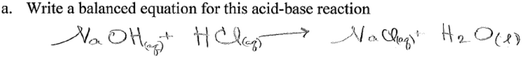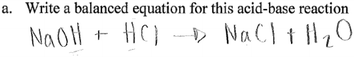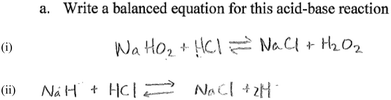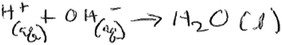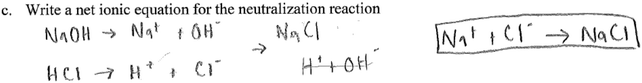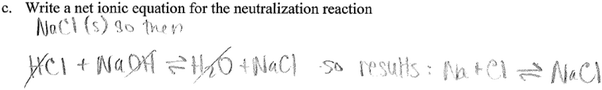General chemistry students' conceptual understanding and language fluency: acid–base neutralization and conductometry
James M.
Nyachwaya
Department of Chemistry and Biochemistry, and School of Education, North Dakota State University, 155B EML Hall, P.O. Box 6050, Fargo ND 58108, USA. E-mail: james.nyachwaya@ndsu.edu
First published on 9th June 2016
Abstract
The objective of this study was to examine college general chemistry students' conceptual understanding and language fluency in the context of the topic of acids and bases. 115 students worked in groups of 2–4 to complete an activity on conductometry, where they were given a scenario in which a titration of sodium hydroxide solution and dilute hydrochloric acid was tracked by measuring electrical conductivity. Students were asked to write a balanced equation for the reaction, provide a particulate level drawing of the reactants and products, write a net ionic equation for the reaction, predict how electrical conductivity would change with the addition of sodium hydroxide to the acid, provide a sketch of their prediction, and explain their sketch. As students worked on the activity, conversations in their groups were audio recorded. Their written responses and audio conversations were analyzed to decipher conceptual understanding and language fluency. Results showed widespread lack of conceptual understanding as well as a lack of language fluency. Students struggled with very basic ideas regarding acid–base chemistry, such as identifying the right species involved in the neutralization reaction, and providing symbolic and sub-microscopic representations (an aspect of the language of chemistry) of the acid–base reaction. Most students could not accurately predict how electrical conductivity would change as the neutralization reaction progressed. None of the groups provided an accurate sketch depicting the trend of electrical conductivity. Most of the groups did not correctly apply acid–base neutralization ideas to the context of conductometry, indicating that students were not able to transfer knowledge of acid–base neutralization to this new context.
Introduction
Acid–base chemistry is an important topic in chemistry, one that helps explain phenomena observed in the natural world (McClary and Bretz, 2012). In addition, acids and bases are also related to a number of concepts in chemistry, such as chemical equilibrium, redox reactions, solutions and solution concentration among others. Students are exposed to acid–base chemistry as early as elementary school (Lin and Chiu, 2007). Later, acids and bases are part of the high school curriculum in the United States. Furthermore, the foundational understanding of acids and bases in introductory chemistry is essential for upper level courses in chemistry, such as organic chemistry, as well as topics in other subject areas, such as biochemistry. However, despite the importance of this topic, research has shown that it continues to be a problematic topic for students (Chiu, 2004; Huang, 2004; Demircioglu et al., 2005; Kala et al., 2013).A common laboratory experiment for general chemistry students involves acid–base titrations, where in most cases, the concentration of one is known, and therefore used to determine an unknown concentration of the other reagent. At the general chemistry level, the neutralization process can be tracked using an indicator (which changes color at the end point), by changes in pH (using a pH meter) or through electrical conductivity. This study looked at the extent to which second semester general chemistry students applied their knowledge of acid–base neutralization in the context of conductometry. The students in this study covered acid–base neutralization in general chemistry (I). As part of the course, they carried out a laboratory experiment on neutralization involving a strong acid and a strong base, and determined the end-point using an indicator. In this research study, through a scenario provided and series of ‘leading questions’ students were expected to apply their prior knowledge of acid–base neutralization to conductometry, where the acid–base reaction would be monitored using electrical conductivity, a phenomenon students in this study had not experienced. Through the activity, students' conceptual understanding and language fluency in the context of acid–base neutralization was probed. This study was therefore guided by the following broad question:
What is general chemistry (II) students' conceptual understanding and language fluency in the context of acid–base neutralization?
Past studies related to acids and bases have looked at various aspects such as neutralization (Schmidt, 1991), understanding of pH (Watters and Watters, 2006), strength of acids (Smith and Metz, 1994), and properties of acids (Hand and Treagust, 1991). This study adds to the field by looking at whether college general chemistry students can apply knowledge of acid–base neutralization in another context (conductometry). The study also explores students' conceptual understanding and language fluency in the context of acids and bases.Theoretical background
Students' understanding of acids and bases
The topic of acids and bases requires an integrated understanding of a number of processes and concepts of chemistry. For example, these involve identifying compounds as acids, bases or amphoteric substances, and their respective formulas, and understanding of the particulate nature of matter, bonding, ionization (Sheppard, 2006). Complete understanding of the chemistry of acids and bases therefore requires students to not only understand each of the ideas, but to successfully integrate the ideas where necessary. For example, to explain phenomena such as pH changes during an acid–base reaction, one needs to understand particulate level interactions involving ions.One of the most common activities involving acids and bases are acid–base titrations (Sheppard, 2006), to determine an unknown concentration of one of the reactants. To fully make sense of an acid–base titration, students have to navigate the multi-representational nature of the phenomenon (macroscopic, symbolic and sub-microscopic). (Johnstone, 1991, 1993). A color change during an acid–base titration indicates that the equivalence point has been reached. The equivalence point can be understood from the interactions of particles at the sub-microscopic level. The acid base reaction can also be described in terms of formulae of reactants and products. These representations, while critical for true understanding, can be challenging for students (Johnstone, 2000; Talanquer, 2011; Taber, 2013).
Research on students' understanding of acid–base chemistry indicates that students struggle with this topic due to a number of factors, including the existence of misconceptions (Schmidt, 1997; Demerouti et al., 2004; Demircioglu et al., 2005), a lack of understanding of the particulate nature of matter (PNM) (Nakhleh and Krajcik, 1993; Nakhleh, 1994; Smith and Metz, 1994), and the language of chemistry (Schmidt, 1991, 1995). Specifically, students have been found to struggle with qualitative and quantitative aspects of pH (Nakhleh, 1990). In a study involving high school students, Sheppard (2006) asked students to explain what was happening during an acid–base titration, and found that students were not successful at describing ideas such as acid strength and pH. He attributed this to a lack of understanding of the particulate nature of matter and chemical change, as well as the ‘dense’ curriculum of acids and bases, and instruction that emphasizes algorithmic learning instead of conceptual understanding (McClary and Bretz, 2012). Smith and Metz (1994) studied college students' as well as faculty's understanding of acid strength. While undergraduate students who took part in the study showed limited understanding, the researchers found that even graduate students harbored misconceptions, especially related to the strength of acids. Interestingly, even faculty members struggled with the idea of acid strength.
