Reconsidering learning difficulties and misconceptions in chemistry: emergence in chemistry and its implications for chemical education
Halil
Tümay

Gazi University, Department of Science and Mathematics Education – Chemistry Education, Ankara, Turkey. E-mail: tumay@gazi.edu.tr
First published on 4th March 2016
Abstract
Identifying students' misconceptions and learning difficulties and finding effective ways of addressing them has been one of the major concerns in chemistry education. However, the chemistry education community has paid little attention to determining discipline-specific aspects of chemistry that can lead to learning difficulties and misconceptions. In this article, it is argued that emergence plays a critical role in the epistemology and the ontology of chemistry and hence it should be taken into account for understanding learning difficulties and finding ways of addressing them in chemistry. It is particularly argued that one of the fundamental sources of learning difficulties and chemical misconceptions is learners' failure to understand the emergent nature of chemical entities, their properties, and interactions. In this article, an interpretive analysis framework is suggested for identifying specific learning demands and the sources of learners' misconceptions about the emergent chemical properties and phenomena. Findings from previous research on learners' misconceptions regarding emergent chemical properties are reanalyzed and interpreted through this framework. Inadequacies of typical teaching practices and their consequences are discussed from an emergentist perspective. Finally, implications of the emergentist perspective for more meaningful chemical education are discussed.
Introduction
Model-based accounts of conceptualization in science and learning science have been increasingly emphasized by many scholars from diverse fields such as philosophy of science, cognitive science, and science education. According to these accounts, models play a pivotal role in science and school science, and our conceptual learning is dominated by building, testing and revising models (Gentner and Stevens, 1983; Johnson-Laird, 1983; Giere, 1988, 1991; Gilbert and Boulter, 1998; Nersessian, 1999, 2002, 2009; Greca and Moreira, 2000, 2002; Halloun, 2004). These accounts provided us with a useful and productive perspective for understanding how we conceptualize our experiences, how we construct, validate, and represent our knowledge of the world in science, school science, and ordinary situations.From a distributed and situated cognition perspective, there are significant differences between the knowledge production and validation processes in science and the learning and reasoning processes in school science especially with respect to social-institutional norms, resources, and interactions in these contexts (Brewer and Samarapungavan, 1991; Brewer et al., 1998; Nersessian, 2009). Despite such differences, the core of conceptualization in science, school science and other ordinary situations is based on modelling processes (Gentner and Stevens, 1983; Johnson-Laird, 1983; Gilbert and Boulter, 1998; Nersessian, 1999, 2002, 2009; Greca and Moreira, 2000). Moreover, many scholars emphasized that conceptualization, learning, and reasoning of scientists and ordinary people are similar in many respects (e.g., Carey, 1985; Brewer and Samarapungavan, 1991; Brewer et al., 1998; Nersessian, 2009). According to these accounts, both scientists and students use the same basic cognitive processes to make sense of experiences. Nersessian (2009, pp. 137–138) clearly emphasized this point: “cognitive practices of scientists are extensions of the kinds of practices humans employ in coping with their environment and in problem solving of a more ordinary kind. … Basic cognitive strategies are extended and refined in explicit and critically reflective attempts to devise methods for understanding nature. As with mundane modes of inquiry, the success of those created by science is rooted in human nature and the nature of the world.”
From the modeling perspective we continuously create, test, and revise models that represent significant aspects of our world in order to make sense of our experiences. Models can be classified from many different perspectives, however in the context of science education Gilbert and Boulter (1998) offered a very useful classification of models based on their contextual and functional characteristics. They classified models as mental models, expressed models, consensus models, and teaching models. A mental model is a personal and cognitive representation of the target system being modelled. It is formed by an individual, either on their own or within a group. An expressed model is an external representation of the target generated from mental models and expressed through any mode of representation, such as action, speech, or writing. A consensus model is an expressed model that has been tested by scientists and which has been socially agreed by at least some of them as having some explanatory merit. A teaching model is a specially constructed model used by teachers to aid learners' understanding of a consensus model (Gilbert and Boulter, 1998). Based-on Gilbert and Boulter's (1998) classification of models we can say that one of the main aims of science education is to support learners in constructing more accurate and adequate mental models of scientific models through teaching models. From this perspective, determining the challenges and learning difficulties in constructing mental models of scientific models and then developing effective teaching interventions to overcome the determined difficulties is critically important.
Research on learning difficulties and misconceptions in chemistry
Students' misconceptions and learning difficulties in understanding scientific models has been one of the major concerns in chemistry education research (Teo et al., 2014). Studies on students' conceptions have revealed that many students at all levels of education have misconceptions about basic chemical concepts, even after years of instruction (e.g., Nakhleh, 1992; Garnett et al., 1995; Taber, 2002). However, many of these studies have only revealed lists of students' common misconceptions without sufficiently clarifying their underlying sources (Taber, 2000; Talanquer, 2006). Such an inventory or catalogue approach was criticized by many chemistry educators who strongly emphasized that developing effective instructional approaches to overcome misconceptions requires identifying and taking into account the underlying sources of these misconceptions, rather than merely listing them (Gilbert and Watts, 1983; Taber, 2000; Talanquer, 2006; Tümay, 2014, 2016).Whereas the chemistry education community has devoted substantial attention to identifying and documenting students' misconceptions, it has paid little attention to determining domain-specific aspects of chemistry that can lead to learning difficulties and misconceptions. In many studies on students' conceptions, authors frequently referred to generic factors drawn from educational psychology (and especially from constructivism) such as learners' prior knowledge, everyday experiences, confusion of scientific and everyday terminology, and incorrect representations in textbooks as the possible sources of misconceptions in chemistry (e.g., Schmidt, 1991; Wandersee et al., 1994; Sanger and Greenbowe, 1999; Schmidt et al., 2003; Demircioğlu et al., 2005). Although it is well established that these generic factors obviously affect learners' conceptualizations, and should be taken into account in teaching; they alone do not give us a clear mechanism for understanding why specific misconceptions emerge and not other possible alternatives. Consequently, these generic factors have usually been ambiguous propositions in understanding specific learning difficulties and consequently unproductive in developing effective teaching interventions for meaningful learning and conceptual change.
Although ideas from educational psychology and constructivism provides us with a general theoretical framework for understanding the effects of previous knowledge on learning new knowledge, we need more productive domain-specific theoretical frameworks to understand, explain, and predict domain-specific learning difficulties, misconceptions and corresponding teaching interventions in the chemistry context. Therefore, we should focus on the nature of chemistry and chemical knowledge to clarify the possible sources of misconceptions and learning difficulties that stem from unique characteristics of our discipline. Determining learning difficulties in a discipline and finding ways of addressing them meaningfully requires a sound understanding of the discipline and nature of disciplinary knowledge (Shulman, 1987; Scerri, 2001; Erduran and Scerri, 2002; Erduran, 2007; Tümay, 2016). As emphasized by Scerri (2001), understanding the nature of chemistry and chemical knowledge is of great importance for chemical educators: “Chemical educators will gain a great deal from familiarizing themselves with such research since it will enable them to be clearer in the way in which they present various aspects of chemistry to their students and colleagues. It is not enough to train chemistry teachers about just the contents of chemistry courses and perhaps a little educational psychology. Chemical educators need to be introduced to the study of the nature of chemistry.”
Based on the modeling perspective and arguments on the necessity of understanding the nature of discipline, we can say that, in the scientific models → teaching models → learners' mental models chain, in order to understand the sources of learners' faulty mental models and misconceptions we should firstly focus on what is modeled in chemistry, how it is modeled, and how it is represented. To date, the most fruitful contribution in this respect has been Johnstone's notions regarding the role of macroscopic, microscopic, and symbolic (MMS) levels of thought or representations in chemistry (Johnstone, 1991, 2000). In this framework, the macroscopic level includes observable substances, processes or phenomena; the microscopic level includes unobservable entities such as atoms, molecules and ions that are conceptualized to explain macroscopic observations; and the symbolic level includes symbols, formulas, and equations that represent macroscopic or microscopic entities and processes. In fact, Johnstone's macroscopic, microscopic, and symbolic levels framework has been the most impressive discipline-specific paradigm in chemistry education research (Gilbert and Treagust, 2009; Talanquer, 2011; Taber, 2013).
Many studies based on the MMS level framework significantly improved our awareness about the critical role of understanding the particulate nature of matter and multiple representations in chemistry education. These studies have led to many fruitful pedagogies for supporting meaningful understanding of chemistry (e.g., Gabel, 1993; Kozma and Russell, 1997; Kozma et al., 1997; Tasker and Dalton, 2006; Gilbert and Treagust, 2009), and indicated the necessity and fruitfulness of such discipline-specific perspectives for clarifying learning difficulties and developing meaningful teaching approaches. In this article, based on ideas from the philosophy of chemistry, it is argued that the emergence is one of the most critical aspects of chemistry that affects the epistemology and the ontology of chemistry (Luisi, 2002; Scerri, 2004, 2007; Lombardi and Labarca, 2005; McIntyre, 2007; Newman, 2013), and hence it should be taken into account for understanding many learning difficulties and misconceptions in chemistry education. In the following sections, I tried to clarify this argument and its implications for chemistry education through examples from basic topics of chemistry.
