Cognitive apprenticeship as a vehicle for enhancing the understanding and functionalization of organic chemistry knowledge
Katarina
Putica
b and
Dragica D.
Trivic
*a
aDepartment for Chemistry Education, University of Belgrade Faculty of Chemistry, Studentski trg 12-16 Belgrade 11000, Serbia. E-mail: dtrivic@chem.bg.ac.rs; Tel: +381 11 333 6 854
bInnovation Center of the Faculty of Chemistry, Belgrade, Serbia
First published on 2nd December 2015
Abstract
This paper presents a pedagogical experiment with parallel groups through which the effectiveness of the cognitive apprenticeship model of dealing with the teaching topic Carboxylic acids and their derivatives was compared with the traditional approach to the elaboration of this topic. This experiment featured the participation of 241 students aged 17, attending their third year of grammar school, natural sciences stream. The experimental group consisted of 118 students, whereas the control group was made up of 123 students. Within the framework of the experiment, a pre-test consisting of items that resembled regular textbook items was used as an instrument for checking how balanced the previously acquired knowledge concerning the teaching topic Carboxylic acids and their derivatives of the students in the two groups was. A post-test was used as an instrument for comparing the effectiveness of the two approaches, and it mostly consisted of items that required the application of the knowledge concerning the teaching topic Carboxylic acids and their derivatives in solving real-life problems. In the pre-test, no statistically significant difference in the overall percentage of correct answers given by the two groups of students was established. In the post-test, the students from the experimental group scored a statistically significant higher percentage of correct answers compared to the students from the control group. On the basis of this, it can be concluded that the applied cognitive apprenticeship approach has the potential to improve the level of students’ understanding of the concepts from the topic Carboxylic acids and their derivatives, as well as the students’ ability to apply the knowledge on the examples from real life.
Introduction
Understanding and the ability to apply scientific knowledge in various situations are central to a young person's preparedness for life in modern society, which is strongly shaped by science and technology (American Association for the Advancement of Science (AAAS), 1993; OECD, 2009; EURYDICE, 2011). Organic chemistry has enormous economic significance and it is an essential part of everyday life. That is why it is important to increase the awareness of secondary-school students of the relevance of organic chemistry in real life (Bailey and Bailey, 1971; Beasly, 1980; O'Dwyer and Childs, 2014). However, they usually find the organic chemistry curriculum difficult to understand (Bojczuk, 1982; Ratcliffe, 2002; Jimoh, 2005; Childs and Sheehan, 2009).The use of topics that are relevant to students' lives promotes both the motivation to learn science (Osborne et al., 2003; Vaino et al., 2012; Linnenbrink-Garcia et al., 2013) and a better understanding of scientific concepts (Winther and Volk, 1994; Demircioğlu et al., 2009; Schwartz-Bloom et al., 2011; Godin et al., 2014). However, traditional school science is weakly connected to everyday life, technology and society (Ebenezer and Zoller, 1993; Aikenhead, 2006; Van Berkel et al., 2009), as teachers “tend to favor abstract decontextualized ‘pure science’” (Aikenhead, 2006, p. 63). This is not the only problem that the students are faced with. Little attention is paid to developing the strategies that experts use in order to acquire new knowledge and solve complex problems in real life. Usually, it is essentially a matter of mastering a few clichéd procedures for solving textbook problems. On the other hand, improving students' problem-solving skills requires “both to understand the nature of expert practice and to devise methods that are appropriate to learning that practice” (Collins et al., 1989, p. 455).
It has been shown that students learn more deeply and perform better on complex tasks if they have the opportunity to engage in “authentic” learning – projects and activities that require them to employ subject knowledge to solve real-world problems (Newmann et al., 1995). By introducing authentic situations that show ways in which new knowledge is linked with real life in the teaching process, the acquisition of this knowledge is facilitated (Brown et al., 1989). Additionally, learning that occurs within the context of application is considered “more likely to result in improved practice” (Dennen and Bruner, 2008). As a way to achieve authenticity that reflects the way in which knowledge can be used in real-life, a teaching model called cognitive apprenticeship has been proposed. Cognitive apprenticeship is based on the model of traditional apprenticeship, and it is designed to “enculturate students into authentic practices through activity and social interaction” (Brown et al., 1989, p. 37).
Cognitive apprenticeship
During the greater part of man's history, acquisition of new knowledge was based on the traditional apprenticeship model. In traditional apprenticeship, the learning process begins by having an expert show the apprentice how the procedure to be mastered is carried out. Following this, the apprentice tries to perform the procedure independently, all the while under the oversight and with the support of the expert. Acting upon the expert's advice and guidance, the apprentice gradually becomes more skilled and less dependent on the expert's help. In the end, help is withdrawn altogether and the apprentice is capable of performing the given procedure independently.Cognitive apprenticeship is a model of instruction “that goes back to traditional apprenticeship but incorporates elements of schooling” (Collins et al., 1989, p. 453). Within the framework of the cognitive apprenticeship model, new knowledge is acquired with the same aim as in the case of the traditional model, which is to become equipped for implementation of knowledge in real-life. That is why knowledge is acquired through a presentation of the ways of implementing it for the purpose of solving real problems, “encouraging both a deeper understanding of the meaning of the concepts and facts themselves, and a rich web of memorable associations between them and the problem solving contexts” (Collins, 2006, p. 48). In this way, students have the opportunity to learn by actively using knowledge, rather than passively receiving it. Also, they can be confronted with different conditions under which acquired knowledge can be applied (Collins et al., 1989). Students can be encouraged to verbalize their own way of thinking during authentic problem solving, which uncovers their understanding and misconceptions and ultimately refines their conceptual understanding (Cave, 2010).
The cognitive apprenticeship approach focuses on four dimensions that constitute any learning environment: content, teaching methods, sequencing, and sociology (Collins, 2006):
1. Content
• Domain knowledge: academic knowledge specific for a given subject
• Heuristic strategies: general strategies that are effective when it comes to problem-solving
• Control strategies: general strategies for directing one's solution process
• Learning strategies: strategies for learning domain knowledge, heuristic and control strategies
2. Teaching methods
• Modeling: teacher performs a task in order for students to observe how it is done
• Coaching: teacher observes how students solve a task and provides advice and guidelines which help them in the process of solving
• Scaffolding: teacher provides support to help the student perform a given task
• Articulation: teacher encourages students to verbalise their knowledge and manner of thinking
• Reflection: teacher encourages students to evaluate their own performance
• Exploration: teacher encourages students to formulate the problems that they are going to solve on their own
3. Sequencing
• Increasing complexity: the complexity of the requirements is gradually increased
• Increasing diversity: acquisition of knowledge in real-life contexts that are as diverse as possible, in order to develop students' awareness of the various ways of its application
• Global to local skills: conceptualizing the whole task before executing the parts
4. Sociology
• Situated learning: students acquire new knowledge through solving real-life problems
• Community of practice: communities within which members communicate about ways to accomplish meaningful tasks
• Intrinsic motivation: students are motivated to solve the problems because they consider them as relevant to themselves and their surroundings
• Cooperative problem-solving: students work together in order to solve real-life problems.