A number of other studies have documented instances where students were not able to identify acids and bases in a reaction when given formulae of reactants and products (Sheppard, 2006). In this study, students struggled to translate written text into symbols (syntax). Past research has also shown that students think of the solutions obtained from neutralization of acids and bases as having no H+ or OH− ions since the ions fully consume each other (Schmidt, 1991; Demerouti et al., 2004; Demircioglu et al., 2005). In a study involving high school students, Sheppard (2006) asked students to predict how, in a conductometric titration of a strong acid with a strong base, the pH of the reaction mixture would change with the addition of the base. In the study, all students predicted a decrease in pH, which they attributed to the fact that since acids have low pH values, the pH of the resulting mixture would be low. It is interesting in this study that students focused only on the low pH of acids, and not the pH of a base. The researcher concluded that students lacked predictive accuracy, as well as a lack of understanding of underlying chemistry concepts.
One of the challenges that college chemistry students may face when learning about acids and bases is the fact that different models of acids and bases are presented. These models are Arrhenius, Bronsted Lowry, and the Lewis model. While students may come into college classrooms with a belief that all acids have a H+ ion and all bases have an OH− ion (Nyachwaya et al., 2014), the Bronsted Lowry and Lewis models, which extend the definition of acids and bases, can be confusing to students, especially when students have to simultaneously use the three ideas. In fact, research has shown that when the Arrhenius theory/model is presented before the other two theories, in the end, students' understanding is found to be dominated by the Arrhenius theory (Hawkes, 1992). Another challenge related to the three theories is that in some cases, chemistry textbooks for high school and college may not present a coherent view of acids and bases (De Vos and Pilot, 2001).
Language in chemistry
Language in chemistry comprises vocabulary, syntax and discourse patterns (Markic et al., 2013). Vocabulary encompasses words used in a discipline, which have specific meanings within that discipline. Syntax refers to conventions such as graphs, tables or equations, which are used to organize and present information. Discourse on the other hand refers to how members of a particular discipline, such as chemistry, write or talk. One of the challenges of learning chemistry is that sometimes students are required to entertain all three forms of language simultaneously, or translate from one to another. This places a huge burden on the learner. Fluency in the language of chemistry is not only a pre-requisite for further learning, it also enculturates learners into the community of chemists (Hodson and Hodson, 1998). Understanding the language of science also contributes to scientific literacy (Wellington and Osborne, 2001). According to Norris and Phillips (2003), scientific literacy enables one to infer meaning from text, and use language adequately during discourse on important issues related to life and society.Most of the teaching and learning activities that we and our students engage in science classrooms are mediated by language (Fang, 2005; Markic et al., 2013). Language is central to the learning of science, and can thus hinder or foster learning (Lee, 2001). In a classroom, students engage in reading, writing, problem solving, asking questions, communicating results of investigations and collaborative discourse, among other activities, which require the use of, and fluency in scientific language (Markic et al., 2013). Students need language in order to understand a subject, communicate what they understand, and perform tasks related to their content. Language enables students to think, ask questions, hold conversations, and ultimately learn in their discipline. Given the myriad of activities that take place in the classroom, such as reading, writing, answering questions, listening to the teacher and peers, it is important for students to understand language in order to participate in these activities.
Every discipline in school has a special language (academic language), in which concepts in various topics are presented, and conversations held (Snow, 2010). In particular, in science, in addition to communicating scientific information, language helps us understand the processes of science (Fang, 2005). For young scientists, part of their induction into science involves learning the academic language. Developing conceptual understanding in the science disciplines requires learning and understanding the language of science (Wellington and Osborne, 2001; Fang, 2005; Brown, 2013). Understanding the academic language of science makes science accessible to learners (Gilbert and Yerrick, 2001; Varelas et al., 2002; Brown, 2004; Brown and Spang, 2008), helps students develop scientific literacy (Wellington and Osborne, 2001) and ultimately affects their performance in school science (Beck et al., 2002; Varelas et al., 2002).
Mastering the language of science is one of the biggest hurdles students face in learning science (Brown and Ryoo, 2008). One of the reasons for this observation is the fact that scientific language is not native to any student, meaning that for students, learning the scientific language is akin to learning a new language (Wellington and Osborne, 2001). Scientific language is often different from students' everyday language (Wellington and Osborne, 2001). Much of the scientific language is laden with ‘heavy’ vocabulary, much of which is new to students. For example, Yager (1983) noted that a high school chemistry student will be exposed to at least 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scientific terms. The challenge is bigger when combined with the fact that scientific concepts themselves can be hard for students to master.
000 scientific terms. The challenge is bigger when combined with the fact that scientific concepts themselves can be hard for students to master.
Learning the language of science occurs at the same time that learning of science (concepts) occurs. This is one of the reasons why science is difficult. Students experience problems when learning science in a language other than students' first language (e.g. English) (Yong, 2003; Romaizah, 2009). In fact, some believe that language is a bigger barrier to learning science than content itself (Gabel, 1999; Yong, 2003). Indeed, research in chemistry education has shown that language comprehension affects student achievement in science (Lewis and Lewis, 2007, 2008).
Terminology that students encounter in chemistry takes on different meanings from every day usage. Such terminology takes on a specialized meaning when used in the context of science, (Cassels and Johnstone, 1984; Schmidt, 1991; Johnstone and Selepeng, 2001; Jaisen, 2010, 2011; Markic et al., 2013). Examples of these words that are problematic for students to use include neutralization (Schmidt, 1991; Jaisen, 2010), and strong (Jaisen, 2011). One of the challenges posed by scientific vocabulary pertains to the fact that words can and do take on different meanings based on context (Gee, 2005; Duschl et al., 2007). Such words have been called dual meaning vocabulary (DMV) (Song and Carheden, 2014). Dual or multiple meaning words have the potential to confuse students since they have context specific, narrow meanings that are different from the everyday meanings that students know of (Duschl et al., 2007).
Methodology
Context and data collection
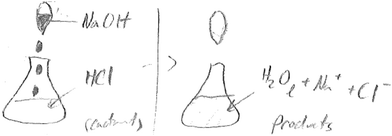
The work reported here came from a class activity in a general chemistry (II) class (115 students) from a university in mid-western United Sates in the spring semester of 2015. The class met three times a week for a total of 150 minutes of instruction. As part of the course, group discussions were routinely used to engage students. The activity occurred right before a second semester general chemistry topic of acids and bases, but draws on material that was covered in general chemistry (I) in a previous semester. It is worth mentioning that in general chemistry (I), as part of the topic on “reactions in aqueous solution” students learn about neutralization reactions, both qualitatively and quantitatively. This work therefore also looks at the aspect of retention in addition to transfer. Institutional review board approval was sought and received for this study. Consent was also sought from students to allow use of their responses as data for this research.The following questions were posed to the class, and students were asked to discuss in their groups and write down a group response. Student conversations in their groups were also recorded to give an idea of participation, nature of conversation and language use (Fig. 1).