Emergence in chemistry
The real world is very complicated and each scientific discipline focuses on different aspects of the world. The practitioners of a discipline attempt to understand and control the world from their disciplinary perspective by constructing models of how the concerned systems work. A model can never capture reality in all aspects, but a good model can capture the major features of the target system, thereby allowing realistic predictions about the system, the practitioners' reasoning approaches in the modeling process and the conceptualized entities, their properties and relations/interactions in the constructed models shaped by the disciplinary perspectives and the nature of examined phenomena. So, understanding the key concepts, theories, laws, and models in a discipline requires a meta-understanding of discipline-specific perspectives. Only by acquiring such a meta-understanding, one can meaningfully understand the ontology of the discipline, epistemological approaches of practitioners and consequently the nature of resulting knowledge in the discipline.Chemistry is essentially concerned with the composition, properties, reactions and changes of substances that make up our world. One of the most widely recognized characteristics of chemistry is that chemists try to explain the macroscopic world by means of microscopic entities (Johnstone, 1991, 2000; Jensen, 1998). Our experiences at the macroscopic level include solutions, their colors, precipitations, formation of gases, change in electrical conductance, etc. These observations are explained by hypothesized theoretical entities (such as atoms, molecules and ions), their properties and interactions at the microscopic level. These chemical entities, their properties and interactions are unobservable and this poses serious learning difficulties for students (Johnstone, 1991, 2000; Gilbert and Treagust, 2009). As Kozma and Russell (1997, p. 949) point out, “understanding chemistry relies on making sense of the invisible and the untouchable.” In many studies this abstract nature of chemistry has been emphasized as one of the most important sources of learners' misconceptions and frequently it was proposed that macroscopic, microscopic and symbolic representations should be used in coordination to overcome this difficulty (Gilbert and Treagust, 2009).
Another important, but currently neglected aspect of chemistry is the emergent characteristic of chemical entities, their properties and interactions. In the current debates on the nature of chemistry and chemical knowledge it is widely acknowledged that chemical entities such as atoms and molecules have many emergent properties that cannot be deduced or predicted from the knowledge of their components (Luisi, 2002; Scerri, 2004, 2007; Newman, 2013). An emergent property is a novel property of a whole, which is neither possessed by its constituent parts nor can be predicted from them.
Emergent properties differ from non-emergent or additive properties which can be deduced from properties of the components (Luisi, 2002; Talanquer, 2008). Physical properties such as mass or charge are examples of additive properties. For example, the mass of a molecule is equal to the sum of the masses of its constituent atoms. In contrast to an additive property, an emergent property of a whole cannot be deduced from the presence or properties of the constituent entities. As emphasized by Scerri (2004, 2007), electronic configurations for atoms and molecules, empirical order in which the atomic orbitals are filled, and the periodic table cannot be predicted or deduced fully from the knowledge of lower level entities using quantum mechanical calculations.
The mostly known example of emergence is the formation of water from the reaction of hydrogen and oxygen. The properties of water such as its ability to make hydrogen bonds, its high surface tension, and its chemical reactivity are emergent properties. Just like any other molecule, the properties of water are not present in its constituent atoms (hydrogen and oxygen atoms), but emerge from the configuration and interactions of oxygen and hydrogen atoms in this particular molecular structure (Luisi, 2002). Another example of an emergent property is the aromaticity of the benzene molecule, which is not present in the constituting atoms, but arising as a result of their structural relations and interactions (Luisi, 2002).
As can be seen from the given examples, particular structural configurations and resulting constrained interactions among the constituent entities can lead to the emergence of new properties of the whole. Therefore, chemists treat each chemical entity as an organized system with a particular structure and interpret its emergent properties by considering a network of relations in the system (Reiher, 2003; Villani, 2014). In other words, chemists unavoidably view chemical entities such as atoms and molecules as systemic entities rather than mere aggregates of constituent entities in order to make sense of emergent chemical properties. Such a perspective has led chemists to organize and examine chemical entities at different levels of complexity.
According to Jensen (1998), all chemical entities and their properties from the most fundamental entities to more complex structures can be considered at three levels: electrical, molecular, and molar. However, when we consider practicing chemists' ways of reasoning and conventional teaching practices in chemistry, the chemical entities can be considered at four levels in terms of emergence relation (Tümay, 2016): electrical, atomic, molecular, and molar (see Table 1). From a chemical perspective, the electrical level includes the most basic constituents of chemical entities which are electrons, protons and neutrons. The atomic level includes isolated single atoms and ions. The molecular level includes molecules and compounds that consist of atoms or ions. The molar level includes aggregates of atomic or molecular level entities. When we consider these levels in terms of emergence relation, entities and their properties at each higher-level emerge from the relations and interactions of lower-level entities.
| Level | Entities | Examples of emergent properties |
|---|---|---|
| Molar | Aggregates of atomic or molecular level entities (e.g., H2(g), HCl(g), NaCl(k), He(g)) | – Tendency to expand in all directions for gas samples |
| – Surface tension | ||
| – Viscosity | ||
| – Phases and phase transition | ||
| Molecular | Molecules and compounds consisting of atomic level entities (e.g., H2, HCl, NaCl) | – Molecular structure and isomerism |
| – Acidity/basicity, acid strength | ||
| – Molecular polarity | ||
| – Aromaticity | ||
| – Chemical bond and its properties (ionic/covalent character, strength and polarity) | ||
| – Magnetic properties | ||
| Atomic | Isolated atoms and ions consisting of electrical level entities (e.g., H, He, Na, Na+, Cl, Cl−) | – Electron configuration |
| – Chemical reactivity | ||
| – Oxidation states | ||
| – Atomic line spectra | ||
| – Metallic character | ||
| – Magnetic properties | ||
| Electrical | The most basic entities in the chemical ontology: electron (e−), proton (p+), and neutron (n0) | |
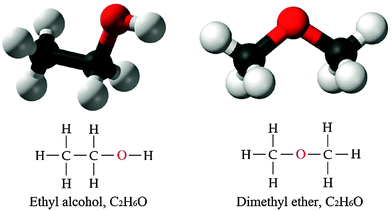
As has been pointed out, in chemistry, emergence is generally associated with the appearance of novel properties with increasing structural complexity from sub-atomic particles to atom, molecule, and molecular complexes (Luisi, 2002). Chemists have long been aware of the fact that the structural configuration of the constituents forming a chemical entity plays a crucial role in determining the properties of the entity. Relating properties of chemical entities to their structures has been one of the major chemical heuristics in predicting and interpreting properties of chemical entities. We can exemplify the role of structure–property relationship in chemical explanations with the isomerism or with explaining the reactivity/stability of atoms or molecules with their electron configurations. For instance, we can consider a typical example of isomerism by comparing and contrasting ethyl alcohol (a colorless liquid alcohol) and dimethyl ether (a colorless gaseous ether). Both ethyl alcohol and dimethyl ether have the molecular formula C2H6O; they consist of the same kind and number of atoms, however, they differ in both physical and chemical properties. These differences result from different structural configurations of constituent atoms as seen in Fig. 1. Different arrangement of atoms in the molecular structure changes constraints and relational interactions among the constituent atoms, and in turn, properties of the molecules as a whole.
Structural configuration of constituents constraints their interactional relations and these constrained relations can give rise to the emergence of unforeseen properties. From that perspective, chemical properties of an atom emerge from interactions of constituting electrons, protons and neutrons in the atomic structure. For example, existence of electrons in quantized energy levels, electronic configurations and unique line spectra of atoms can be regarded as emergent properties that arise from interactions of constituent parts in specific structural configurations of atoms. Similarly, chemical properties of a molecule emerge from interactions of constituting atoms in the molecular structure. For example, acidic character of a molecule and the strength of acidity cannot be deduced or predicted from the constituent atoms; these properties emerge from constrained interactions of constituent atoms in the molecular structure (Tümay, 2016). The properties of chemical compounds are not the sum of the properties of their constituent atoms and we cannot predict the properties of chemical compounds from a knowledge of the properties of their constituent atoms in isolation or in other compounds. Clearly, chemical entities such as atoms and molecules are more than just simple aggregates of constituents. Therefore chemical entities should be regarded as systems that have emergent properties as a result of specific structural configurations and constrained interactions of constituent entities.
In contrast to the emergence at the atomic and molecular levels, the emergence at the molar level derives not from structurally constrained interactions of constituents but from relatively dynamic and random interactions among the atomic or molecular level entities in their aggregates (Chi et al., 2012). Emergent molar properties can be considered as the macroscopic properties that are observed in aggregates and they derive from collective interactions of entities in dynamic, random motion rather than relatively stable interactions that are constrained in an organized structure. For example, macroscopic gas samples have a tendency to expand in all directions. Whereas individual gas particles do not have such a property, it emerges at the molar level as a result of random collisions between gas particles (Rappoport and Ashkenazi, 2008).
Effects of emergence on chemical epistemology and practicing chemists' ways of reasoning
In each discipline, the nature of entities, their properties, relations and interactions not only affect the ontology of the discipline, but also epistemological reasoning approaches of practitioners and the modeling process in that discipline. The emergent nature of chemical entities, their properties, and interactions considerably influences and shapes chemists' ways of reasoning about chemical systems, and subsequently the nature of chemical explanations (Tümay, 2016). As has been discussed, chemical entities are complex systems that have emergent properties, and even though we classify different substances as acids, bases, alkali metals, etc. based on their similarities in some respect, we know that similarity is not identity or equality. Any change in constituent entities and/or their structural configurations in a system changes constraints and relational interactions among the constituents; and in some cases, completely unexpected results (new emergencies) can appear.Because of this unavoidable nature of chemical systems, chemistry is an inductive science more than a deductive science. In turn, chemistry typically lacks precise mathematical formalisms that are found in deductive sciences (such as F = kq1q2/r2 in physics). Due to the issue of emergence, we cannot determine exact rules for chemical properties that are valid in all cases. For instance, we cannot formulate a precise formula that gives us exact conclusions for acid strength of a substance such as “acid strength = k1A + k2B − C” (k1 and k2 are constants, A, B, and C are measurable properties of entities). In many cases, all we can do is inferring qualitative factors (such as relative bond polarity and bond strength) that can explain the emergence in question when brought together in a systemic manner.