Results of the study, which involves an environment of authentic science inquiry, indicated that the students' conceptual knowledge increased and their beliefs about the nature of science changed to a more tentative perspective when students apprenticed with expert scientists on the contemporary questions of molecular genetics (Charney et al., 2007). This kind of work enables students to develop more sophisticated ways of thinking which include an increased ability to generate hypotheses, consider alternative hypotheses, implement models and logical argumentation in explanations, connect ideas, extend concepts, and ask questions. Another study in which the new knowledge was acquired in the same way, but in the area of health-related research has shown that the research experiences increased student interest in science careers (Davis, 1999).
Instruction according to the cognitive apprenticeship approach encompasses students working under the oversight and with the support of a teacher, especially at the beginning of the learning process. Several studies in which new knowledge was gained within the framework of authentic learning situations created in the classroom deal with identifying the most effective scaffolding procedures. With the assumption that scaffolding in the form of reflection can help students to become autonomous integrators of their knowledge, the effectiveness of different reflection prompts has been considered. It has been found (Davis, 2003) that through generic prompts (asking students to “stop and think”) students develop a more coherent understanding as they work on a complex science project than within the framework of scaffolding with directed prompts (hints indicating potentially productive directions for student's reflection). Also, it has been realized (Davis and Linn, 2000) that self-monitoring prompts, which encourage planning for and reflection on activities, help students to demonstrate an integrated understanding of the relevant science while activity prompts, which guide the inquiry process, are less successful in prompting knowledge integration.
It is not easy for a teacher to provide continuous help and support for each student in the classroom. Learning based on cooperative problem solving “provides students with an additional source of scaffolding, in the form of knowledge and processes distributed throughout the group” (Collins et al., 1989, p. 489). The results of a study in which students solved real-life problems in small groups, with subsequent discussion with other students and teachers, confirmed the contribution of this approach to better training students to face ethical challenges in scientific research (Mabrouk, 2007). A computer-based interactive learning environment allows adequate assistance to students at any time during the learning process. In this way, it is possible to simulate situations from the real world, especially those that are not easy to demonstrate in the classroom (Stockhausen and Zimitat, 2002). It has been shown (Hwang et al., 2009) that scaffolding based on context-aware ubiquitous learning which integrates wireless, mobile and context-awareness technologies for the detection of situation of learners in the real world, provides adaptive support or guidance to students, promoting their learning efficiency and effectiveness in performing complex science experiments.
Scaffolding may include a combination of different forms of assistance in learning. For example, the combination of written material, oral consultations with experts, as well as the teacher's evaluation form as a basis for their reflection, can contribute to improving the quality of scientific reading and writing (Kolikant et al., 2006). Consideration of the most suitable type of real-life problems in physics teaching has shown that these are the problems that students set by themselves (Roth and Bowen, 1995). A similar conclusion has also been reached within the field of mathematics teaching (Schoenfeld, 1985).
The results of the study in which cognitive apprenticeship was used in order to teach the concept of causality showed that this approach can increase immediate learning effects (Hendricks, 2001), i.e. the students improved their understanding of the concept of causality. Many of them said that instruction was fun and different from standard science instruction. However, the results of this study did not support the expectation related to the transfer of knowledge to real-life situations, due to instructional conditions.
As can be seen, none of these studies dealt with the effectiveness of the cognitive apprenticeship approach when it comes to overcoming many of the problems concerning organic chemistry learning in high school. For instance, it has been shown that high-school students perceive organic chemistry as an abstract subject (O'Dwyer and Childs, 2011) which they fail to learn primarily due to their lack of conceptual understanding (Duffy, 2006). Low levels of conceptual understanding have been mainly associated with a rote memorization-orientated learning which occurs when students simply memorize new information without considering how it relates to the knowledge they already possess. Therefore it is necessary to foster meaningful learning, in which the learner makes significant connections between new information and prior knowledge (Bretz, 2001; Novak, 2002; Grove and Bretz, 2012). Alongside rote memorization, the main cause of the lack of the conceptual understanding of organic chemistry lies in an algorithmic-orientated approach to learning that presupposes using a limited number of problems with gradually-progressing solutions, through which the students are not able to generate alternative ways of thinking (Nakhleh, 1993; Sanger, 2005).
When it comes to the determination of the most prominent misconceptions concerning organic chemistry knowledge, it has been shown that the students have difficulty in understanding the dependence of physical properties of organic compounds such as solubility and boiling point of the presence of certain functional groups in their molecules. Even though students were able to discern the functional groups, they were not able to sufficiently understand and correlate the concepts regarding their properties (Akkuzu and Uyulgan, 2015).
Problems concerning the understanding of the concepts electrophile and nucleophile (Akkuzu and Uyulgan, 2015) as well as students' difficulties in explaining the relation of these concepts to acids and bases have also been reported (Cartrette and Mayo, 2011; Cruz-Ramirez de Arellano and Towns, 2014). Consequently it has been recommended that the concepts of acid and base should be taught through various learning techniques by associating them with the concepts of the nucleophile and the electrophile with respect to the reaction mechanisms (Anderson, 2009; Anzovino and Lowery Bretz, 2015). However, it has been shown that the students are also faced with great difficulties when it comes to the understanding of the mechanisms of organic chemistry reactions, and that they fail to use mechanistic thinking to predict the products of reactions, even when explicitly directed to do so (Grove et al., 2012).
The cognitive apprenticeship approach could be a good way of promoting students' understanding of the key concepts from the organic chemistry domain. Development of understanding of these concepts through solving real-life problems makes the subject less abstract and students can become aware of why the knowledge that they acquire is relevant for their lives. Learning based on the cognitive apprenticeship model is not rote memorization or algorithmic-orientated. Given that the real-life problems are usually very complex and impossible to solve through the application of a single algorithm, the students have an opportunity to connect the new knowledge with the knowledge that they already possess in new and diverse ways in order to find an adequate solution. Conceptual understanding occurs when the new knowledge is connected with existing knowledge by using alternative ways of logical thinking.
The review of literature showed that the researchers were mainly focused on studying the effectiveness of only one or two elements of the cognitive apprenticeship model, for example, comparing the effectiveness of various types of scaffolding or real-life problems that the students had to solve. Not a single study reported the effects of an intervention in which learning situations were designed according to all four dimensions of the cognitive apprenticeship model. Consequently, none of the studies reported the effects of the application of such an intervention in teaching organic chemistry to secondary-school students.
