007.07.005.
- Lemke J. L., (1990), Talking science: Language, learning, and values, Westport, CT: Ablex Publishing.
- McNeill K. L. and Krajcik J., (2008), Scientific Explanations: Characterizing and Evaluating the Effects of Teachers' Instructional Practices on Student Learning, J. Res. Sci. Teach., 45(1), 53–78, DOI: http://10.1002/tea.
- McNeill K. L., Lizotte D. J., Krajcik J. S. and Marx R. W., (2004), Supporting students' construction of scientific explanations using scaffolded curriculum materials and assessments, Paper presented at Annual Meeting of the American Educational Research Association, San Diego, CA.
- McNeill K. L., Lizotte D. J. and Marx R. W., (2006), Supporting students' construction of scientific explanations by fading scaffolds in instructional materials, J. Learn. Sci., 15(2), 153–191, DOI: http://10.1207/s15327809jls1502.
- Moje E. B., (2007), Developing socially just subject-matter instruction: A review of the literature on disciplinary literacy teaching, Rev. Res. Educ., 31(1), 1–44.
- Moje E. B., Peek-brown D., Sutherland L. M., Marx R. W., Blumenfeld P. and Krajcik J., (1997), Explaining explanations: Developing scientific literacy in middle school project-based science reforms, in Strickland D. and Alvermann D. E. (ed.) Bridging the gap: Improving literacy learning for preadolescent and adolesecent learners in grades 4–12, New York: Teachers College Press.
- Mortimer E. and Scott P., (2003), Meaning making in secondary science classrooms, UK: McGraw-Hill Education.
- National Research Council, (2012), A framework for K-12 science education: Practices, crosscutting concepts, and core ideas, Washington, DC: The National Academies Press.
- Osborne J. and Patterson A., (2011), Scientific argument and explanation: A necessary distinction?, Sci. Educ., 95(4), 627–638, DOI: http://10.1002/sce.20438.
- Osborne J., Erduran S. and Simon S., (2004), Enhancing the quality of argumentation in school science, J. Res. Sci. Teach., 41(10), 994–1020, DOI: http://10.1002/tea.20035.
- Rottman B. and Keil F., (2011), What matters in scientific explanations: Effects of elaboration and content, Cognition, 121(3), 324–37, DOI: http://10.1016/j.cognition.2011.08.009.
- Salmon W. C., (1978), Why ask, “why?”? An inquiry concerning scientific explanation, Proceedings and Addresses of the American Philosophical Association, 51(6), 683–705, DOI: http://10.2307/3129654.
- Sandoval W. A. and Millwood K. A., (2005), The quality of students' use of evidence in written scientific explanations, Cognition and Instruction, 23(1), 23–55, DOI: http://10.2307/3233896.
- Sandoval W. A. and Reiser B. J., (2004), Explanation-driven inquiry: Integrating conceptual and epistemic scaffolds for scientific inquiry, Sci. Educ., 88(3), 345–372, DOI: 10.1002/sce.10130.
- Stefani C. and Tsaparlis G., (2009), Students' levels of explanations, models, and misconceptions in basic quantum chemistry: A phenomenographic study, J. Res. Sci. Teach., 46(5), 520–536, DOI: http://10.1002/tea.20279.
- Swales J., (1990), Genre analysis: English in academic and research settings, Cambridge University Press.
- Taber K. S. and Watts M., (2000), Learners' explanations for chemical phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- Talanquer V., (2007), Explanations and teleology in chemistry education, Int. J. Sci. Educ., 29(7), 853–870, DOI: http://10.1080/09500690601087632.
- Tang K.-S., (2015), The PRO instructional strategy in the construction of scientific explanations, Teaching Science, 61(4), 15–21.
- Tang K.-S., (in press), How is disciplinary literacy addressed in the science classrooms? A Singapore case study, Australian Journal of Language and Literacy.
- Tang K.-S., Ho C. and Putra G. B. S., (2016), Developing Multimodal Communication Competencies: A Case of Disciplinary Literacy Focus in Singapore, in Hand B., McDermott M. and Prain V. (ed.) Using multimodal representations to support learning in the science classroom, Springer International Publishing, pp. 135–158, DOI: http://10.1007/978-3-319-16450-2_8.
- Unsworth L., (2001), Evaluating the language of different types of explanations in junior high school science texts, Int. J. Sci. Educ., 23(6), 585–609, DOI: http://10.1080/09500690010006473.
- Wang C.-Y., (2014), Scaffolding middle school students' construction of scientific explanations: Comparing a cognitive versus a metacognitive evaluation approach, Int. J. Sci. Educ., 37(2), 237–271, DOI: http://10.1080/09500693.2014.979378.
- Wellington J. and Osborne J., (2001), Language and literacy in science education, Buckingham: Open University Press.
- Yin R. K., (2013), Case study research: Design and methods, 5th edn, Washington D.C.: Sage.
Footnote |
| † This paper refers to data obtained from the research project “Developing Disciplinary Literacy Pedagogy in the Sciences” (OER 48/12 TKS), funded by the Education Research Funding Programme, National Institute of Education (NIE), Nanyang Technological University, Singapore. The views expressed in this paper are the authors' and do not necessarily represent the views of NIE. |
| This journal is © The Royal Society of Chemistry 2016 |