This tool was designed in such a way as to guide students in thinking about acids and bases, to ultimately be able to answer the last three questions.
Data analysis
Data from all groups was first analyzed for conceptual correctness. Specifically, student responses were coded for (i) the right equation for the acid base reaction, with appropriate state symbols, (ii) appropriate particulate representation of the equation, showing ions in the acid and base, and water molecules with the right geometry and relative atomic sizes (iii) the right net ionic equation (iv) the correct prediction of the trend in electrical conductivity (v) correct sketch of the predicted trend and (vi) the correct explanation for the expected trend in conductivity. Audio data was transcribed verbatim, and analyzed for language fluency (such as vocabulary used and how it was used).Grounded theory was used to analyze data collected for this study. In grounded theory, themes emerge from the data (Cohen et al., 2011). Data analysis occurred at two levels. Student written responses were examined for conceptual correctness. For example, for question a, a correct response would have the correct formulae of reactants and products, with associated state symbols. Group discussions (audio) data was also analyzed for both conceptual understanding and language fluency. Decisions on conceptual understanding and language fluency emerged from or were based on data from student work.
Results
In the following sections, results will be presented by question. Sample student written responses, and in some cases where appropriate, group discussion transcripts will be used to illustrate results.a. Writing a balanced equation
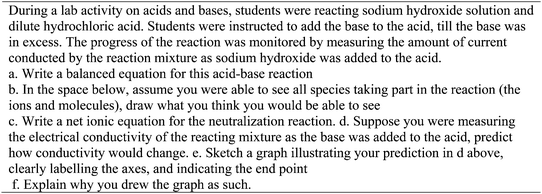
This question assessed students' ability to transfer written text (information) to symbols. Specifically, students were expected to provide the right formula for each reactant and product, including the appropriate state symbols, and balance the equations. The expected answer was NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l). Twenty three out of twenty six groups wrote the chemical equation with the right formulas of reactants and products, with fifteen groups correctly balancing the equation. Out of the twenty three groups, only four groups included state symbols for reactants and products in their equations. The following is a transcript of the discussion by one of the groups who provided an appropriate equation for the reaction.S1: So basically it would be sodium hydroxide (NaOH) plus the hydrochloric acid which is
S2: You can just put it here
S1: I just want you to be able to see
S3: Okay as long as we can still see
S1: HCl aqueous and then
S4: Is it like a double displacement reaction?
S2: I believe mmmh… am not sure. Double displacement so would it be like
S3: So it would be like switching then we would have H2O and NaCl
S1: Yea. NaCl is a liquid right? Or is it a solid?
S3: I believe it is aqueous
S4: And I believe the equation is balanced
The group came to a consensus on the following equation:
This group successfully translated text into symbols, showing ability to navigate the two languages. The conversation in the group reveals their use of language, in vocabulary such as ‘double displacement’ and ‘aqueous’. In the group, student struggles with very fundamental ideas are apparent. For example, one student does not know what a double displacement reaction is, while the student who is not sure whether NaCl is a liquid or solid lacks an understanding that NaCl is a soluble salt (therefore it would not be a solid), and that given the reaction, the physical state of the salt should be aqueous as opposed to liquid. It is also interesting that in the conversation, students who seem to have a better understanding in the group do not seem to help their peers with ideas they are struggling with and come to a shared understanding as a group. In the transcript above, when one of the students (S4) asks whether it is a double displacement reaction, the group does not address the question when the second student is not sure. When another student (S1) asks whether NaCl is liquid or solid, another student responds by saying it is aqueous, without further explanation.
As noted above, most groups did not include state symbols, which convey important information about the physical states of reactants and products in an equation of a chemical reaction. Omitting state symbols is an indication of lack of understanding of the meaning of terms such as aqueous, and solution, and how they translate to symbols. For example, one group who omitted state symbols provided the following equation:
The inability to provide correct formulas for some reactants and products is an indication of students' inability to navigate from written text to symbols, an important aspect of the language of chemistry. Also notable in both equations is the use of the equilibrium sign, which is not correct for a reaction involving a strong acid and a strong base. Students in the two groups whose responses are shown above did not know the correct formula of sodium hydroxide and water, in addition to not knowing the products of an acid–base reaction, specifically that water is produced. These students also failed to recognize the fact that because the reaction involves a strong acid and a strong base, the reaction is not reversible.
In another group, students' struggles with very fundamental, elementary ideas were evident in their conversation. For example, some students did not know which one of the reactants was the acid and which was the base:
S1: Which is the base and acid again?
S2: NaOH is the base and HCl is the acid.
While other studies have documented student difficulties with identifying acids and bases in a reaction when given formulae of reactants and products (Sheppard, 2006), in this study, some students were not able to translate written text into symbols (syntax).
b. Drawing of particulate representations
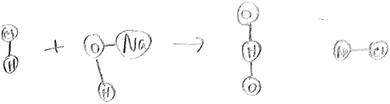
This question required students to translate from the symbolic to the particulate levels of representation. This requires an understanding that in an aqueous solution, there would be ions of the acid, base and salt, in addition to water molecules (product). Drawing these particles requires an understanding of the appropriate symbolic language. The expected response was to have Na+(aq), OH−(aq), H+(aq) and Cl−(aq) ions on the reactant side. On the products side, Na+(aq) and Cl−(aq) ions as well as H2O(l) molecules (product) were to be drawn. (Students were familiar with this convention in the course). Because of what students had covered in the course, they were not expected to draw solvent (water) molecules as part of their particulate drawing.None of the twenty six groups drew appropriate particulate representations of the reactants and products, with the right ionic species, charges, and relative atomic sizes in the case of water especially for students who chose to explicitly show the relative sizes of hydrogen and oxygen in water. The drawings provided by the groups varied widely. For example, two groups provided a macroscopic drawing of a titration apparatus, as shown in Fig. 2 below. The following transcript shows a conversation in one of these two groups, detailing their decisions as they solve the problem and plan the drawing:
S1: So I think he is trying to say that on the molecular level what is happening
S2: Do you want to draw that
S1: Sure. So on one side, you have the reactants
S3: And this would be like inside the cup of reactants
S4: Yes, yes
S1: So we have the reactants which are going to be the NaOH and HCl
S2: So the NaOH is already in there right?
S3: No that is the base
S2: So the HCl is already in the Erlenmeyer flask
S3: And then they are adding NaOH from the top
S1: Okay. And it is probably better to do like in steps. So here we have the little drip thing. This is the NaOH and once they get dripped in, then
S2: Looks good
S4: And then it breaks into ions right?
S3: So would these then be Na ions and Cl ions? Is that how this will work?
S1: I think so. I will fix the ions, so we have Na plus and then Cl minus. Okay we got it.
S4: Then in that case, we have them mixing and then making that liquid as well.
S2: So this is are they both solid or are they liquid?
S1: They are ions
S2: Ions. So they just exist in there
S1: So they are kinda chilling in there
S2: Sweet. All liquids still. So this is the H2O and Na plus Cl (referring to the mixture in the flask).