Since changes in constituents, their structural configurations and resulting relational interactions in the system might give rise to novel factors that have not been considered before, there are always exceptions to general rules in chemistry. As a result, chemists regard pre-established factors and principles more as heuristics rather than exact rules that are valid in all cases (Taber, 2009; Tümay, 2016). For example, when considering bond polarity between the same atom pairs, such as C and H, we tend to focus on pre-established electronegativity values of these atoms, and to assume that electronegativity values are constant. However, electronegativity of an atom is affected by various factors such as oxidation state and hybridization. When the hybridization of the C atom changes in a different molecule system; it will be an in situ factor that should be taken into consideration.
As another example, we can consider the “Aufbau principle” that is used to predict the electron configurations of elements. Despite its usually correct predictions, chromium and copper, for example, do not agree with the predictions. Detailed studies show that the configurations of chromium and copper result from complex electron interactions that are not taken into account in the simple model. However, this does not mean that we should discard the simple model that is so useful for most atoms. Instead, we must apply it with caution and not expect it to be correct in every case.” (Zumdahl and Zumdahl, 2012, p. 119). Chemists interpret all chemical knowledge with such a cautionary approach, and we almost inevitably attach caution adverbs (such as “usually”, “frequently”, and “generally”) to our generalizations and principles. Consider for example the generalization “metals and nonmetals form ionic compounds”. We can show numerous examples that agree with this generalization. However, as usual in chemistry, there are also exceptions. For instance, SnCl2 and SnCl4 are typical metal-nonmetal compounds. Although SnCl2 is an ionic compound, SnCl4 is a covalent compound because of strong polarization effects of Sn4+. Consequently, it is more correct to state this generalization as “metals and nonmetals usually form ionic compounds”. “Usually”, “generally”, and similar qualifiers warn us about the heuristical nature of given generalizations or principles, and inevitable use of such qualifiers is a natural consequence of the emergent characteristic of chemical properties. When reasoning about an emergent chemical property, chemists should determine and consider all relevant factors (both pre-established ones and the ones that arose in situ) and their effects in the system simultaneously to reach a conclusion that is more consistent with the empirical findings.
The issue of emergence makes chemistry very difficult but also fascinating science. When chemists face with unforeseen emergent properties that need explanation, conjecturing previously unknown parameters that lead to the emergence is the most difficult task they face. In these cases, pre-established explanatory chemical factors serve as heuristics for chemists. When the pre-established chemical factors are insufficient to make an adequate explanation, chemists reanalyze the system by considering constituent entities, their properties, possible relations and interactions to infer newly emerged factors that will fill the gap in explanation. Such a process of inferring emerged features or factors in the considered system can be called backward inference (from empirical results to explanatory factors) or retrospective reasoning.
We can trace the development of an emergentist perspective in many topics such as chemical bonding, hybridization of atomic orbitals in chemical bonding, the empirical order in which the atomic orbitals are filled, and Ligand Field Theory. In all of these and similar cases in which an emergentist perspective has been adopted to explain the property in question, proposed conceptualizations about the properties, relations and interactions of constituent entities in organized chemical structures were not derived from known properties of constituent entities, rather they were inferred to explain observed empirical findings (Scerri, 2004, 2007). This implies that, in many cases, our knowledge about chemical properties of substances did not result from deductive inferences from known entities and properties, but from retrospective inferences of an emergence mechanism that explains observed findings.
In determining the properties of a chemical compound there is no theoretical shortcut or exact rule that is valid in all cases, therefore we must empirically study the sample and then infer emergence factors and the emergence mechanism through a retrospective analysis when needed. As Caldin (1959) clearly specified in his examination of the role of hypothetico-deductive methods in chemistry, experiments in chemistry are not typically used to test and falsify a hypothesis, model or theory, but are used to make it more precise. As stated by Caldin, although some observations are made to test chemical theories, making observations in order to test hypotheses is neither the central activity nor the main purpose of chemical research. Caldin illustrated this situation by the study fields in physico-chemistry (the determination of the structures of molecules, thermodynamic investigations on physical or chemical equilibria, and kinetic investigations on the rates of physical or chemical change). He emphasized that in each of these fields chemists begin their investigations with a molecular model of some kind, and they do not test this molecular model, rather they seek to make it more precise. According to him this characteristic of chemical research stems from the fact that, in contrast to physics where theories are general in scope, and mathematically exact, so that precise deductions could be made from them; “many of our theoretical hypotheses are by contrast restricted in scope, contain undetermined parameters, require additional hypotheses in application, and do not give numerically exact predictions.” (p. 221). Apparently, this unique characteristic of chemical research and practicing chemists' ways of reasoning stems from the emergent nature of chemical entities, properties, and interactions.
Learning demands of understanding emergent properties in chemistry
Without considering the nature of chemistry and clarifying discipline-specific constraints, all of our attempts, whether aimed at understanding learning difficulties or developing effective instructional approaches, will be unrealistic and less meaningful. As has been discussed, emergence plays a crucial role in the epistemology and the ontology of chemistry, and consequently meaningful understanding of chemistry requires adopting an emergentist perspective. Understanding, predicting, and explaining an emergent property requires taking into account of all constituent entities, their structural relations and interactions simultaneously in a holistic and systemic manner. However, developing such an emergentist perspective is not easy and places specific learning demands on learners of chemistry. These learning demands involve at least the following understanding:– Conceptualizing chemical entities as structured systems that have emergent properties.
– Becoming aware of the fact that isolated chemical entities and their properties are modified in some way when they become part of a system because of constrained relational interactions in the system.
– Realizing that constrained relations/interactions of constituent entities can lead to the emergence of unforeseen new properties in the system.
– Recognizing that every change in constituent entities and their structural relations affect the properties of the system. Therefore, even very similar chemical entities have differences, no matter how similar they are.
– Understanding that the emergent properties of a system cannot be attributed to any constituent entity or a single parameter. All constituent entities, their structural relations and interactions in the system should be taken into account in an integrated manner.
– Becoming aware of the fact that chemical principles and generalizations are heuristic principles rather than exact, determining rules.
If students fail to develop such an emergentist perspective, they will inevitably have many misconceptions in chemistry. In that case, students' chemical misconceptions about an emergent property may not derive from a failure to understand isolated entities, their properties and interactions or the parameters of emergence in isolation, but from a failure to think all constituent entities, their structural relations and interactions in a holistic and systemic manner. Consequently, one of the fundamental sources of the learning difficulties and chemical misconceptions is the failure to understand the emergent nature of chemical entities, their properties, and interactions. Learners' chemical misconceptions that derive from a failure to understand the emergence in chemistry may appear in the following forms.
– Attributing an emergent property of a system to particular components.
– Viewing an emergent property of a system as the sum of the properties of constituent entities.
– Viewing chemical heuristics as exact determining rules.
– Focusing on salient factors or cause–effect relationship in isolation instead of systemic consideration of all relevant ones.
These items can be used as an interpretive analysis framework for understanding learning difficulties and identifying the sources of learners' misconceptions about emergent chemical properties and phenomena. Many chemical misconceptions regarding emergent chemical properties that are documented in previous studies can be analyzed and interpreted through this framework. Such an analysis can give clear insights into the sources of examined misconceptions and likely to be effective teaching interventions for conceptual change. Some of the well-known chemical misconceptions were reinterpreted from this perspective in the following section.
Attributing an emergent property of a system to particular components.
– CH4 and NaH are acids because the substances that contain H are acids (Demircioğlu et al., 2005).
– CH3CH2–OH is a base because the substances that contain OH are bases (Demircioğlu et al., 2005).
– Acid strength increases as the number of H in the acid molecule increases (Demircioğlu et al., 2005).
– CH3CH2CH2F is a gaseous compound because it consists of fluorine, which is a gaseous element (Levy Nahum et al., 2004).
– Copper is red-brown and malleable because the copper atoms are red-brown and malleable (Ben-Zvi et al., 1986).
– Gases expand when heated, because gas particles expand when heated (Griffiths and Preston, 1992; Lin et al., 2000).
Viewing an emergent property of a system as the sum of the properties of constituent entities.
– Salts are neutral because they contain neither H nor OH (Demircioğlu et al., 2005).
– A neutralization reaction produces a neutral solution (Schmidt, 1991; Demircioğlu et al., 2005).
– The color of a reaction product is the weighted sum of the colors of reactants. If a yellow and a blue reactant react, the color of the reaction product will be green (Talanquer, 2008).
– CH4 does not conduct electricity because it consists of two elements: H2(g) and C(s), both of which do not conduct electricity (Levy Nahum et al., 2004).
Viewing chemical heuristics as exact determining rules.
– BeCl2 is an ionic compound, because metals and nonmetals always form ionic compounds (Luxford and Bretz, 2013; Enawaty and Sartika, 2015).
– Na7−, C4+ and even Cl11− ions are more stable than their corresponding neutral atoms, because chemical species with an octet structure, or a full outer shell, are more stable than the related species without such an electronic configuration (Taber, 2009).