Given secondary-school students' problems with understanding and functionalization of organic chemistry knowledge, and the potential of the cognitive apprenticeship approach to overcoming them, which remains entirely unconfirmed in literature, we found it important to conduct a study that would establish whether an approach designed according to all four dimensions of the cognitive apprenticeship model can contribute to secondary-school students' better understanding and transfer of organic chemistry knowledge to real-life situations.
The purpose of the study and research hypotheses
The purpose of this study was to examine the effects of the application of the cognitive apprenticeship model in organic chemistry teaching in secondary schools. We started from the assumption that the cognitive apprenticeship approach has the potential to contribute to overcoming some of the problems linked to the teaching of organic chemistry in secondary schools, such as the students' view that the subject is abstract and of little relevance to their lives, as well as their low conceptual understanding of it. The construction of organic chemistry knowledge through solving of real-life problems which represents that the core of the cognitive apprenticeship approach prevents rote memorization and algorithmic-orientated learning which are perceived as the principal causes of the poor conceptual understanding of organic chemistry. Also the students could become more aware of the various ways in which the knowledge can be applied in real life. Therefore our aim was to determine to what extent cognitive apprenticeship approach contributes to a better understanding of the organic chemistry content and to the students being better equipped to apply knowledge acquired in this way in real life, compared to the traditional approach.In keeping with the aim of the study, the following research hypotheses were posed:
1. There is a significant difference in the effectiveness of the cognitive apprenticeship approach and the traditional approach in terms of their contribution to a better understanding of the content of organic chemistry in secondary school students, in favour of the cognitive apprenticeship approach.
2. There is a significant difference in the effectiveness of the cognitive apprenticeship approach and the traditional approach in terms of making secondary school students better equipped to apply their knowledge of organic chemistry in real life situations, in favour of the cognitive apprenticeship approach.
Methodology
Research design
According to the aim of the study and the research hypotheses, we conducted the pedagogical experiment with parallel groups. The experiment was conducted within the framework of dealing with the teaching topic Carboxylic acids and their derivatives. This topic was chosen according to the previously mentioned problems associated with the understanding of organic chemistry concepts. Work with students from both groups encompassed a total of five regular classroom periods, each lasting 45 minutes, two such periods per week. The schedule of activities and the teaching units in both groups are presented in Table 1.| Classroom period no. | Activities in the experimental group (taught by the researcher) | Activities in the control group (taught by the regular teacher in each of the schools) |
|---|---|---|
| 1 | Pre-testing | Pre-testing |
| Dealing with the teaching topic Carboxylic acids and their derivatives according to the principles of the cognitive apprenticeship model | Dealing with the teaching topic Carboxylic acids and their derivatives according to the principles of the traditional teaching approach | |
| 2 | Carboxylic acids: structure, IUPAC and common names, physical properties, acidity of carboxylic acids | Carboxylic acids: structure, IUPAC and common names, physical properties, acidity |
| 3 | Esters: structure, IUPAC and common names, mechanism of the esterification reaction, physical and chemical properties | Esters: structure, IUPAC and common names, mechanism of the esterification reaction, physical and chemical properties |
| 4 | Acyl halogenides, carboxylic acid anhydrides and amides: structure, IUPAC and common names, physical and chemical properties | Acyl halogenides, carboxylic acid anhydrides and amides: structure, IUPAC and common names, physical and chemical properties |
| Comparing the reactivity of all classes of carboxylic acid derivatives | Comparing the reactivity of all classes of carboxylic acid derivatives | |
| 5 | Post-testing | Post-testing |
Within the framework of the study, the students from both groups completed the pre-test during the first classroom period (Appendix 1). The pre-test was used as an instrument for checking how balanced the previously acquired knowledge concerning the teaching topic Carboxylic acids and their derivatives of the students in the control and the experimental group was. The items in the pre-test required the academic knowledge and its' application in solving usual academic problems. The next three classroom periods were devoted to the elaboration of the teaching topic Carboxylic acids and their derivatives, which was conducted according to the principles of the cognitive apprenticeship model with the students from the experimental group, while the students from the control group elaborated this teaching topic according to the principles of the traditional teaching approach. The first of these three classroom periods, for students in both groups, was devoted to the elaboration of the teaching unit Carboxylic acids, the second was devoted to the elaboration of the content concerning esters, while the third classroom period of the elaboration of this teaching topic was devoted to the remaining carboxylic acid derivatives, i.e. acyl halogenides, acid anhydrides and amides (Table 1). Upon completing the elaboration of the teaching topic Carboxylic acids and their derivatives in this way, within the framework of the fifth and final classroom period of our study, students from both groups took the post-test (Appendix 2). According to the aim of our study and the research hypotheses the items on the post-test were conceived so as to require the understanding and the application of knowledge concerning the above mentioned teaching topic in solving real-life problems.
The intervention vs. the traditional teaching approach
A detailed presentation of the elaboration of the teaching topic Carboxylic acids and their derivatives with the students in the experimental group is provided in Appendix 3. Each activity in the experimental group is preceded by the teacher's introductory presentation. The students carry out all activities in pairs, in accordance with the sociological principle of cooperative problem solving. Each activity block presented in Appendix 3 contains the corresponding tasks in the worksheet. While the students solve the task, the teacher monitors their work and obtains from them information on why they opted for a particular way of solving the task. On the basis of this, the teacher provides feedback, guidelines (application of coaching method) and specific forms of assistance in solving the task (application of the scaffolding method). When all the pairs of students complete the tasks, the representative of each pair reports to the other students on the solution that the pair found out. The teacher then asks the students to assess the correctness of the solution, and also to propose alternative solutions with appropriate explanation. In this way, the strategies that various students used to solve the task become visible to both the teacher and their peers (application of the articulation method). Through active communication that develops at the level of the class with a common aim – finding a solution to a particular problem, the class is transformed into a community of practice, which also contributes to stimulating the students' intrinsic motivation.The traditional teaching approach in the control group is teacher centered and involves the teacher presentation of the academic content about carboxylic acids and their derivatives. Students listen to the teacher's explanations, write notes and at the end of each classroom period solve the textbook items related to the teaching unit that has just been elaborated. These items are either open-ended or multiple choice types similarly like the items in the worksheet for the experimental group.