Even though the question explicitly directed students to ions and molecules, this group chose to draw flasks, reminiscent of what they do in a typical laboratory experiment involving a titration. It is interesting to see a water molecule and Na+ and Cl− ions identified as the products of the reaction. In the transcript, students use various relevant vocabulary words, such as reactants, and ‘on the molecular level’. In the transcript the students do not know whether sodium chloride would be a solid. An understanding of solubility rules, which were covered in general chemistry I, would lead to the conclusion that the salt is soluble, which would mean that the salt is aqueous. In addition, on a general level, students struggled to communicate using appropriate scientific language (academic language). For example, instead of correct, specific vocabulary, the students used slang, such as ‘they are kinda chilling in there’ (S1) and generic visual descriptions, such as ‘the little drip thing’, (S1) to communicate.
In another group, students provided the following drawing:
Notable in this drawing is the use of a covalent bonding model for ionic compounds (sodium hydroxide and sodium chloride), the drawing of dyslexic water, in addition to using an incorrect geometry for water. Another critical student error was the drawing of NaOH and HCl, since in reality, there are no NaOH and HCl particles in the reaction mixture. Instead, NaOH and HCl exist as Na+(aq), OH−(aq), H+(aq) and Cl−(aq) ions respectively. These results confirm past research which found that even successful chemistry students have problems visualizing particulate level interactions in titration experiments (Suits et al., 2005).
c. Writing the net ionic equation
To correctly respond to this question, students should know the meaning of the terms ‘neutralization’ and ‘net ionic equation’. Correctly identifying the particles involved in the reaction (and visualizing these particles) in question ‘b’ above was an important step in answering this question. In addition, this question was also meant to help students come to the conclusion that in the neutralization reaction, the net reaction involved hydrogen (H+) ions and hydroxide (OH−) ions reacting to form water (H+(aq) + OH−(aq) → H2O(l)). Eight groups wrote the correct net ionic equation, as in the example below.In conversations of groups who did not provide a correct net ionic equation, student struggle was evident as students wrote their net equation. For example, the transcript below shows a discussion in one of the groups that did not write the correct ionic equation:
S1: So the net ionic that after that is like is like the ionizing that will be after the ionizing and then you subtract like what like the changes or something
S2: It has been a long time
S3: I think I remember that HCl will break up, then you get rid of something. I can't remember.
S4: NaOH will break into this (silence) and HCl into this (silence) and water into this (silence). Then you have NaCl.
S1: That makes sense.
S4: Then you subtract water so this H plus and this OH minus so we are left with this Na plus and Cl minus to give NaCl. The group provided the following response:
Students in this group were initially successful in determining the ions in the reaction mixture. The interview transcript reveals underlying struggles in students' understanding of the process of ionization in solution. The fact that they settle on sodium ions and chloride ions reacting to form sodium chloride shows a lack of understanding neutralization, a fundamental concept in the reaction of acids and bases. Another elementary piece of knowledge missing is how to go from a complete ionic to a net ionic equation, specifically the idea of ‘spectator ions’. In the transcript, it is evident that one of the students (S3) is struggling to remember a process of writing ionic equations from previous experience. This might be an indication of reliance of rote memorization instead of conceptual understanding. Two other groups wrote the same net ionic equation as this group.
In one of the groups, the students provided the complete ionic equation, identifying the particles involved in the reaction. They however stopped at the complete ionic equation, indicating they did not understand the idea of a ‘net ionic equation’, an aspect of vocabulary in chemistry. They provided the following response
This group also mistakenly assigned the symbol ‘g’ to the hydroxide (OH−) and hydrogen (H+) ions. It is interesting that the group decided to assign different states to Na+ and OH−, both from NaOH(aq). It is not clear if this is a mistake due to carelessness. The group also assigned a negative charge to the water molecule in the equation.
Two other groups reproduced the complete molecular equation meant for part ‘a’ above, again showing a lack of understanding of the terms ‘ionic equation’. For example, one group provided the following equation:
This group made a first mistake of labelling NaCl as a solid, pointing to a lack of understanding of solubility (a connection to this being table salt would have told students that it is soluble). It is not surprising that this group did not assign physical states (symbols) in their equation. This decision most likely led to the next incorrect step. This group cancelled out hydrogen, hydroxide and water, the very species that are indeed part of the net ionic equation in the context of a neutralization reaction. At the core of this response is a lack of understanding of particulate level interactions in a neutralization reaction.
In this question, students were expected to write a complete ionic equation, identify and cancel out spectator ions, and then write down the net ionic equation. A number of groups did not correctly answer this question, confirming a finding from previous studies involving high school students (Dumon and Laugier, 2004). From these results, one can hypothesize that some students answered the question algorithmically. Students who wrote the net ionic equation as involving sodium and chloride ions to produce sodium chloride might have just been going through the mechanics of writing a net ionic equation, not informed by an understanding of the process of neutralization, specifically in the context of the reactants in the scenario provided.
d. Prediction of electrical conductivity
This question required students to think about particulate level interactions in the reaction between the acid and base, and how the ionic composition in the reaction mixture changed with the titration. The correct response takes into account the fact that as the reaction progresses towards the equivalence point, electrical conductivity would decrease, and would be minimum at the equivalence point, beyond which it will begin to rise again, with the addition of excess sodium hydroxide.Sixteen groups predicted that conductivity would increase as the titration progressed, six groups predicted a decrease, two groups predicted that the mixture would not conduct any current, one group did not make a prediction, while the remaining group provided a response that was considered irrelevant. In effect, based on the instructions in the question where the base was added till in excess, none of the groups provided a correct prediction as all of the groups that predicted a decrease did not account for the statement that the base was added till in excess. As an example, the following is a transcript from one of the groups who predicted decreasing electrical conductivity, and reasoning thorough their answer:
S1: So electrical conductivity will decrease because you are having the ions come together to form a neutral, because this is neutral, I mean this is dipolar but (silence)
S2: This is a strong electrolyte. What makes a strong electrolyte?
S3: They are both liquid
S4: They are not conductive by themselves. I just know in my notes all acids and all bases by themselves are electrolytes, but when you add them together, they are neutralized they don't have electric
S3: Yea
S4: Because these aren't
S3: These aren't acids and bases (probably referring to the salt and water).
S1: I thought this NaCl will conduct?
S4: Okay, I am overthinking again. So this is a salt. Is it not going to be an electrolyte again? Yea if there are ions if you have water breaking apart the ions in salt water, now it is an electrolyte so it can conduct from negative to positive but
S2: Well, just thinking like is salt water more conductive than regular water?
S4: Yes it is.
S2: It is?
S4: Yes it is, salt water is. It is not a strong electrolyte but it still decreases because salt water is a very low electrolyte.
After the discussion, the group provided the following written explanation (this is also part of question f below):
Electrical conductivity would decrease because a neutral salt is formed in an aqueous solution but NaCl in water is still an electrolyte.