– 1,1-Dichloroethane dissolves in water, and it is immiscible with pentane, because it is a highly polar molecule and “like dissolves like” (Ashkenazi and Weaver, 2007).
Focusing on salient factors or cause–effect relationship in isolation instead of systemic consideration of all relevant ones.
– HF is a stronger acid than HCl, HBr, and HI because acid strength increases as the H–X bond polarity increases (Tümay, 2016).
– Vapor pressure of a liquid increases as the total number of vapor particles increases (Tümay, 2014).
– Exothermic reactions are spontaneous because the spontaneity of a reaction depends on the enthalpy change (Sozbilir, 2002).
– Polarity of molecules depends only on the electronegativity difference between the constituent atoms. ClF3 is a polar molecule because fluorine atoms are highly electronegative (Furió and Calatayud, 1996; Wang and Barrow, 2013).
– Ionization energy depends only on the distance of the electron from the nucleus. Sodium has a higher first ionization energy than aluminum because the 3p electron of aluminum is further away from the nucleus compared to the 3s electron of sodium (Tan et al., 2005).
– Hydrogen has the smallest atomic radius because the atomic size depends only on the number of electrons in the system (Talanquer, 2006; Salame et al., 2011).
As can be seen from these examples, the suggested emergentist framework can be more informative and meaningful for identifying specific difficulty areas in learning emergent chemical properties and phenomena. It can also reasonably be argued that this perspective will be more beneficial to chemistry educators in developing effective pedagogies and learning materials to overcome the identified difficulties and misconceptions since it reveals their underlying sources rather than proposing ambiguous generic factors as causes of misconceptions.
Research on learners' understanding of emergent properties in chemistry
Despite its critical importance in chemistry and chemical understanding, the issue of emergence and its relation to learning difficulties and misconceptions have been largely overlooked in chemical education research. Many studies on learners' conceptions in chemistry have examined learners' understanding of the relationship between the macroscopic world and the microscopic world of chemical entities (e.g., Ben-Zvi et al., 1986; Andersson, 1990; Gabel, 1998). Although these studies were actually focused on students' understanding of the particulate nature of matter and the relationship between the macroscopic, microscopic, and symbolic representations; their results were informative in gaining insights into students' understanding of emergence relations between the macroscopic and the microscopic world. Results of these studies clearly showed that learners have difficulty in understanding the emergent properties of macroscopic aggregates and many students attributed a wide range of the macroscopic properties and changes shown by bulk matter to the microscopic entities (atoms, molecules, etc.) (Ben-Zvi et al., 1986; Griffiths and Preston, 1992; Nakhleh, 1992). For example, many high school students and prospective elementary teachers believed that particles of a matter expand or get larger as the matter changes from liquid to gaseous state or when heated (Griffiths and Preston, 1992; Valanides, 2000); 10th grade students proposed that copper atoms are malleable and have a reddish-brown color (Ben-Zvi et al., 1986); and some students suggested that soft substances are made up of soft particles (Andersson, 1990).Students' tendency to attribute the macroscopic properties of matter (such as hardness, coldness, color, and physical state) to its microscopic level particles seems to be an intuitive way of making sense of observable macroscopic properties and changes. From the learners' perspective, such explanations might be meaningful and at first sight explain the macroscopic world in terms of learned microscopic entities such as atoms, molecules, and ions. However, these intuitive conceptions contradict with scientific ideas that explain the emergence of macroscopic properties in terms of arrangement, motion, and interaction of constituent microscopic entities. A few recent studies that examined explicitly whether students understand the macroscopic properties as emergent properties have confirmed this conclusion (Rappoport and Ashkenazi, 2008; Talanquer, 2008).
Rappoport and Ashkenazi (2008) examined how graduate and undergraduate chemistry students and faculty members connected macro, submicro, and symbolic representations. Participants were asked to solve conceptual problems and a think-aloud interview protocol was used to reveal their ideas. Rappoport and Ashkenazi used a ‘levels of complexity’ framework to analyze responses. In this framework, they considered the macro and symbolic modes as system-level representations, and the submicro mode as component-level representation. The results of the study indicated that faculty members thought of system-level properties as emerging from mechanistic interactions between particles on the component level. However, it was found that students generally adopted a ‘submergent’ perspective and either failed to connect the system and component levels, or thought of system-level properties as determining the behavior of particles on the component level.
The findings of these studies have demonstrated that many chemistry learners fail to understand the emergence of macroscopic properties of matter and consequently develop various misconceptions about the relationship between the macroscopic and the microscopic world. Unfortunately, learners' conceptualizations of the emergence of chemical properties at the atomic and molecular levels, related learning difficulties and misconceptions have not been sufficiently investigated up to now. Only recently a few research studies focused on this topic (Talanquer, 2008; Tümay, 2016). For example, Talanquer (2008) investigated college chemistry students' ideas about the expected properties (color, smell, and taste) of the products of chemical reactions represented at the molecular level. Using multiple-choice questions and individual interviews, Talanquer explored the extent to which novice learners intuitively used an additive framework to predict the properties of the product, rather than an approach that recognizes the emergent nature of the properties of chemical compounds. The results of the study indicated that most students relied on an additive heuristic to predict the properties of chemical compounds, overlooking the possibility of emergent properties resulting from the interaction of the atoms that compose the system. In his review on chemistry students' implicit assumptions and heuristics, Talanquer (2013) emphasized that attribution of the macroscopic properties to the microscopic entities, as well as viewing properties of chemical compounds as the weighted sum of properties of constituent atoms, indicates that students tend to use additive thinking rather than emergentist thinking. Many students who used additive thinking seemed to implicitly assume that atoms have fixed properties and the properties of a compound result from the sum of the properties attributed to the constituent particles (Talanquer, 2013).
In a more recent study, Tümay (2016) investigated undergraduate chemistry students' conceptions of acids and acid strength, and examined whether or not they conceptualized acid strength as an emergent chemical property. The results of the study showed that many learners failed to conceptualize acid strength as an emergent property that arises from interactions of multiple factors. Rather than considering all relevant factors in a holistic and systemic manner, the majority of students focused on a limited number of factors (especially bond polarity) when making predictions and constructing explanations about acid strength. The findings indicated that many misconceptions about acid strength stemmed from learners' failure to conceptualize acid strength as an emergent property, and consequently their failure to recognize and consider all factors that affect acid strength. These relatively few research studies on learners' conceptualizations of emergent chemical properties revealed the critical importance of emergence as a disciplinary lens for understanding many learning difficulties and misconceptions in chemistry education.
Implications for chemical education
Identifying students' misconceptions and learning difficulties, and finding effective ways of addressing them has been one of the major concerns in chemistry education research. As has been emphasized by many scholars from diverse fields including chemical education, philosophy of chemistry, and cognitive psychology, determining learning difficulties in a discipline and finding ways of addressing them meaningfully requires a sound understanding of the discipline and nature of disciplinary knowledge (Shulman, 1987; Scerri, 2001; Erduran and Scerri, 2002; Erduran, 2007; Tümay, 2016). In this article, it is proposed that emergence plays a critical role in the epistemology and the ontology of chemistry (Luisi, 2002; Scerri, 2004, 2007; Lombardi and Labarca, 2005; McIntyre, 2007; Newman, 2013) and hence it should be taken into account for understanding learning difficulties and ways of addressing them in chemistry. It is particularly argued that one of the fundamental sources of learning difficulties and chemical misconceptions is learners' failure to understand the emergent nature of chemical entities, their properties, and interactions.As has been pointed out, conceptualizing chemical entities, their properties and interactions from an emergentist perspective and interpreting examined chemical phenomena accordingly place specific learning demands on learners of chemistry. In order to understand emergent properties, learners should view chemical entities as structured systems and take into account all constituents, their constrained relations and interactions in a holistic and systemic manner. However, this is not an easy task and learners might focus only on the perceived salient factors while ignoring other relevant ones. In such cases, learners' misconceptions about an emergent property may not derive from their lack of knowledge or misunderstanding of the constituent entities, their properties or emergence related factors and their effects in isolation, but may derive from the failure of conceptualizing the emergence of the property in a systemic manner by considering all relevant factors holistically. As will be discussed in the following sections, this argument has epistemologically sound and critical implications for more meaningful chemical education.
Inadequacies of typical teaching models and practices in the absence of an emergentist perspective
We want our students to construct mental models that are closer to chemical models, and through these models understand, explain, and predict the chemical world. This aim can be accomplished only by learners' adoption of chemists' perspectives and ways of thinking about the concerned entities, their relations, properties, and interactions. This, in turn, necessitates students to adopt an emergentist perspective to understand and use chemical models meaningfully. Learners' mental models are essentially qualitative conceptualizations of entities, their properties, relations and possible interactions in a system (Johnson-Laird, 1983; Greca and Moreira, 2002). They construct their mental models from available experiences and information sources. These experiences can be either first-hand empirical experiences or indirect experiences that are obtained through conveyed representations of real or hypothetical systems.In chemistry, conceptualized explanatory entities, their properties, relations, and interactions are typically at the atomic or molecular level. They are unobservable and learners have no chance of gaining direct experience with them that can be used as a base for abstracting mental models or testing and refining them (Johnstone, 1991, 2000; Kozma and Russell, 1997; Coll and Treagust, 2003; Laszlo, 2013). In most cases, the only experience base that is available to students regarding chemists' models of the underlying entities, their properties, and relations is expressed teaching models. Therefore, learners acquire new information in chemistry primarily by instruction or by reading (Taber, 2001; Nakiboğlu, 2003). This point has been clearly emphasized by Taber (2008, p. 1041): “students' ideas about the nature and properties of atomic level particles are not abstractions from their own direct experiences of the phenomena. Knowledge of the atomic world is knowledge of models developed by humans, and communicated within our culture through talk, books and so forth.”