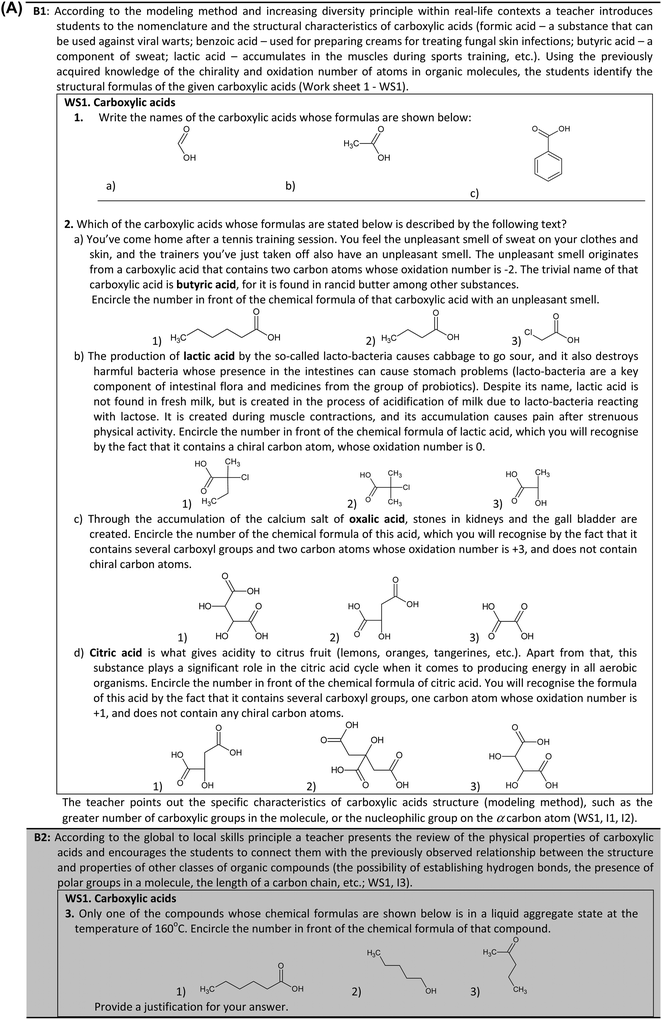
The elaboration of the teaching unit Carboxylic acids with the students from the experimental group encompasses activity blocks B1, B2 and B3 (Appendix 3). As can be seen within the framework of the activity block B1, the students in the experimental group learn where and how the selected carboxylic acids can be found and used in real-life (application of the sociological principle of situated learning) even before they consider the chemical formulas, IUPAC and common names of these acids. In order to write the chemical formulas of the carboxylic acids students have to apply their previous knowledge concerning chirality of atoms and oxidation number of elements in organic compounds. In this way the rote memorization of this content could be avoided and students are encouraged to link the previously acquired knowledge with the new knowledge in a meaningful way. In order to make the lesson about carboxylic acids less abstract for the students and to promote their intrinsic motivation, the examples of the application of carboxylic acids are chosen with regard to their relevance for the students, from the perspective of their real life experience and interest. For example, lactic acid is dealt with as a substance which accumulates in the muscles during sports training sessions and causes pain. According to the increasing diversity principle, the students learn how the same carboxylic acid can have different roles in different real-life situations. For example, citric acid is dealt with as a substance that gives acidity to citrus fruits, as well as a substance which, within the framework of the citric acid cycle, has a significant role in the production of energy in all aerobic organisms. In contrast to this approach, the teacher in the control group presents to the students a list of the structural chemical formulas of various mono-, di- and tricarboxylic acids and their IUPAC and common names. Real-life application of the carboxylic acids is illustrated at the end of the lesson by a teacher.
The elaboration of the structure and nomenclature of carboxylic acids is followed by the elaboration of their physical properties. The students in the experimental group are encouraged to presume and state the physical properties of carboxylic acids on the basis of the relationship between the structure (the possibility of establishing hydrogen bonds, the presence of polar groups in a molecule, the length of a carbon chain, etc.) and physical properties of the previously elaborated organic compounds. This approach is in accordance with the global to local skill principle (activity block B2). On the other hand, the students in the control group listen to the teacher's presentation and explanation of the physical properties of carboxylic acids (boiling point, solubility in water, and smell).
The elaboration of the acidity of carboxylic acids with the students from the experimental group is based on the increasing complexity principle (activity block B3). As previously discussed acidity of the organic compounds represents one of the key organic chemistry concepts that the students find difficult to understand. In order to overcome this problem the teaching/learning situation in which the students start to develop their understanding of this topic by applying the knowledge of general chemistry concepts (the dissociation of weak acid, Ka and pKa values) and the knowledge of organic chemistry (the properties of acetic acid) from primary school is designed. The students write the chemical equation of the dissociation of acetic acid, then the equilibrium constant expression and, based on the given value of this constant, they calculate the pKa value of acetic acid. This is followed by the teacher's presentation of the pKa values of other aliphatic carboxylic acids and the explanation of the influence of the electronegative group on the α carbon atom on the pKa values of chloroacetic acid, dichloroacetic acid and trichloroacetic acid (the application of the modeling teaching method). The students then apply the new knowledge in order to determine the pKa value of lactic acid. Following this, the teacher reminds the students of the rule that a stronger acid can “squeeze out” a weaker acid from its salt and presents them with a problem of discerning which one of the two given substances is propanoic acid (or which one is ethanol), on the basis of their reactivity with sodium bicarbonate. Zhou et al. (2015) documented that the students have difficulty in comparing the acidity of acetic acid with the acidity of inorganic acids, and in particular with the acidity of carbonic acid. Therefore, in order to promote students' understanding of this concept, we presented them with a problem that requires the application of their previous knowledge concerning the acidity of alcohols and carbonic acid. In order to additionally promote their conceptual understanding the students solve this problem according to the principles of the Itakamura method of articulation. Firstly, they propose hypotheses concerning which of the two substances would react with the sodium bicarbonate and why. This is followed by the teachers' demonstration of what really happens when sodium bicarbonate is added to propanoic acid and ethanol. After that, in accordance with the sociological principles of cooperative problem solving and community of practice a discussion in which all of the students and their teacher take part is conducted in order to draw conclusions on the strength of aliphatic carboxylic acids compared to carbonic acid and alcohols.
On the other hand, the students in the control group listen to the teacher's explanation about the factors that influence the acidity of acetic acid and how the acidity of the aliphatic carboxylic acids can be compared with the acidity of carbonic acid and alcohols. Also, they observe the demonstration of what happens when sodium bicarbonate is added to propanoic acid and ethanol.