In the transcript, students invoke relevant vocabulary, such as electrolyte, neutral, and ions. Student 4 (S4) notes correctly notes that the acid and base are electrolytes, and the fact that in water, NaCl will dissociate into Na+ and Cl− ions (hence an electrolyte) which will conduct current. Worth noting are instances where student statements indicate a lack of understanding of associated chemistry concepts. For example the notion that when acids and bases react, the resulting product is a non-electrolyte is not accurate especially in the context of the reaction provided. It is possible that the student is confused by the terms neutral and electrolyte. One student notes that salt water is not a strong electrolyte, and goes on to note that it is a ‘low electrolyte’, showing a lack of understanding of what electrolyte can be considered strong or weak. The explanation provided by this group, while partially correct, is not backed by sound reasoning. The decrease in conductivity is due to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 replacement of fast moving H+(aq) ions by slower moving Na+(aq) ions, a fact which the group does not refer to. Their answer implies that formation of NaCl hinders conductivity.
1 replacement of fast moving H+(aq) ions by slower moving Na+(aq) ions, a fact which the group does not refer to. Their answer implies that formation of NaCl hinders conductivity.
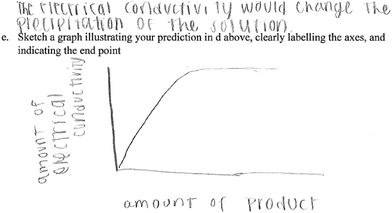
As an example, one of the groups who provided an irrelevant prediction provided the following response and the sketch in Fig. 3.
In this group, students predicted that conductivity would change the precipitation of the solution, an irrelevant response to the question. Also, this response, shows a lack of understanding of the products of the reaction, or that sodium chloride, NaCl, is a soluble salt. Given that the question identified variables that students were to base their prediction on, it is not clear whether students in this group understood what was required of them in the question.
The results here show that most students lacked predictive accuracy (Sheppard, 2006). This is possibly due to a lack of understanding of the underlying chemistry, specifically the interaction of particles during the acid base reaction.
e. Sketch of the predicted trend
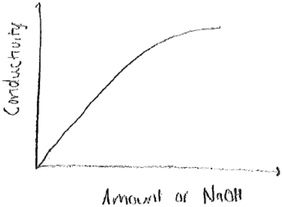
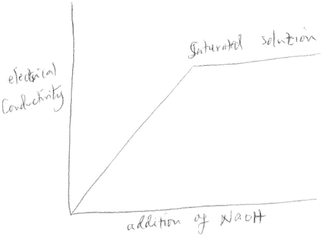
This question required students to demonstrate an understanding of particulate level interactions of ions, and what trend would be observed if the interactions were tracked. If students made the correct prediction, then they would translate their prediction into a sketch, which is an element of the symbolic language. The correct sketch would be labelled ‘conductivity’ on the y-axis, and volume or amount of NaOH on the x-axis. The sketch should indicate a decrease in electrical conductivity with the addition of NaOH, till the equivalent point, and then it would begin to rise with addition of excess sodium hydroxide. A U-shaped or V-shaped sketch was acceptable.None of the twenty six groups provided a sketch that would be considered appropriate. The sixteen groups who predicted an increase in conductivity therefore provided the most common sketch, where conductivity increased and levelled off at some point. At the core of this mistake is a lack of understanding of the particulate level interaction of H+(aq) from the acid and OH−(aq) from the base, and how this would affect electrical conductivity. Another common mistake made by most of the groups was showing electrical conductivity starting at zero. Since the acid is fully ionized already, there would be current conducted right at the start of the measurement. Fig. 4 below is an example of such a sketch:
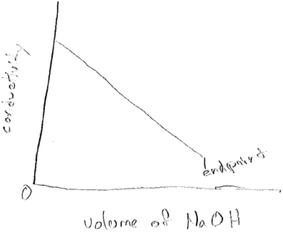
Six of the twenty six groups predicted a decrease in electrical conductivity with the addition of the base to the acid. Four of the six groups drew sketches showing electrical conductivity only up to the end point, and therefore did not account for the effect of excess sodium hydroxide on conductivity. Two out of the six groups drew sketches showing zero conductivity at the end-point. Although some sketches indicated an initial trend of decreasing electrical conductivity, they did not account for the effect of the excess sodium hydroxide on conductivity. As an example, one group provided the sketch in Fig. 5 below:
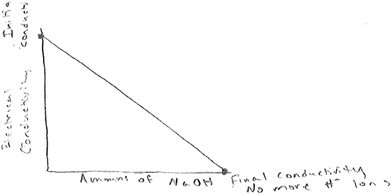
Two of the six groups who predicted decreasing electrical conductivity drew sketches showing zero conductivity at the end-point. Fig. 6 below is an example of such a sketch.
This drawing (Fig. 6) points to a lack of understanding of the composition of the reacting mixture at the end point, so that even if there were no more H+ ions as the group notes in the drawing in Fig. 6, there would be Na+ and Cl− ions that would conduct current. It also raises the question of whether students missed or did not take into account the implication of the explicit statement that sodium hydroxide is added in excess. The drawing also points to a potential for confusion from the terms ‘end point’. The students wrote the statement ‘no more H+ ions’, which is true in this context. Their statement ‘final conductivity’ is inaccurate given the composition of the reacting mixture at the end point. Past research has also shown that students think of the solutions obtained from neutralization of acids and bases as having no H+ or OH− ions since the ions fully consume each other (Schmidt, 1991; Demerouti et al., 2004; Demircioglu et al., 2005).
Other notable errors in students' drawings or sketches included missing labels or wrong labels for exes, as shown in Fig. 7 below.
Twenty four groups provided sketches that were consistent with their predictions (regardless of whether the predictions were correct or not), with the remaining groups providing sketches that were not consistent with their predictions. For example, in Fig. 8 below, the group provided a prediction that is not consistent with what they drew, specifically since their sketch has two lines.
f. Explanation of graphical sketch
The expected correct response is that as NaOH is added to the acid, OH− ions from the base react with H+ ions from the acid. At the same time, H+ ions are replaced by Na+(aq) ions from the base. The decrease in conductivity is due to the slower movement of the electric field by Na+ ions which replace faster moving H+(aq) ions. At the equivalence point, a solution of sodium chloride is formed. Beyond the equivalence point, as more base is added (excess NaOH), more Na+(aq) ions and OH−(aq) ions lead to increased conductivity, hence the rise in conductivity beyond the end point. Since none of the groups provided a correct prediction, and therefore correct sketch, none of the explanations were considered correct, even though most groups' explanations were consistent with their sketches. For example, one group that provided the sketch in Fig. 9 below had the following conversation as they formulated an explanation:S1: Okay so (silence while the student draws)
S2: Is that how you draw it
S3: Yea I think that is probably how it should look. It should get less conductive and then more conductive because it would take a little bit before the reaction actually starts taking place, right?