Unfortunately, it seems that our typical teaching models, instructional resources and practices in chemistry education are inadequate for supporting learners in developing an emergentist perspective through comprehension of the emergent characteristic of chemical entities, their properties, relations, and interactions. In the absence of an emergentist perspective, students can easily interpret teaching models and explanations intuitively based-on perceived salient features. In such cases, they unavoidably construct flawed mental models and develop misconceptions in predictable forms. In order to exemplify how typical teaching models and practices can lead to misconceptions in the absence of an emergentist perspective, we can consider the functional group approach in organic chemistry, explanations of changes in acid strength in periods and groups, and the octet rule.
Functional group approach
Understanding and explaining an emergent property of a chemical system is not an easy task especially when the complexity of the system is increased. For each system, we do not start from the most basic level entities. For example, when considering properties and interactions of H2O we do not start from electrons, protons, and neutrons and then proceed to the atoms and then H2O as a whole. Instead, for reducing the complexity to a manageable degree, we tend to consider proximal lower-level entities as basic units. For the H2O example, in order to explain the properties, relations, and interactions of H2O we consider H and O atoms, their structural relations, and interactions in the H2O molecule. If such a treatment is not sufficient for the explanation of the concerned emergent properties, we proceed to the next lower-level entities.Another strategy adopted by chemists to reduce complexity is to determine subsystems that are commonly encountered and for which structural/relational interactions of components and resulting consequences are well established. The functional group approach in organic chemistry reflects this strategy. We can consider R–COOH as an example. Carboxyl group (–COOH) can be considered as a subsystem. Although changes in alkyl groups (R–) affect imposed constraints to a degree, resonance, bond polarities, and bond strengths in the –COOH subsystem are relatively stable and well established properties. The functional group approach is commonly used in organic chemistry as an explanatory and predictive framework. In this approach, chemical properties of organic compounds are attributed to the presence of functional groups.
Nothing wrong with this approach and we all use it in the context of organic chemistry. However, we use this way of thinking with caution because we are aware of the emergent nature of chemical properties. We know that chemical properties of compounds that have the same functional groups are similar but not identical. They are not identical because chemical properties are also modified by other factors, such as the position of the functional group or branching in the carbon chain. However, novice students might interpret given examples and explanations reductively and they can infer that chemical properties of compounds can be determined only by the presence of particular atoms or atom groups. But, this is not the case in reality and students should be warned to consider all constituents, their relations and interactions in the molecule to reach more valid inferences. For example, all of the following compounds include the –O–H group but their chemical properties are not determined only by the –O–H group.
Na–O–H a base
CH3–O–H an alcohol
Cl–O–H an acid
CH3CO–O–H a carboxylic acid
H–O–H a neutral compound
This example clearly shows that the chemical properties of entities cannot be attributed to the presence of particular components reductively. Without adopting a systemist emergentist perspective, it is very difficult if not impossible to make sense of these empirical facts. In contrast to an emergentist perspective, a reductionist approach may work well with the learned examples and seem to be consistent and compatible with the teachers' explanations. However, learners can be easily mislead by such reductionist inferences and construct various misconceptions. For example, in explaining acidity if the emergence mechanism of acidic properties is not explicitly clarified and understood by students, they can misleadingly attribute acidity to H atoms in the molecule and think that as the number of H atoms in the molecule increase, acidity increases.
Explanations of periodic trends in acid strength
Typical teaching practices and misconceptions regarding acid strength are also indicative of the critical importance of the emergentist perspective (Tümay, 2016). Instruction on acids–bases in introductory chemistry courses tends to center on algorithmic problem solving rather than conceptual understanding of the scientific models or the interconnected consideration of factors that affect acid strength (de Vos and Pilot, 2001; Furió-Más et al., 2005). Explanations of acid strength and related factors in textbooks' are also ineffective and misleading in some respects. In his examination of nine general chemistry textbooks, Moran (2006, p. 800) identified that the following trends in acid strength are usually cited in the textbooks:– For binary hydrides HnA across a period, acid strength is in the order of increasing electronegativity of A, for example: NH3 < H2O < HF.
– For binary acids within a group, acid strength increases as the electronegativity of A decreases. For example, in the hydrohalogenic acids the acid strength order is HF < HCl < HBr < HI.
– For oxoacids (HO)nXOm with a given central atom (X), the stronger acid has the greater number of “extra” O atoms (m in the formula).
– Among oxoacids with the same m and n and different central atoms (X), acid strength increases with increasing electronegativity of X.
The explanations for these trends generally involved H–A bond polarity and bond strength (Moran, 2006). In these explanations, it is proposed in some cases that the more polar the bond, the stronger the acid; while in other cases bond strength is emphasized and increasing acid strength is attributed to decreasing H–A bond energy. In some textbooks bond strength and bond polarity are mentioned together misleadingly: the electronegative groups withdraws electron density from the H–A bond, thereby weaken it (Moran, 2006).
In fact, abstracting the underlying factors that affect chemical properties from periodic trends is commonly used and in most cases an almost inevitable approach in chemistry. As has been discussed, chemical entities are complex systems and any change in constituent entities and/or their structural relations correspondingly changes the system as a whole and its chemical properties. Because of the emergent characteristic of chemical entities, their properties, and interactions we cannot formulate precise rules regarding chemical properties; instead we attempt to identify qualitative parameters that are capable of explaining emergence in such a way that simulation of their integrated effects can give rise to the observed properties.
In the teaching practice, we try to get students to understand the parameters of emergence and then use them in the relevant systems to predict and explain emergence. When there are multiple parameters, the most straightforward way to show the effects of each parameter would be to fix all parameters other than the concerned one and changing the parameter in question and then observing its effects just like as we do in controlled experiments. However, controlling and manipulating all variables is not possible in chemistry. Because, as has been discussed, any change in a parameter means the change of a system as a whole and this also makes fixing other parameters impossible. For example, in examining the effects of bond polarity and bond strength on acid strength we cannot control all variables and manipulate only chosen ones when making comparisons. In such cases, we tend to compare chemical systems that have variability regarding the concerned parameter and are similar in terms of other variables as much as possible. One of our common heuristics to meet this requirement for binary compounds is making comparisons across periodic groups and periods.
Even though we are aware of the fact that we cannot infer simple/direct causal relations from such comparisons as if they were controlled experiments, and the inferred relations should be used heuristically with caution in a systemist–emergentist perspective; it might not be so obvious for learners. Unfortunately, in typical teaching practices, only a single dominant factor is emphasized for each trend in these comparisons, without adequately clarifying other relevant factors in a holistic manner. Such teaching practices seemingly do not promote the understanding of the chemical property in question as emerging from the integrated effects of multiple factors. In such cases, students' tendency to figure out heuristic factors as determining causal factors should not be surprising. If we cannot clarify and present the emergence mechanism clearly and comprehensibly, students create their explanations on the spot based on perceived salient features, their background knowledge and intuitive heuristics. And, when this is the case, they usually think constituent entities as property carriers (for example, attributing acidity to H atoms) or infer incorrect deterministic causal linkages between an emergent property and its isolated parameters (for example, thinking bond polarity as only determinative of acid strength) (Tümay, 2016).
The octet rule
Another example of how learners might construct misconceptions from typical teaching practices in the absence of an emergentist perspective comes from the chemical bonding topic. As has been pointed out, because of the issue of emergence, chemists tend to treat chemical principles and rules (such as the Aufbau principle or the octet rule) as chemical heuristics that are not always but usually valid for similar systems (Taber, 2009; Tümay, 2016). For example, chemists were well aware of the fact that the octet rule is not valid for all chemical systems and should be used with caution, even in the times of the first formulation of this rule (see, Lewis, 1923). However, it is well-established that, typical teaching models and associated accounts of chemical bonding give an impression that “the octet rule” is an exact, determining rule rather than a chemical heuristic that is valid for limited chemical systems (Taber, 2001, 2009; Taber and Coll, 2002). Research on students' understanding of chemical bonding has revealed that learners' conceptualization of “the octet rule” as an exact rule rather than a chemical heuristic leads to many learning difficulties and misconceptions about chemical bonding and the stability of chemical species (Taber, 2001, 2009; Taber and Coll, 2002).Explicitly representing and emphasizing the emergence in chemistry
Based on learners' difficulties in understanding the emergence and the brief overview of typical teaching practices, we can say that what is missing in our typical teaching is presenting the emergent characteristic of chemical entities, properties, and interactions in an accessible and comprehensible manner and developing a systemist–emergentist perspective among students. The findings of studies on students' understanding of emergent properties in various disciplines imply that understanding emergent properties and developing an emergentist perspective are often beyond the intuitive reach of learners unless explicitly taught (Wilensky and Resnick, 1999; Rappoport and Ashkenazi, 2008; Talanquer, 2008; Chi et al., 2012; Tümay, 2016). Obviously, we as chemical educators have an important role in making explicit the emergent characteristic of chemical entities, properties, and interactions which has been implicit in typical instruction. Therefore, in order to develop an emergentist perspective, one clear suggestion is presenting and emphasizing the emergent characteristic of chemical entities, properties, and interactions explicitly in a comprehensible manner.As has been pointed out, understanding emergence in chemistry is closely related to understanding chemical entities as systems at various complexity levels. Although it is not explicit in typical teaching resources and practices, system thinking is one of the fundamental characteristics of all molecular sciences and especially chemical science (Reiher, 2003; Villani, 2014). According to Villani, chemistry is the first true systemic science and the main chemical concepts such as the molecule and the compound are systemic concepts: “A molecule ‘is not an atomic aggregate’, but ‘is an atomic system’, a structured piece of world, which leads to the emergence of ‘new’ properties.” (Villani, 2014, p. 109). Viewing chemical entities as systems rather than simple aggregates will apparently facilitate conceptualizing the emergent nature of chemical entities, properties and interactions. In fact, promoting system thinking for facilitating comprehension of emergence has been emphasized by many researchers in other disciplines as well (e.g., Wilensky and Resnick, 1999; Assaraf and Orion, 2005).