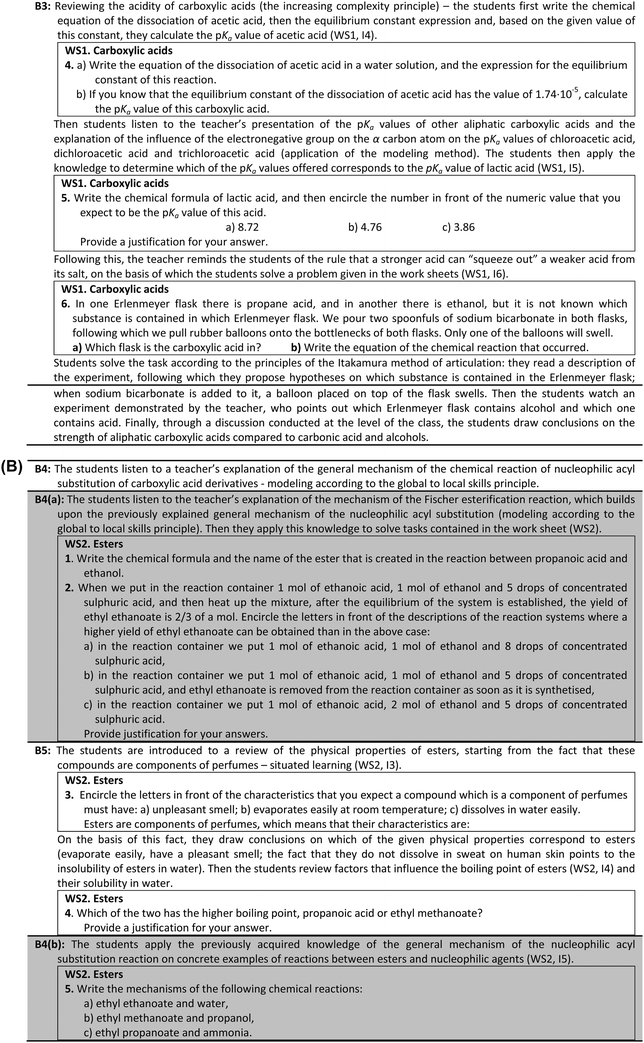
The next classroom period is devoted to the elaboration of the teaching unit concerning esters. The elaboration of this teaching unit with the students in the experimental group encompasses activity blocks B4 and B5 (Appendix 3). In order to introduce the students to both the reactions of esterification and the chemical properties of esters, the teacher elaborates the general mechanism of the nucleophilic acyl substitution reaction (activity block B4) according to the global to local skill principle. In accordance with the recommendation that students' understanding of the concepts nucleophile and electrophile is best promoted if they are taught with respect to the reaction mechanisms, whilst elaborating the general mechanism of the nucleophilic substitution reaction, the teacher also elaborates the electrophilic/nucleophilic nature of its reactants and products. After this, the students are encouraged to apply the general mechanism on the examples of reactions between alcohol and carboxylic acids, or between the esters and the nucleophiles. By the application of the modeling method the general structure of esters, their IUPAC and common names are explained to the students by the teacher. Following this, within the framework of the activity block B5, esters are presented to the students as the components of perfumes. In this manner according to the sociological principle of situated learning the awareness of the importance of the new knowledge in real life, as well as the intrinsic motivation of students, are encouraged. Based on this introduction the students are expected to conclude about the physical properties of esters, such as esters evaporate easily, have a pleasant smell and do not dissolve in water, which is why they cannot be easily washed away from skin by sweat. This is followed by a theoretical elaboration of these physical properties, based on the understanding of relationships between the physical properties and structures of already known molecules of organic compounds. The elaboration of the esters in the control group comprises the teacher's presentation of the structure and IUPAC and common names of esters, examples of the esterification reaction, as well as the examples of reactions of esters with nucleophiles. Through the elaboration of these examples, the students in the control group have the opportunity to realize that these reactions follow the nucleophilic acyl substitution reaction mechanism. Also, the students listen to teacher's explanation of the physical properties of esters (boiling point, solubility in water, and smell).
The final classroom period of the elaboration of the teaching topic Carboxylic acids and their derivatives is devoted to the other classes of carboxylic acids derivatives, i.e. acyl halogenides, carboxylic acid anhydrides and amides. In accordance with the principle of situated learning, the students in the experimental group are introduced to the various compounds from the aforementioned classes of carboxylic acids derivatives through examples of their application in real-life (activity block B6). This includes the use of urea in agriculture, acyl halogenides as lachrymatory substances, and the use of acetic acid anhydrates in the synthesis of heroin. The teacher in the control group presents the structural chemical formulas of these substances and their physical properties. At the end of lesson the students in the control group listen to teacher's presentation of the examples of real-life application of the carboxylic acid derivatives.
The elaboration of the chemical properties of the carboxylic acid derivatives is conducted in accordance with the global to local skill principle, i.e. according to the general mechanism of the nucleophilic acyl substitution reaction the students in the experimental group write the mechanism of reactions of the given carboxylic acid derivatives with various nucleophiles (activity block B8a). The situated learning is enabled by teacher's explanation of these reactions in real-life contexts, e.g. the irritation of the wet surfaces of the human organism, such as the surface of the eye or the mucous membranes of bronchial tubes, is caused by the reaction between acyl halogenides and water. The students then compare the reactivity of the carboxylic acid derivatives based on the fact that esters exist independently in nature, whereas acyl halogenides do not. Also, students in the experimental group should conclude which class of these compounds is the most convenient one for quick and easy organic syntheses in chemical industry (activity block B8b). In comparison to this approach, the teacher in the control group writes a series of examples of reactions of various carboxylic acid derivatives with nucleophiles, and arranges the classes of derivatives according to their growing reactivity.