S1: Probably
S4: I think you are not talking about the reaction. Like the dissociation occurs instantaneously. I mean I think it does. Aside from the time it takes to dissolve. Well that is it. It is instantaneous. In fact, it is already dissociated probably
S2: Yes but if it would have dissociated, you would have the two ions. Sodium as a cation and chlorine as an anion, you should still be able to have
S3: Yea yea
S2: So I don't think your DC will ever go down.
S3: Yea I don't think it will ever go down
S2: So it will just go maybe more
S1: So it will go up maybe it plateaus or maybe it does not I don't know
S2: So what if
S4: I found a graph that says as you add it conductivity goes down (this student probably tried to google the answer).
S2: It is the same. Conductivity goes down but then once you continue to add more NaOH to the solution, when it is saturated, conductivity goes back up. It plateaus because ions are added constantly.
In drawing their sketch, this group labeled their axes correctly. Looking at the conversation in the transcript, students in the group invoked vocabulary, such as ‘conductive’, ‘instantaneous’, ‘cation’ and ‘anion’, ‘dissociated’ and ‘saturated’. However, there are problems with how they use the terms. The acid and base are already dissociated, a fact that the students do not seem to realize. Student 2 refers to ‘chlorine’ anion, which is technically incorrect. The use of the term ‘saturated’ is not relevant for this context. In the transcript, as the group grapples with whether the reaction starts when the base is added to the acid, they seem to imply that solid sodium chloride is formed, dissolves and then dissociates. Student 4 (S4) talks about the time it takes to dissolve, finally saying that it would ‘probably’ be already dissociated, finally deciding that it is instantaneous. Their explanation of the trend in electrical conductivity does not make any reference to H+ and OH− ions. Instead, this group says that current would plateau off since ions are added constantly. A major error, showing a lack of understanding of the nature of aqueous solutions is the fact that their sketch shows an initial current of zero. It is interesting to note how often students interrupted each other during their conversation, so that in some statements, some students did not get a chance to present their ideas. For example, student 2 (S2) was interrupted by students 3 and 4 (S3 and S4) in the transcript. From the transcript, students in this group did not reach consensus in some ideas. This may be attributed to the fact that students in this study had not been explicitly instructed on how to carry out a group discussion.
In another group, the explanation provided for their sketch was that “as the reactants ionize and dissolve in the solution, the conductivity will increase. When excess NaOH is added, the solution would become more basic, which will in turn increase conductivity”. In this response students do not realize that the reactants are already ionized. It is not clear whether the group is implying that ionization leads to dissolving. In addition to a lack of understanding of particulate level interactions in the acid–base reaction, and its implications on conductivity, students in this group do not realize that the acid and base are already ionized. They also do not seem to realize that when the acid and base react, new products are formed, so that the acid and base are not dissolving in the solution. The last statement, though accurate, seems to imply that the increased conductivity is due to the ‘solution’ becoming more basic in its literal sense.
Even for groups that predicted and drew a sketch indicating decreasing electrical conductivity, the explanations for their sketches were not correct. For example, one of the groups explained their sketch, saying that “because the base would balance out the acid and keep it at a constant electrical ampage”. In addition to the explanation being inconsistent with their drawing, this group's response does not account for the initial trend of decreasing conductivity that they sketched. Another group whose sketch indicated decreasing conductivity noted that “the graph reaches the end point at equilibrium”. The explanation makes no reference to the neutralization reaction and its connection to the trend in conductivity. Also, the reaction involving a strong acid and a strong base does not involve an equilibrium. This group also seems to explain the shape of the graph, as opposed to the trend they depicted, possibly because they did not interpret the question as intended.
Discussion and conclusion
This study probed students' conceptual understanding of aspects of acid–base chemistry, and language fluency in the context of acid–base, neutralization. Specifically, the language fluency aspect pertains to students' ability to translate text to symbols, their understanding and use of vocabulary, such as neutralization, ionic equations, conductivity, and end-point of a reaction. The exercise also probed students' fluency in representing information in multiple formats, such as text and symbolic representations, like a graphical sketch. From writing the equation of the reaction, to sketching a graph of their predicted trend of electrical conductivity, students demonstrated their understanding of the symbolic language. Since students dialogued in their groups, they were expected to use relevant academic language. From the results above, students were not fluent in using the academic language relevant to this activity. Their use of language in their groups also shed light on their conceptual understanding, which as seen above, is shaky.Translating text to symbols demonstrates students' facility with the language of chemistry, to take information in text format and translate it into symbols. While most groups in this study were successful at writing equations with the right formulas, most students did not provide state symbols, which communicate important information such as the solubility of reactants or products, and are an important part of the language of chemistry. Past research has shown that students are not successful at identifying acids and bases from an equation of an acid–base reaction (Sheppard, 2006). In this study, some of participants were not able to write formulas from text, especially the formulas of water and sodium hydroxide. In chemistry, students are either expected to ‘know’ the formula of a compound by memory, or determine it from the name, such as sodium hydroxide. Even though this might look to be a simple exercise, writing the correct formula requires an integrated understanding of a number of ideas (Sheppard, 2006). Such ideas in this case include knowing the formula of an element or ion (such as Na, Na+ and OH−) and how to determine the formulas using this information, especially if these formulas have not been memorized. Besides knowing the formulas, students had to also know the products of the acid–base reaction.
This study uncovered many instances where students used language inappropriately. For example, using an ‘equilibrium’ sign in the acid–base reaction equation is incorrect, especially given that the reaction involves a strong acid and a strong base. In one of the transcripts provided above, where students are reasoning about their sketch, they invoke the term ‘saturation’ or ‘saturated’, which is not relevant in the context of the acid–base reaction here. Knowing the relevant terminology is therefore not sufficient if students do not use the vocabulary in appropriate ways.
This study also highlights the inconsistency and potential for confusion in how language is used in chemistry. In this study, specifically in writing the equations, one runs into language issues, particularly the meaning of the (aq) symbol. This symbol means ‘hydrated’ or ‘in solution’. A species in the aqueous state (hydrated) is expected to be surrounded by water molecules. With this in mind, one might interpret NaOH(aq) to mean NaOH surrounded by water molecules. However, there is no NaOH in the reaction mixture, but rather Na+(aq) and OH−(aq) ions. It is not clear if the confusion is the reason students drew NaOH instead of the constituent Na+ and OH− ions in question ‘b’ above where they were asked to provide a drawing of the species taking part in the reaction.
This study confirmed findings from past research that a lack of conceptual understanding often underlies struggles with language fluency (e.g.Lewis and Lewis, 2007, 2008). For example, not knowing that an acid–base reaction (as in the scenario provided) forms a salt and water meant students would not predict the right products. Not understanding that a neutralization reaction at the core involves a reaction between H+ ions and OH− ions underlies student's inability to provide the correct net ionic equation, or make the right prediction of electrical conductivity, which would lead to an appropriate sketch and correct explanation for the trend in electrical conductivity. A lack of understanding of the particulate level interactions is also partly responsible for students who provided sketches with a current of zero, as they did not realize that the acid has ions which will conduct current initially.