Conceptualizing chemical entities as systems, recognizing structural constraints and relational interactions of components, and correspondingly emergence of the system's properties is not easy for students. Especially, as is often the case in chemistry, when the emergence is a result of multiple causal factors/parameters some of which work against each other, dominant or arise only under particular conditions, understanding emergence will be very difficult for students. However, the development of a systemist–emergentist perspective for thinking about chemical entities, their properties, relations and interactions can be supported through appropriate representations. A valid explanation of an emergence mechanism (as opposed to reductionist explanations or one-factor cause–effect relations) requires carefully designed representations which specify how the integrated effects of structurally constrained relations and interactions of the constituent entities in the system produce the emergent property in question. Accordingly, effective representations of emergent properties and the emergence mechanism should basically convey the following information:
– chemical systems in question and their composition and structure,
– properties, relations and interactions of constituents under the structural constraints of their systems,
– emergence of properties of the system as a result of the integrated effects of structurally constrained relations and interactions of constituents.
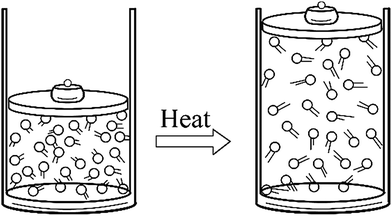
Since models and associated representations cannot represent all aspects of the considered system we use pluralistic models and different representations in accordance with our purpose. These representations include a variety of molecular models and diagrams, structural formulas, Lewis structures, electron density distribution graphics, particulate drawings, analogies, and dynamic computer models (Shusterman and Shusterman, 1997; Gilbert and Boulter, 1998). Each representation has a different functionality with respect to representing entities, their properties, relations, and interactions in the considered chemical system (such as a molecule or a reaction system). In this respect, the task of a chemical educator is to select or develop, and use most suitable representations by considering what to represent and how it can be represented. For example, following particulate drawing is an effective representation that can be used to illustrate the mechanism of thermal expansion of gases (Fig. 2).
This representation illustrates the components of the system, the effect of heating on particles' energy and the space between them. With these features, it effectively illustrates how the expansion of a macroscopic gas sample emerges from changes in energy and distances between the gas particles. In explaining thermal expansion we don't need to consider the atomic or molecular structure of gas particles, and thinking each molecular entity as a particle is sufficient. However, in some cases we need to consider the molecular structure and constrained interactions of entities in order to understand the emergence mechanism. We can consider “negative thermal expansion” of water as an example.
Many substances expand when heated and contract when cooled. But water is one of the few exceptions to this behavior. Even though water does expand when heated and contract when cooled at most temperatures, it expands when cooled and contracts when heated between 4 and 0 degrees Celsius. This unexpected behavior emerges from the particular molecular structure of water, its ability to make multiple hydrogen bonds, and the hexagonal arrangement of the water molecules in ice crystals at lower temperatures due to the network of hydrogen bonds. The following representation can be used to illustrate the emergence of this property as a result of the structural properties and interactions of water molecules (Fig. 3).
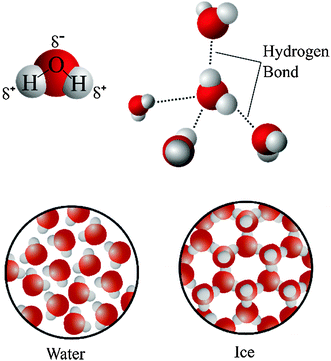 | ||
| Fig. 3 Illustration of negative thermal expansion of water as a result of structural properties and interactions of water molecules. | ||
In order to explicitly emphasize components of the system and emergence mechanisms in chemical systems, we can also use generic representational tools such as concept maps to indicate components of a system, and causal diagrams to facilitate the integrated consideration of all emergence related causal factors/parameters. The framework, proposed in this article, can significantly facilitate this process by serving both as an analytical tool for defining the considered chemical phenomena in terms of systems, constituent entities, their properties, relations, and interactions; and as a crafting tool for explaining the emergence mechanism based on the analyzed structure and properties of the system. As an example, we can consider the emergence of acidic properties. The explanation of this emergent property depends on the adopted acid–base theory. According to the Arrhenius model, a substance is an acid if it gives hydrogen ions (H+) as a result of its ionic dissociation in water. Based on this model, the acidic properties of a substance emerge depending on the following parameters:
1. It must have at least one hydrogen atom (H–X),
2. The H–X bond must be broken. It can be broken homolytically or heterolytically in three different ways:
H–X → H· + ·X
H–X → H:− + X+
H–X → H+ + :X−
3. The X atom must be more electronegative than the H atom to give H+.
As can be seen, all of these factors are necessary but, of themselves, not sufficient for the emergence of acidic properties. The dependence of acidic properties on the integrated effect of these parameters can be represented with a causal diagram as follows (Fig. 4).
 | ||
| Fig. 4 A causal diagram to illustrate the parameters of acidic properties based on the Arrhenius model. | ||
A complementary suggestion for supporting system thinking and comprehension of emergence is using and emphasizing the terms “emergence” and “system” explicitly whenever appropriate while teaching different classes of chemical entities, their properties, relations and transformations (Tümay, 2016). For example, we might use the term “atom system” instead of “atom”; “a molecule system that has acidic properties” instead of “acid molecule”, “an acid–base reaction system” instead of “an acid–base reaction”, and “emergence of acidic properties” instead of “acidic properties”. The “emergence” and the “system” terms are in fact meta-level terms in chemical reasoning and they will presumably differentiate learners' perceptions about chemical entities and processes by guiding students about how to think chemically in this discipline.
Using argumentation as a scaffold for the development of the emergentist perspective
Explicitly representing and emphasizing the emergence through appropriate teaching models is necessary but might not be sufficient for the comprehension of the emergence. Because of our cognitive constraints, we are unable to mentally represent and consider all aspects of a system. Instead, our mental models typically include limited number of entities, properties, relations and interactions that we perceived as important and explanatory (Johnson-Laird, 1983). And, our perceptions and conceptualizations are considerably affected from our prior knowledge. Sophisticated prior knowledge of scientists (such as the relevant scientific theories and models, empirical facts, meta-level epistemological and ontological understandings) enriches their repertoire of effective entities, properties and possible interactions in particular contexts and thus enables them to more correctly explain and predict the considered system and its emergent properties. Contrary to scientists, novice students do not have sophisticated knowledge of the domain that facilitates drawing sound inferences from their experiences; however, they have the basic cognitive skills needed to generate mental models such as making analogies, idealizations and abstractions (Brewer and Samarapungavan, 1991; Brewer et al., 1998; Greca and Moreira, 2000; Taber, 2002, 2008; Nersessian, 2009). Therefore, it is important to scaffold the learning process in such a way that it focuses students' attention on key aspects of scientific models and provide indications and evidence for the emergent characteristic of chemical entities, their properties, and interactions. In this respect, epistemologically sound argumentation practices can be a fruitful pedagogy for supporting chemical understanding and the development of an emergentist perspective (Erduran, 2007; Erduran and Jiménez-Aleixandre, 2008; Tümay, 2016).Argumentation on carefully selected paradigmatic examples that can highlight the emergent nature of chemical entities, properties, and interactions can serve as a catalyst for understanding the emergence and its consequences in chemistry. Engaging learners in argumentation about emergent chemical properties can also provide us with opportunities to increase their meta-level awareness of emergence in the epistemology and the ontology of chemistry by immersing learners in chemical ways of thinking and doing (Izquierdo-Aymerich, 2013). In order to support an efficient and meaningful argumentative discourse, besides using carefully selected paradigmatic cases, the relevant experimental data should be made readily accessible to learners as well. Additionally, in order to model chemists' mindset and ways of reasoning, we should argue not only on explaining general trends in chemical properties with isolated dominant factors, but also on comparative examples including conflicting cases to make students consider why any single factor is not enough for explaining given cases. For example, following argumentative discussions can be used to support learners' understanding of the emergent nature of acidic properties and acid strength.
– Given the parameters of acidic properties based on the Arrhenius model, which of the following substances in water are expected to show acidic properties? Explain the reasons for your claims.
H2, KH, CH4, HBr
– NH3 contains 3 H atoms and H atoms are bonded to the highly electronegative N atom. Why NH3 does not show acidic properties in water? Explain your reasoning regarding the parameters of acidic properties.
– PH3 contains 3 H atoms bonded to P, and the P–H bond strength is less than that of H–Cl. Why PH3 does not show acidic properties? Explain your reasoning regarding the parameters of acidic properties.
– Can acidic properties be attributed to a particular element or parameter?
– Predict the acid strength of the following compounds and explain your reasoning.