Participants
A total of 241 third-year grammar school students (aged 17), attending the natural sciences course of studies, from three grammar schools in Serbia, participated in the study. Two of these grammar schools are located in Belgrade, while the third one is in Šabac. The schools' science committees were informed about the study. In order to obtain the consent from the schools, the research proposal, i.e. the aims of research, who will be the participants in the research, the research methodology, and the way of data usage after the research, was presented during the first meeting with the science committee in each school. During that meeting we discussed the benefits for the school related to the introduction of the new approach to the elaboration of one theme which is present in the chemistry curriculum and the effects of this way of work on the students' achievements, as well as the expected activities of all participants according to the research design. After that the necessary permissions were obtained from the schools' governments and the contracts which regulate the collaboration between the Faculty of Chemistry and each school were signed by the Faculty dean and the principal of each school. The research sample was made up of 10 classes: four classes in each of the Belgrade grammar schools and two classes from the Šabac grammar school. The classes for the experimental and control groups were chosen randomly among all classes of the third-year in each grammar school. This is a weaker design of the experiment (Taber, 2014), but it is in line with agreed ways of working with schools' science committees and schools' principals. The experimental group was made up of five classes (118 students), namely: two classes from each Belgrade grammar school and one class from the Šabac grammar school. The control group, encompassing 123 students, was made up of two classes from each Belgrade grammar school and one class from the Šabac grammar school. One teacher from each of the above-mentioned grammar schools participated in the study. The working experience of these teachers exceeds 20 years. They worked with the students from the control group, whereas one of the researchers worked with the students from the experimental group. Such a teaching arrangement was made primarily because, upon discussing the organization of the experiment, the teachers had showed little enthusiasm for adopting the new approach themselves. Despite their vast teaching experience, most of the teachers had heard about this kind of approach for the first time. They expressed that they prefer to continue teaching in their usual way, while the researcher should be the one who try to do it in a “new way”. Such an attitude can be attributed to several factors. It is well known that experienced teachers are less receptive to proposed changes in their teaching (Hargreaves, 2005). We had to take into account the weekly workload of the teachers and respected their attitude toward the new approach. Compared to more than two decades of the school teachers' experience, the researcher who worked with the students in the experimental group, a PhD student, is a relative novice with less than five years of teaching experience. On the other hand, successful implementation of the cognitive apprenticeship approach in the classroom requires both a thorough theoretical knowledge of the approach and the ability to devise various ways for presentation of any given academic content according to its principles. Developing these skills requires a lot of time and effort which, given the teachers' already substantial workload, only added to their reluctance to apply the approach in practice.There is no getting around the fact that the researcher was well informed about the cognitive apprenticeship approach and that therefore she could be considered as an expert when it comes to its application. On the other hand, the teachers whose teaching experience exceeds 20 years can certainly be considered as experts when it comes to teaching in a traditional way. Therefore, rather than making either side teach according to the approach that they were not enthusiastic about to one group, and at the same time according to the approach in which they specialized to the other, we anticipated that it would be more effective to simply compare the learning outcomes of the two different expert teaching practices, even if it meant having two different teachers working with the students in the control and the experimental group. Such a teaching arrangement, however, could have influenced the final outcome of our experiment.
At the very beginning of the study the students from each class in the experimental and the control group in the research sample were informed about the working plan during the next five classroom periods. We explained to the students in both groups the purposes of the pre-test and the post-test as the opportunity to get insight into the previous knowledge and to monitor progress after the elaboration of the new theme. But, students were volunteers in the sense that they could give up on solving the pre-test and the post-test, because these tests were the additional activities in comparison with the school plan established at the beginning of the school year. On the other side, the elaboration of the theme Carboxylic acids and their derivatives was completely in accordance with the school plan and the existing national curriculum. According to the definition of the Hawthorne effect, being exposed to new factors in their working environment and to the realization that they are participants in an experiment and thus objects of special attention may prompt individuals to temporarily improve their working performance. In order to overcome this effect the students in the experimental group were informed by their regular teacher that the next teaching topic will be taught to them by a young colleague as a part of a mandatory teaching practice session of her PhD training program. No emphasis was placed upon the novel approach that the new teacher will implement. The students from both groups previously worked with graduate students who completed mandatory teaching practice sessions in their schools. Within these sessions graduate students taught lessons according to the various teaching approaches and administered tests of their own, so this was not a new experience for them. As in the case of the tests administered by the graduate students, their regular teacher assured the students in the experimental group that the marks they get on the tests administered by their new teacher will not be known to him/her or officially registered in their school portfolios. Knowing that their performance will not be officially documented or known to their regular teacher eased the performance pressure that the students ordinarily feel upon taking any other school test. We expected that this, in turn, helped minimize the influence of the Hawthorne effect. As for the students in the control group, their regular teacher repeated the story of a young colleague teaching the next teaching topic to some of the other classes as a part of her mandatory teaching practice session. The teacher assured them that the colleague will not be working with them simply because her work schedule was incompatible with their chemistry lessons timetable. However, out of courtesy to the visiting colleague, they will “try out” two of her tests. The marks they get on these tests will not be known to their regular teacher, or officially registered. No mention was made of the fact that their performance was being compared to the performance of the students working with the new teacher, or that the new teacher will implement a novel teaching approach.
It was not possible to prevent communication between the students taught by different teachers and that could have easily led to comparisons of the ways in which their lessons were taught. Therefore, as a way of equalizing the influences of the experimental intervention between different groups, which is noted in the literature as a powerful tool for minimizing the influence of the Hawthorne effect (Cook, 1967), we made sure that the general organization of the lessons in both groups, at least at a first glance, appeared to be the same. For example, the teaching topic Carboxylic acids and their derivatives was elaborated for the same amount of time in both groups, with the identical order of the teaching units. Students from both groups completed worksheets during these lessons (which is a type of schoolwork that they were already familiar with), and within the elaboration of the acidity of carboxylic acids the same experiment was performed by a teacher in both groups. All of the lessons were realised according to the regular school schedule in the school laboratory – the regular place for chemistry lessons.
Data collection
The pre-test items referred to carboxylic acids and their derivatives, which the students had dealt with in the eighth year of primary school, the protolytic theory of acids and bases, which had been taught to them in the first year of grammar school, and also to the material making up the teaching topics Alkenes, Alcohols and Carbonyl compounds, which they had dealt with in the course of the current academic year. The test items were of various types:• four multiple choice items (I1a, I3, I4, I11)
• three alternative choice items (I8a, I10b, I10c)
• 15 open-ended items (the remaining items).
The students had previous experience in solving the above-mentioned types of tasks.
Given that the control and the experimental group should be as similar as possible before the experimental intervention is introduced (Shadish et al., 2002), the results of the pre-test represent an important indicator of how well the students in the two groups are matched when it comes to the previously acquired knowledge concerning a selected teaching topic, at the beginning of the experiment. However, it has been shown that the pre-test can sensitize the students in the experimental group to intervention. Additionally, taking the pre-test may influence the outcome of the subsequent identical post-test for students in both groups (Martella et al., 2013). In order to overcome these obstacles, we applied one of the guidelines for devising the two tests (Cohen et al., 2007), which states that the pre-test and the post-test may differ in form or wording, as long as they refer to the same content. Therefore, in our experiment the pre-test and the post-test were two different tests. In this way, we avoided having the students from both groups doing the same test twice, and by carefully constructing the items of our pre-test so as to resemble regular textbook items that do not require the application of academic content in solving real-life problems (e.g. encircle the letter in front of the chemical formula of a compound whose oxidation can produce carboxylic acid), we avoided sensitizing the students in the experimental group to the experimental intervention. On the other hand, in order to collect data to verify the second research hypothesis, the majority of the items in the post-test were conceived so as to require the application of the acquired knowledge concerning the teaching topic Carboxylic acids and their derivatives in solving real-life problems (e.g. instead of the straightforward academic request to determine whether a given carboxylic acid is an α- or β-hydroxycarboxylic acid, the students were asked to select an appropriate shampoo for persons with different skin types, on the basis of the type of the hydroxycarboxylic acid that the proposed shampoos contained). For the purpose of gaining insight into the strategy applied in solving the problems posed and the students' manner of reasoning, in the last item of each task the students were required to provide an explanation of the solution. In this way, we tried to collect data to verify the first research hypothesis. Two items (I6, I8) in the post-test had the sole purpose of checking the understanding of academic knowledge. They refer to areas which secondary school students find rather difficult to understand. Those are the acid-based characteristics of organic substances (Furio-Mas et al., 2007) and the mechanisms of organic reactions (Bojczuk, 1982; Ratcliffe, 2002; Jimoh, 2005; Childs and Sheehan, 2009).