Most students in this study were not fluent at using multiple representations to depict phenomena. Students were asked to write an equation of the reaction, draw particulate level representations of the reaction, write a net ionic equation of the reaction, and sketch a graph of their predictions. One danger of students having limited understanding of multiple representations of phenomena as shown in this study is the likelihood of struggling in other topics in chemistry or with upper level chemistry courses, especially related to acids and bases (Bhattacharyya and Bodner, 2005).
A major goal of science instruction and learning is to equip students with problem solving skills (Graulich, 2015). Transfer, an aspect of problem solving, requires one to apply ideas learned in one context to an unfamiliar setting or context. In this study, students were assigned an activity based on acids and bases, requiring students to apply their knowledge of acid–base titrations to conductometry. From the results, students in the general chemistry (II) course were not successful in many aspects of the exercise. The results show a lack of conceptual understanding on a very fundamental topic in chemistry. Given that the participants had encountered ideas of acids and bases, and conducted titrations as part of their general chemistry (I) laboratory experiments, the results show that the students failed to transfer that knowledge to the context of conductometry. This is most likely caused by a lack of conceptual understanding of acid–base neutralization-specifically particulate level interactions (PNM) that lead to neutralization (Nakhleh and Krajcik, 1993; Nakhleh, 1994; Sheppard, 2006).
It is evident from the transcripts in the results section that students in this study did not always take turns in talking; they often interrupted each other, and at times, when a question was posed in a group by one of the students, it was not always addressed. It is worth noting that students had not been explicitly instructed on these aspects of group work. It was assumed that they would know how to productively engage in group discussions, to give each other time to express their ideas, and that when one of their peers asked a question, the group would take time help their peer. It is not surprising that these qualities of a good group discussion were lacking in some groups, as seen in the transcripts above.
Implications
This study highlights the central nature of language in chemistry. Indeed, in the collaborative discourse/discussions that occurred in groups, students used language to communicate with each other as well as to respond to the questions they were asked. Engaging students in collaborative discourse, as shown here gives them a chance to use the language of chemistry, as well as giving instructors a window into both their fluency with the language, as well as conceptual understanding of particular chemistry concepts. Opportunities for students to use multiple representations and the academic language of chemistry, are necessary in order to help students become fluent in the language of chemistry, as well as to deepen their conceptual understanding.Often, during instruction, students are exposed to both content and ‘academic’ language at the same time, a factor that can make learning challenging (Brown and Ryoo, 2008). It is important to not assume that students will implicitly understand the language we use in our lessons. Instead, as an example, it is necessary to take time and explicitly point out and/or model to students how to switch from one language form to another, such as going from equations to text or from text to equations as in this study. In an exercise in writing equations from statements, it is important to explicitly point to students how words translate to symbols. For example, in addition to writing the formula of a reactant or product, it is important to explicitly point out the meaning of terms such as aqueous solution, and how that is presented in an equation of a reaction.
Results from this study highlight an important area and goal of science education, where students are expected to apply disciplinary knowledge in multiple contexts (Graulich, 2015). We also highlight the use of collaborative cooperative learning, where students solve problems together, learning from each other as well as gaining vital skills such as communication and problem solving. As researchers, the approach used in this study can be applied to other fields of science, or used to see the extent to which students solve inter-context problems. The results will be of interest to chemistry instructors and science education researchers, especially in the context of helping our students apply ideas across multiple contexts, and researching the extent to which students are able to accomplish this goal.
Common titration experiments are done using indicators to signify the equivalence point, or through the use of a pH meter. It may be necessary to expose students to methods such as conductometry, which are not as common, but which will help students think about phenomena in multiple ways, such as particulate level interactions as they explain observations. It is also important to present phenomena in multiple formats. In addition to recording conductivity data in a table, the same data could be plotted to see the trend displayed in a different format.
This study highlights one of the challenges to assessing student understanding, such as handling assessments from large enrolment classrooms. In the transcripts presented in the results section above, there are instances where students would have benefited from immediate feedback in the groups, so that besides the activity being an assessment, it would also be a learning opportunity. While this is a challenge, it is important to recognize the potential of collaborative group activities such as the one reported here to develop language skills in students. As students discuss in their groups, they get a chance to teach and learn from each other. One way we have addressed this challenge is through the use of student learning or teaching assistants, so that there are more of us in the classroom. As we go around the classroom, we listen in on group discussions, answer clarifying questions, and if necessary provide feedback to students such as in the use of academic language.
Given the link between conceptual understanding and language fluency demonstrated by this and other past studies, an important implication of this study is that in addition to assessing for conceptual understanding, we need to find ways to assess language fluency, especially through ‘talk’. Also, if our students have to become fluent in the ‘academic’ language of chemistry, we have to provide opportunities for students to practice using the language. As noted above, we have to assess student fluency in language alongside content, and provide feedback. In the process, we have to explicitly instruct our students on best practices of group work, such as taking turns, addressing each other's questions, and arriving at shared understanding through consensus.
References
- Beck I. L., McKeown M. G. and Kucan L., (2002), Bringing words to life: robust vocabulary instruction, New York: Guilford Press.
- Bhattacharyya G. and Bodner G. M., (2005), ‘It gets me to the product’: how students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Brown B., (2004), Discursive identity: assimilation into the culture of science and its implications for minority students, J. Res. Sci. Teach., 41(8), 810–834.
- Brown B. A., (2013), The language-identity dilemma: an examination of language, cognition, identity, and their associated implications for learning, in Bianchini J. A., Akerson V. L..
- Brown A. B. and Ryoo K., (2008), Teaching Science as a Language: A content-First Approach to Science Teaching, J. Coll. Sci. Teach., 45(5), 529–553.
- Brown B. and Spang E., (2008), Double talk: synthesizing everyday and science language in the classroom, Sci. Educ., 92, 708–732.
- Cassels J. R. T. and Johnstone A. H., (1984), The Effect of Language on Students Performance on Multiple Choice Test in Chemistry, J. Chem. Educ., 61(7), 613–615.
- Chiu M.-H., (2004), An investigation of exploring mental models and causes of secondary school students' misconceptions in acids-bases, particle theory, and chemical equilibrium, Annual report to the National Science Council in Taiwan, Taiwan: National Science Council.
- Cohen L., Manion L. and Morrison K., (2011), Research methods in education, 7th edn, London: Routledge.
- Demerouti M., Kousathana M. and Tsaparlis G., (2004), Acid–base equilibria, part I. upper secondary students' misconceptions and difficulties, Chem. Educ., 9, 122–131.
- Demircioglu G., Ayas A. and Demircioglu H., (2005), Conceptual change achieved through a new teaching program on acids and bases, Chem. Educ. Res. Pract., 6(1), 35–51.
- de Vos W. and Pilot A., (2001), Acids and bases in layers: the stratal structure of an ancient topic, J. Chem. Educ., 78(4), 494–499.
- Dumon A. and Laugier A., (2004), The equation of a reaction: a cluster of obstacles which are difficult to overcome, Chem. Educ. Res. Pract., 5(3), 327–342.