CH4, NH3, H2O, HF
HF, HCl, HBr, HI
– Observed relative acid strengths of these compounds are as follows:
HF > H2O > NH3 > CH4
HI > HBr > HCl > HF
Did your prediction agree with the findings? If not, reconsider and revise your answers by examining the following table. Can you infer which parameters can affect acid strength, and in which way they affect it?
| pKa | ΔEN | D(H–X) (kJ mol−1) | D(H+X−) (kJ mol−1) | |
|---|---|---|---|---|
| ΔEN: Pauling electronegativity difference. D(H–X): gas phase homolytic H–X bond dissociation energy. D(H+X−): gas phase heterolytic H–X bond dissociation energy. | ||||
| H–CH3 | 49 | 0.4 | 438 | 1744 |
| H–NH2 | 34 | 0.9 | 450 | 1688 |
| H–OH | 14 | 1.4 | 498 | 1633 |
| H–F | 3.2 | 1.9 | 570 | 1554 |
| H–Cl | −3 | 0.9 | 432 | 1395 |
| H–Br | −6 | 0.7 | 366 | 1353 |
| H–I | −7 | 0.4 | 298 | 1314 |
Through such argumentative discourses we can get students to recognize that no one factor alone can determine emergent chemical properties and they emerge from the integrated effects of multiple factors. They can also recognize that rules and principles in chemistry are unavoidably used heuristically by chemists.
These experiences, then, can be used as a pedagogical resource for clarifying the role of emergence in chemistry and meaningfully modeling authentic chemical thinking by indicating how chemists think about entities, their properties, relations, and interactions at different levels from an emergentist perspective. Argumentation practices can support these learning gains by providing a quality control mechanism for learners' mental models as well as enriching their repertoire of alternative conceptualizations of the underlying entities, their properties, relations, and interactions. The availability and consideration of alternative well developed conceptualizations will support the conceptual change significantly (Thagard, 1992; Taber, 2001, 2008). Throughout the argumentation process, learners will effectively make their mental models of the system structure and behavior explicit, will have opportunities to critically evaluate their own and others' conceptualizations and thus will be aware of the limitations and strengths of each one in terms of empirical and conceptual consistency, and will be encouraged to revise their mental models from an emergentist perspective.
Using historical cases to support the adoption of the emergentist perspective
Since the conceptualized entities, their properties and relations/interactions in the scientific models have always evolved and shaped in response to the specific contextual parameters such as the research problems, available facilities, empirical data at hand, knowledge background and accepted paradigms at the time, presenting these contextual parameters along with the scientific model in question may help learners in understanding the model and the conceptualization of the emergence mechanism. In this respect, using the history of science in such a way that learners can recognize why and how the scientists focused on specific entities, properties, and relations, how they conceptualized them, and consequently how the conceptualized model enables them to describe, explain, predict and control the chemical systems can be very beneficial to students. Such an approach may help us to clarify the evolution of chemists' conceptualizations from a reductionist to an emergentist perspective, and thereby facilitate learners' adoption of an emergentist perspective.In all fields of chemistry we can exemplify the struggling processes of making sense of empirical observations regarding emergent properties and gradual but evolutionary progression in our conceptualizations from a reductionist perspective to an emergentist perspective. For example, we can consider the historical development of conceptualizations on acid–base concepts (Jensen, 1980). Initially, chemists tried to explain acidity from a reductionist perspective by attributing acidic properties to constituent parts. For instance, Lavoisier attributed acidic properties to the oxygen element, while Liebig attributed them to hydrogen. However, empirical results revealed that these explanations could not capture all cases of acidity, and there is no simple causal linkage between constituent parts and the properties of the whole. Gradually, with the new empirical findings and developments in our theoretical conceptualizations on properties, possible relations and interactions of entities, both in isolation and in organized chemical structures, more sophisticated acid–base models (such as Brønsted–Lowry's and Lewis' models) were developed and our conceptualizations of acidic properties have evolved towards seeing acidic properties as emergent properties.
Conclusion
In this article, I tried to clarify the critical role of emergence in the epistemology and the ontology of chemistry, its consequences for chemical reasoning and explanations, and its implications for chemical education. As outlined in this article, the emergentist perspective represents a meta-level understanding regarding the epistemology and the ontology of chemistry, and give us clues for chemically meaningful education. Since the emergentist perspective represents a more authentic disciplinary lens for understanding chemistry and chemical knowledge (Luisi, 2002; Newman, 2013), it provides an epistemologically and ontologically sound framework for identifying the sources of learning difficulties and developing chemically meaningful and effective instructional approaches for addressing them.As has been clarified in this article, in the absence of an emergentist perspective learners unavoidably construct flawed mental models and particular types of misconceptions (such as attributing an emergent property to particular components of the system or thinking an emergent property as an additive property). In order to support the development of the emergentist perspective among chemistry learners, it was proposed to explicitly present the emergent nature of chemical entities, their properties, and interactions in an accessible and comprehensible manner by clarifying all emergence related factors and their causal relations in a holistic and systemic manner through appropriate representations and pedagogical approaches.
Considering chemistry from an emergentist perspective can make significant and chemically meaningful contributions to chemistry education. As briefly exemplified and discussed in this article, the emergentist perspective has a great potential to make sense of many learning difficulties and misconceptions that derive from this discipline-specific characteristic of chemistry as well as to develop chemically meaningful and authentic teaching interventions to overcome these difficulties. At this point it should be noted that, by no means am I suggesting that learners' failure to understand the emergent nature of chemical entities, properties, and interactions is the only source of misconceptions in chemistry. However, apparently many misconceptions derive from this failure and therefore the emergentist perspective will guide us in developing and implementing epistemologically sound teaching models and instructional approaches in this respect.
As an epistemologically and ontologically sound disciplinary perspective for understanding discipline-specific learning difficulties, sources of misconceptions, and likely to be effective teaching interventions, the emergentist perspective offers a productive avenue for further research in chemical education. Possible areas for further research from this perspective include the following:
– Clarifying the emergence mechanism and accordingly identifying the learning demands and possible difficulty areas for the key chemical properties and phenomena.
– Interpretively analyzing learners' misconceptions regarding the emergent chemical properties and phenomena in terms of the clarified emergence mechanism, and learning demands.
– Examining the appropriateness and adequacy of existent teaching models and practices from an emergentist perspective.
– Developing, implementing, and assessing the effects of new teaching models and practices that are informed by the emergentist perspective.
– Clarifying the historical development of the emergentist perspective for the key chemical properties and phenomena. Assessing the effects of presenting these historical developments on developing the emergentist understanding of chemistry.
References
- Andersson B., (1990), Pupils' conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Ashkenazi G. and Weaver G. C., (2007), Using lecture demonstrations to promote the refinement of concepts: the case of teaching solvent miscibility, Chem. Educ. Res. Pract., 8, 186–196.
- Assaraf O. B.-Z. and Orion N., (2005), Development of system thinking skills in the context of earth system education, J. Res. Sci. Teach., 42, 518–560.
- Ben-Zvi R., Eylon B.-S. and Silberstein J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63, 64–66.
- Brewer W. F. and Samarapungavan A., (1991), Children's theories vs. scientific theories: differences in reasoning or differences in knowledge, in Hoffman R. R. and Palermo D. S. (ed.) Cognition and the symbolic processes: applied and ecological perspectives, Hillsdale, NJ: Erlbaum, pp. 209–232.
- Brewer W. F., Chinn C. A. and Samarapungavan A., (1998), Explanation in scientists and children, Mind. Mach., 8, 119–136.
- Caldin E. F., (1959), Theories and the development of chemistry, Brit. J. Philos. Sci., 10, 209–222.
- Carey S., (1985), Conceptual change in childhood, Cambridge, MA: Bradford Books, MIT Press.
- Chi M. T., Roscoe R. D., Slotta J. D., Roy M. and Chase C. C., (2012), Misconceived causal explanations for emergent processes, Cognitive Sci., 36, 1–61.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40, 464–486.
- Demircioğlu G., Ayas A. and Demircioğlu H., (2005), Conceptual change achieved through a new teaching program on acids and bases, Chem. Educ. Res. Pract., 6, 36–51.
- De Vos W. and Pilot A., (2001), Acids and bases in layers: the stratal structure of an ancient topic, J. Chem. Educ., 78, 494–499.
- Enawaty E. and Sartika R. P., (2015), Description of students' misconception in chemical bonding, in Proceeding of International Conference on Research, Implementation and Education of Mathematics and Sciences 2015, Yogyakarta State University, 17–19 May 2015.
- Erduran S., (2007), Breaking the law: promoting domain-specificity in chemical education in the context of arguing about the periodic law, Found. Chem., 9, 247–263.
- Erduran S. and Jiménez-Aleixandre M. P., (2008), Argumentation in science education, Dordrecht: Springer.
- Erduran S. and Scerri E., (2002), The nature of chemical knowledge and chemical education, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and van Driel J. H. (ed.) Chemical education: towards research-based practice, Dordrecht: Kluwer Academic Publishers, pp. 7–27.
- Furió C. and Calatayud, M. L., (1996), Difficulties with the geometry and polarity of molecules: beyond misconceptions, J. Chem. Educ., 73, 36–41.
- Furió-Más C., Calatayud M. L., Guisasola J. and Furió-Gómez C., (2005), How are the concepts and theories of acid–base reactions presented? Chemistry in textbooks and as presented by teachers, Int. J. Sci. Educ., 27, 1337–1358.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70, 193–194.
- Gabel D. L., (1998), The complexity of chemistry and implications for teaching, in Fraser B. J. and Tobin K. G. (ed.) International handbook of science education, Great Britain: Kluwer, pp. 233–248.