The tasks in the post-test were of the following type:
• one matching item (I2b)
• 15 open-ended items (the remaining items).
We should note that the answers to both tests, in the items requiring an explanation, were only awarded points if they were completed, that is, only if an explanation was provided, so that when it came to classifying the tasks according to their types, these were presented as open-ended items. The principles for coding of the open-ended items on both tests are presented in Appendix 4.
To confirm the content validity, the pre-test and the post-test were examined by a group of experts comprising two university chemistry educators and five high school chemistry teachers, who have been teaching for over twenty years in grammar schools in the cities of Belgrade and Šabac. Additionally, the pre-test and the post-test were piloted with 103 third-year grammar school students, in order to check its readability and understandability. Then, some minor revisions were made in the light of the results of the pilot study to produce the final versions of tests.
To confirm the internal validity, the value of the alpha reliability coefficient (KR20) was established for both tests. Also, for each item of the pre-test and the post-test, the values of the discrimination and the difficulty index were established. The pre-test had a KR20 value of 0.86 for both the pilot study and the main study. The distribution of values of the discrimination indexes and the difficulty indexes for each item of the pre-test is presented in Fig. 1. As can be seen from Fig. 1, the values of the discrimination indexes range from 0.2 to 0.89, whereas the values of the difficulty indexes range from 0.3 to 0.79.
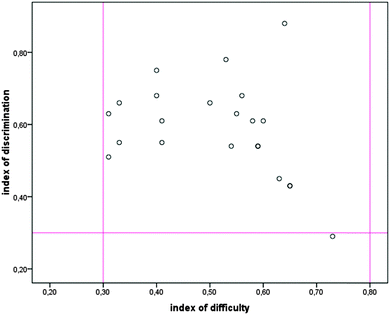 | ||
| Fig. 1 The distribution of the values of the discrimination indexes and the difficulty indexes for each item of the pre-test. | ||
As for the post-test, a KR20 value of 0.85 for the pilot study and a KR20 value of 0.87 for the main study were established. The distribution of the values of the discrimination indexes and the difficulty indexes for each item of the post-test is presented in Fig. 2. As can be seen from Fig. 2, the values of the discrimination indexes range from 0.4 to 0.89, while the values of the difficulty indexes range from 0.3 to 0.79.
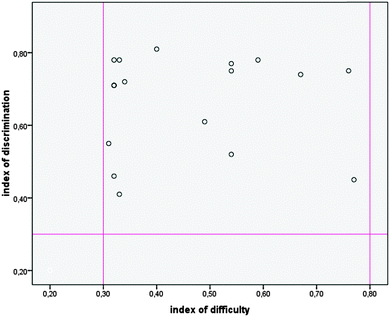 | ||
| Fig. 2 The distribution of the values of the discrimination indexes and the difficulty indexes for each item of the post-test. | ||
On the basis of the established KR20 values of 0.86 for the pre-test and 0.87 for the post-test, which are considerably higher than the lowest value allowed of 0.70 (Nunnally, 1978), we can conclude that both tests have a relatively high degree of intrinsic value. According to that the pre-test and the post-test can be applied without any further revision (Peterson et al., 1989; Özmen, 2008), since their values, except for the values of one discrimination index for the pre-test, exceed 0.3.
Results and discussion
By using the SPSS software program for statistical analysis, we determined the overall percentage of correct answers in both tests, in both groups. The statistical significance of the difference in the overall percentage of correct answers between the groups was examined by means of a t test. Additionally, for each item on both of the tests we provided a 2 × 2 contingency table, as well as the corresponding values of the chi-square test of independence.Table 2 contains the overall percentage of correct answers, pE and pK in each of the groups on the pre-test, as well as the t(240) value of the test. The maximum score on the pre-test is 22.
However, a nonsignificant t-test does not necessarily mean that the two groups are identical. Therefore, in order to determine whether the students from the control and the experimental group had equal knowledge at the beginning of the experiment, we followed the “two one-sided t tests” procedure recommended by Lewis and Lewis (2005). Following this procedure, we determined that t1 and t2 have the identical value of 3.08. Given that the value of both t1 and t2 is higher than the t(1–2α) (α = 0.1) value, it can be concluded that the students from the control and the experimental group had equal knowledge at the beginning of the experiment.
The values of the chi-square test of independence for each item contained in the pre-test are presented in Table 3.
| Items | Number of correct answers in the experimental group | Number of incorrect answers in the experimental group | Number of correct answers in the control group | Number of incorrect answers in the control group | χ 2(1, N = 241) |
|---|---|---|---|---|---|
| a The value of the chi-square test of independence is statistically significant at the level of p < 0.01. | |||||
| I1a | 75 | 43 | 79 | 44 | 0.01 |
| I1b | 61 | 57 | 66 | 57 | 0.09 |
| I2 H2O | 80 | 38 | 73 | 50 | 1.85 |
| I2 H2SO4 | 38 | 80 | 36 | 87 | 0.24 |
| I2 K2Cr2O7 | 69 | 49 | 76 | 47 | 0.28 |
| I3 | 89 | 29 | 86 | 37 | 1.15 |
| I4 | 67 | 51 | 71 | 52 | 0.02 |
| I5 | 45 | 73 | 53 | 70 | 0.61 |
| I6a | 51 | 67 | 46 | 77 | 0.85 |
| I6b | 34 | 84 | 39 | 84 | 0.24 |
| I7a | 48 | 70 | 48 | 75 | 0.07 |
| I7b | 74 | 44 | 68 | 55 | 1.37 |
| I7c | 67 | 51 | 76 | 47 | 0.63 |
| I7d | 38 | 80 | 35 | 88 | 0.40 |
| I8a | 62 | 56 | 68 | 55 | 0.18 |
| I8b | 39 | 79 | 36 | 87 | 0.40 |
| I9a | 62 | 56 | 58 | 65 | 0.70 |
| I9b | 64 | 54 | 68 | 55 | 0.03 |
| I10a | 68 | 50 | 73 | 50 | 0.07 |
| I10b | 77 | 41 | 79 | 44 | 0.03 |
| I10c | 77 | 41 | 79 | 44 | 0.03 |
| I11 | 38 | 80 | 62 | 61 | 8.22a |
Based on these results, it can be concluded that there are no statistically significant differences in the knowledge of the experimental and the control groups concerning the following contents:
• the chemical properties of alkenes, alcohols and carbonyl compounds (I1, I2);
• the spread of carboxylic acids in nature (I3 and I4);
• the physical properties of alcohols and carbonyl compounds (I5, I6);
• the proteolytic theory of acids and bases (I7, I8);
• the terms electrophile and nucleophile (I9, I10).