- Duschl R. A., Schweingruber H. A. and Shouse A. W., (2007), Taking science to school: learning and teaching science in grades K-8, Washington, DC: National Academies Press.
- Fang Z., (2005), Scientific Literacy: A Systemic Functional Linguistics Perspective, Sci. Educ., 89, 335–347.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gee J., (2005), Language in the science classroom: academic social languages as the heart of school-based literacy, in Yerrick R. and Roth W.-M. (ed.) Establishing scientific classroom discourse communities: multiple voices of teaching and learning research, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 19–37.
- Gilbert A. and Yerrick R., (2001), Same school, separate worlds: A sociocultural study of identity, resistance, and negotiation in a rural, lower track science classroom, J. Res. Sci. Teach., 38, 574–598.
- Graulich N., (2015), Intuitive Judgments Govern Students' Answering Patterns in Multiple-Choice Exercises in Organic Chemistry, J. Chem. Educ., 92(2), 205–211.
- Hand B. and Treagust D. F., (1991), Student achievement and science curriculum development using a constructivist framework, Sch. Sci. Math., 91, 172–176.
- Hawkes S. J., (1992), Arrhenius confuses students, J. Chem. Educ., 69, 542–543.
- Hodson D. and Hodson J., (1998), From constructivism to social constructivism: a Vygotskian perspective on teaching and learning science, Sch. Sci. Rev., 79(289), 33–41.
- Huang W. C., (2004), The types and causes of misconceptions of elementary students on acids–bases, Annual Report to the National Science Council in Taiwan (in Chinese), Taiwan: National Science Council.
- Jasien P. G., (2010), You said ‘‘neutral’’, but what do you mean? J. Chem. Educ., 87(1), 33–34.
- Jasien P. G., (2011), What do you mean that ‘‘strong’’ doesn't mean ‘‘powerful’’? J. Chem. Educ., 88, 1247–1249.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70, 701–705.
- Johnstone A. H., (2000), Teaching of chemistry—logical or psychological? Chem. Educ.: Res. Pract. Eur.,, 1, 9–15.
- Johnstone A. H. and Selepeng D., (2001), A Language Problem Re-visited, Chem. Educ.: Res. Pract. Eur., 2(1), 19–29.
- Kala N., Yaman F. and Ayas A., (2013), The effectiveness of Predict-Observe-Explain technique in probing students' understanding about acid–base chemistry: a case for the concepts of pH, pOH and strength, Int. J. Sci. Math. Educ., 11(1), 555–574.
- Lee O., (2001), Culture and language in science education: what do we know and what do we need to know? J. Res. Sci. Teach., 38, 499–501.
- Lewis S. E. and Lewis J. E., (2007), Predicting at-risk students in general chemistry: comparing formal thought to a general achievement measure, Chem. Educ. Res. Pract., 8, 32–51.
- Lewis S. E. and Lewis J. E., (2008), Seeking effectiveness and equity in a large college chemistry course: an HLM investigation of peer-led guided inquiry, J. Res. Sci. Teach., 45,794–811.
- Lin J.-W. and Chiu M.-H., (2007), Exploring the characteristics and diverse sources of students' mental models of acids and bases, Int. J. Sci. Educ., 29, 771–803.
- Markic S., Broggy J. and Childs P., (2013), How to deal with linguistic issues in chemistry classes, in Eilks, I and Hofstein A. (ed.) Teaching Chemistry-A studybook. A practical guide for student teachers, teacher trainees and teachers, Boston: Sense Publishers, pp. 127–152.
- McClary L. M. and Bretz S. L., (2012), Development and Assessment of A Diagnostic Tool to Identify Organic Chemistry Students' Alternative Conceptions Related to Acid Strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- Nakhleh M. B., (1990), A study of students' thought processes and understanding of acid/base concepts during the performance of instrument-based titrations, PhD Thesis, University of Maryland, Maryland.
- Nakhleh M. B., (1994), Students' models of matter in the context of acid–base chemistry, J. Chem. Educ., 71, 495–499.
- Nakhleh M. B. and Krajcik J. S., (1993), A protocol analysis of the influence of technology on students' actions, verbal commentary, and thought processes during the performance of acid–base titrations, J. Res. Sci. Teach., 30, 1149–1168.
- Norris S. P. and Phillips L. M., (2003), How literacy in its fundamental sense is central to scientific literacy. Sci. Educ., 87, 224–240.
- Nyachwaya J. M., Warfa A. M., Roehrig G. and Schneider J. L., (2014), College chemistry students' use of memorized algorithms in chemical reactions, Chem. Educ. Res. Pract., 15, 81–93.
- Romaizah S., (2009), Brunei primary pupils' ideas of water cycle: effects of culture and language, Jur. Pend., 14, 70–80.
- Schmidt H. J., (1991), A label as a hidden persuader: chemists' neutralization concept, Int. J. Sci. Educ., 13, 459–472.
- Schmidt H.-J., (1995), Applying the concept of conjugation to the Brønsted theory of acid–base reactions by senior high school students from Germany, Int. J. Sci. Educ., 17, 733–741.
- Schmidt H.-J., (1997), Students' misconceptions - looking for a pattern, Sci. Educ., 81, 123–135.
- Sheppard K., (2006), High School Students' Understanding of Titrations and Related Acid-Base Phenomena. Chem. Educ. Res. Pract., 7(1), 32–45.
- Smith K. J. and Metz P. A., (1994), Evaluating Student Understanding of Solution Chemistry through Microscopic Representations, J. Chem. Educ., 73(3), 233–235.
- Snow C. E., (2010), Academic language and the challenge of reading for learning about science, Science, 328(23), 450–452.
- Song Y. and Carheden S., (2014), Dual meaning vocabulary (DMV) words in learning chemistry, Chem. Educ. Res. Pract., 15, 128–141.
- Suits J., Kunze S. and Diack M., (2005), Use of Microcomputer-Based Laboratory Experiments to Integrate Multiple Representations of Scientific Phenomena, in Kommers P. and Richards G. (ed.), Proceedings of World Conference on Educational Multimedia, Hypermedia and Telecommunications 2005, AACE: Chesapeake, VA, pp. 1924–1931.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry triplet, Int. J. Sci. Educ., 33(2),179–195.
- Varelas M., Becker J., Luster B. and Wenzel S., (2002), When genres meet: inquiry into a sixth grade urban science class, J. Res. Sci. Teach., 39(7), 579–605.
- Watters D. J. and Watters J. J., (2006), Student understanding of pH, Biochem. Mol. Biol. Educ., 34, 278–284.
- Wellington J. and Osborne J., (2001), Language and literacy and science education, Buckingham, England: Open University Press.
- Yager R. E., (1983), The Importance of terminology in teaching K-12 science, J. Res. Sci. Teach., 20(6), 577–588.
- Yong B. C. S., (2003), Language problems in the learning of biology through the medium of English, J. Appl. Res. Educ., 7(1), 97–104.
| This journal is © The Royal Society of Chemistry 2016 |