- Garnett P. J., Garnett P. J. and Hackling M., (1995), Students' alternative conceptions in chemistry: a review of research and implications for teaching and learning, Stud. Sci. Educ., 25, 69–95.
- Gentner D. and Stevens A. L. (ed.), (1983), Mental models, Hillsdale, NJ: Lawrence Erlbaum.
- Giere R. N., (1988), Explaining science: a cognitive approach, Chicago: University of Chicago Press.
- Giere R. N., (1991), Understanding scientific reasoning, Forth Worth, TX: Holt, Rinehart & Winston.
- Gilbert J. K. and Boulter C. J., (1998), Learning science through models and modeling, in Fraser B. J. and Tobin K. G. (ed.) International handbook of science education, Amsterdam: Kluwer Academic Publishers, pp. 53–66.
- Gilbert J. K. and Treagust D. F., (2009), Multiple representations in chemical education, Dordrecht: Springer.
- Gilbert J. K. and Watts D. M., (1983), Concepts, misconceptions and alternative conceptions: changing perspectives in science education, Stud. Sci. Educ., 10, 61–98.
- Greca I. M. and Moreira M. A., (2000), Mental models, conceptual models, and modelling, Int. J. Sci. Educ., 22, 1–11.
- Greca I. M. and Moreira M. A., (2002), Mental, physical, and mathematical models in the teaching and learning of physics, Sci. Educ., 86, 106–121.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Halloun I. A., (2004), Modeling theory in science education, Dordrecht: Kluwer Academic Publishers.
- Izquierdo-Aymerich M., (2013), School chemistry: an historical and philosophical approach, Sci. & Educ., 22, 1633–1653.
- Jensen W. B., (1980), The Lewis acid–base concepts: an overview, New York: John Wiley & Sons.
- Jensen W. B., (1998), Logic, history, and the chemistry textbook: I. Does chemistry have a logical structure? J. Chem. Educ., 75, 679–687.
- Johnson-Laird P. N., (1983), Mental models, Cambridge, MA: Harvard University Press.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Lear., 7, 75–83.
- Johnstone A. H., (2000), Teaching of chemistry-logical or psychological? Chem. Educ. Res. Pract., 1, 9–15.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 43, 949–968.
- Kozma, R. B., Russell J. W., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74, 330–334.
- Laszlo P., (2013), Towards teaching chemistry as a language, Sci. & Educ., 22, 1669–1706.
- Levy Nahum T., Hofstein A., Mamlok-Naaman R. and Bar-Dov Z., (2004), Can final examinations amplify students' misconceptions in chemistry? Chem. Educ. Res. Pract., 5, 301–325.
- Lewis G. N., (1923), Valence and the structure of atoms and molecules, New York: Chemical Catalog Company.
- Lin H.-S., Cheng H.-J. and Lawrenz F., (2000), The assessment of students and teachers' understanding of gas laws, J. Chem. Educ., 77, 235–238.
- Lombardi O. and Labarca M., (2005), The ontological autonomy of the chemical world, Found. Chem., 7, 125–148.
- Luisi P. L., (2002), Emergence in chemistry: chemistry as the embodiment of emergence, Found. Chem., 4, 183–200.
- Luxford C. J. and Bretz S. L., (2013), Moving beyond definitions: what student-generated models reveal about their understanding of covalent bonding and ionic bonding, Chem. Educ. Res. Pract., 14, 214–222.
- McIntyre L., (2007), Emergence and reduction in chemistry: ontological or epistemological concepts? Synthese, 155, 337–343.
- Moran M. J., (2006), Factors that influence relative acid strength in water: a simple model, J. Chem. Educ., 83, 800–803.
- Nakhleh M. B., (1992), Why some students don't learn chemistry, J. Chem. Educ., 69, 191–196.
- Nakiboğlu C., (2003), Instructional misconceptions of Turkish prospective chemistry teachers about atomic orbitals and hybridization, Chem. Educ. Res. Pract., 4, 171–188.
- Nersessian N. J., (1999), Model-based reasoning in conceptual change, in Magnani L. Nersessian N. J. and Thagard P. (ed.) Model-based reasoning in scientific discovery, New York: Kluwer Academic/Plenum Press, pp. 5–22.
- Nersessian N. J., (2002), The cognitive basis of model-based reasoning in science, in Carruthers P., Stich S. and Siegal M. (ed.) The cognitive basis of science, Cambridge: Cambridge University Press, pp. 133–153.
- Nersessian N. J., (2009), Conceptual change: creativity, cognition, and culture, in Meheus J. and Nicles T. (ed.) Models of discovery and creativity, (pp. 127–166), Netherlands: Springer.
- Newman M., (2013), Emergence, supervenience, and introductory chemical education, Sci. & Educ., 22, 1655–1667.
- Rappoport L. T. and Ashkenazi G., (2008), Connecting levels of representation: emergent versus submergent perspective, Int. J. Sci. Educ., 30, 1585–1603.
- Reiher M., (2003), A systems theory for chemistry, Found. Chem., 5, 23–41.
- Salame, I. I., Sarowar, S., Begum, S. and Krauss, D., (2011), Students' alternative conceptions about atomic properties and the periodic table, Chem. Educ., 16, 190–194.
- Sanger M. J. and Greenbowe T. J., (1999), An analysis of college chemistry textbooks as sources of misconceptions and errors in electrochemistry, J. Chem. Educ., 76, 853–860.
- Scerri E. R., (2001), The new philosophy of chemistry and its relevance to chemical education, Chem. Educ. Res. Pract., 2, 165–170.
- Scerri E. R., (2004), How ab initio is ab initio quantum chemistry, Found. Chem., 6, 93–116.
- Scerri E. R., (2007), The periodic table: its story and its significance, Oxford: Oxford University Press.
- Schmidt H. J., (1991), A label as a hidden persuader: chemists' neutralization concept, Int. J. Sci. Educ., 13, 459–471.
- Schmidt H. J., Baumgärtner T. and Eybe H., (2003), Changing ideas about the periodic table of elements and students' alternative concepts of isotopes and allotropes, J. Res. Sci. Teach., 40, 257–277.
- Shulman L. S., (1987), Knowledge and teaching: foundations of the new reform, Harvard Educ. Rev., 57, 1–23.
- Shusterman G. P. and Shusterman A. J., (1997), Teaching chemistry with electron density models, J. Chem. Educ., 74, 771–776.
- Sozbilir M., (2002), Turkish chemistry undergraduate students' misunderstandings of Gibbs free energy, Univ. Chem. Educ., 6, 73–83.
- Taber K. S., (2000), Multiple frameworks?: evidence of manifold conceptions in individual cognitive structure, Int. J. Sci. Educ., 22, 399–417.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S., (2002), Chemical misconceptions—Prevention, diagnosis and cure: vol. I: theoretical background, London: Royal Society of Chemistry.
- Taber K. S., (2008), Conceptual resources for learning science: issues of transience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ., 30, 1027–1053.
- Taber K. S., (2009), College students' conceptions of chemical stability: the widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31, 1333–1358.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Taber K. S. and Coll R., (2002), Bonding, in Gilbert J. K., Jong O. D., Justi R., Treagust D. F. and Van Driel J. H. (ed.) Chemical education: towards research-based practice, Dordrecht: Kluwer, pp. 213–234.
- Talanquer V., (2006), Commonsense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 83, 811–816.
- Talanquer V., (2008), Students' predictions about the sensory properties of chemical compounds: additive versus emergent frameworks, Sci. Educ., 92, 96–114.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Talanquer V., (2013), How do students reason about chemical substances and reactions? in Tsaparlis G. and Sevian H. (ed.) Concepts of matter in science education, Dordrecht: Springer, pp. 331–345.
- Tan G. C. D., Taber K. S., Goh N. K. and Chia L. S., (2005), The ionisation energy diagnostic instrument: a two-tier multiple-choice instrument to determine high school students' understanding of ionisation energy, Chem. Educ. Res. Pract., 6, 180–197.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Teo T. W., Goh M. T. and Yeo L. W., (2014), Chemistry education research trends: 2004–2013, Chem. Educ. Res. Pract., 15, 470–487.
- Thagard P., (1992), Conceptual revolutions, New Jersey: Princeton University Press.
- Tümay H., (2014), Prospective chemistry teachers' mental models of vapor pressure, Chem. Educ. Res. Pract., 15, 366–379.
- Tümay H., (2016), Emergence, learning difficulties, and misconceptions in chemistry undergraduate students' conceptualizations of acid strength, Sci. & Educ., 25, 21–46.
- Valanides N., (2000), Primary student teachers' understanding of the particulate nature of matter and its transformations during dissolving, Chem. Educ. Res. Pract., 1, 249–262.
- Villani G., (2014), Structured system in chemistry: comparison with mechanics and biology, Found. Chem., 16, 107–123.
- Wandersee J. H., Mintzes J. J. and Novak J. D., (1994), Research on alternative conceptions in science, in Gabel D. L. (ed.) Handbook of research on science teaching and learning, NY: Macmillan Publishing Company, pp. 177–210.
- Wang C. Y. and Barrow L. H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Pract., 14, 130–146.
- Wilensky U. and Resnick M., (1999), Thinking in levels: a dynamic systems approach to making sense of the world, J. Sci. Educ. Technol., 8, 3–19.
- Zumdahl S. S. and Zumdahl S. A., (2012), Chemistry: an atoms first approach, 2nd edn, Cengage Learning.
| This journal is © The Royal Society of Chemistry 2016 |