When it comes to I1, students in both groups were relatively successful in selecting a primary alcohol as a substance that can be oxidized to a carboxylic acid and producing an appropriate formula of that carboxylic acid. However, some of the students in both groups that within I1a correctly selected benzyl alcohol, incorrectly presented the oxidation product as a carboxylic acid in which the benzene ring and the carboxylic group are connected with one –CH2– group. The index of difficulty value of I1a is well above 0.35, so it is unlikely that many of the students guessed the correct answer to this multiple-choice item. When it comes to the students in both groups whose answers on I1a were coded as incorrect, majority made no attempt to solve this item, whilst a few of them selected phenol. These students presented a formula of benzoic acid in I1b, whilst the rest of the students in both groups whose answers were coded as incorrect made no attempt to solve this item. When it comes to I2, students in both groups showed relatively poor knowledge of the fact that sulphuric acid serves as a catalyst for the reaction of alkene hydration (I2 H2SO4). With the exception of two students in the control and eight students in the experimental group who actually wrote the formula of potassium dichromate as a catalyst for this reaction, the remaining students in both groups whose answers were coded as incorrect made no attempt to solve this item. When it comes to I2 K2Cr2O7, the majority of the students from both groups whose answers were coded as incorrect presented the chemical formula of potassium dichromate as K2CrO7, KCr2O7 or K2Cr2O6. When it comes to I3, students in both groups demonstrated good knowledge of the fact that vinegar represents 5% water solution of acetic acid. Students from both groups whose answers were coded as incorrect either selected propanoic acid instead of the ethanoic acid or made no attempt to solve this item. When it comes to I4, students in both groups demonstrated relatively good knowledge of the facts that anthill's characteristic smell and skin irritation in contact with nettles are caused by methanoic acid. Students from both groups whose answers were coded as incorrect either selected benzoic acid instead of the methanoic acid or made no attempt to solve this item. Given that the index of difficulty values of these two multiple-choice items were above the value of 0.35, it is not likely that many of the students in both groups managed to guess the correct answers. Within the framework of I5 and I6 students had to arrange the three given substances in a sequence based on increasing boiling point and increasing solubility in water, respectively. Overall, students in both groups experienced some difficulties with these two items. Majority of students in both groups whose answers were coded as incorrect on I5 made no attempt to solve this item. The remaining few of these students produced an incorrect sequence that had butane as the first, 1-butanol as the second and acetone as the third sequence member, with no corresponding explanation. When it comes to I6a and I6b, majority of students in both groups whose answers were coded as incorrect made no attempt to solve these items, whilst some of them produced a correct sequence with no corresponding explanation for I6a. I7 and I8 checked students' general knowledge concerning acidity/basicity. When it comes to I7a, I7b, I7c and I7d, in general, students in both groups either answered them correctly or made no attempt to solve them. They experienced the greatest difficulty with I7d where they were expected to present the mathematical ratio of the equilibrium constant of the reaction of the dissociation of acid HA and the pKa value of this acid: pKa = −log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka. Within I8a, based on the pKa values, students had to determine which of the two given acids is stronger, whilst in I8b they had to apply this knowledge in order to determine whether the given reaction of acid and salt is possible. As can be seen in Table 3, the number of correct answers on I8b was lower than the number of correct answers on I8a, which could imply that some of the students whose answers were coded as correct on I8a actually guessed the correct solution. However the index of difficulty value for item I8a is 0.54. Therefore, it is more likely that some of the students in both groups, who knew how to determine which of the two acids is stronger, simply had no idea how to apply this knowledge when it comes to the reaction of acid with salt. Within I9a and I9b students were expected to define the terms nucleophile and electrophile. Majority of the students in both groups whose answers were coded as incorrect made the attempt to solve these items, but incorrectly defined nucleophile as a negatively charged atom, group or substance. Some of the students wrote that the –OH group of alcohols represents a nucleophile. In I9b electrophile was incorrectly defined as a negatively charged atom, group or substance, whilst some of the students from both groups simply wrote that the carbon atom in the carbonyl group of aldehydes and ketones is electrophilic. Within I10 students were expected to apply the knowledge concerning electrophiles and nucleophiles on a carboxylic group in the general formula of the carboxylic acids. Since the number of correct answers on I10a, I10b and I10c was slightly higher than the number of correct answers on I9a and I9b in both groups, it seems that in some cases even such inadequate knowledge concerning electrophiles and nucleophiles was sufficient to solve these items correctly. I10b and I10c were alternative choice items, but their index of difficulty values were 0.43 so it is unlikely that many of the students in both groups actually managed to guess the correct answers on these items. The only discrepancy between the two groups in the pre-test occurred in connection with the notion of esters (I11), of which the students learn in the final year of primary school. In the control group, there was a statistically significant higher value of the chi-square test of independence in this item compared to the experimental group. The index of difficulty for this multiple-choice item was 0.55. About a half of the students in the control group whose answer was coded as incorrect selected answer (c) which stated that esters cannot be found in fats, whilst the rest of them made no attempt to solve this item. When it comes to the students in the experimental group whose answers were coded as incorrect, about 30% of them selected answer (a), 45% selected answer (c), whilst the rest of them made no attempt to solve this item.
Ka. Within I8a, based on the pKa values, students had to determine which of the two given acids is stronger, whilst in I8b they had to apply this knowledge in order to determine whether the given reaction of acid and salt is possible. As can be seen in Table 3, the number of correct answers on I8b was lower than the number of correct answers on I8a, which could imply that some of the students whose answers were coded as correct on I8a actually guessed the correct solution. However the index of difficulty value for item I8a is 0.54. Therefore, it is more likely that some of the students in both groups, who knew how to determine which of the two acids is stronger, simply had no idea how to apply this knowledge when it comes to the reaction of acid with salt. Within I9a and I9b students were expected to define the terms nucleophile and electrophile. Majority of the students in both groups whose answers were coded as incorrect made the attempt to solve these items, but incorrectly defined nucleophile as a negatively charged atom, group or substance. Some of the students wrote that the –OH group of alcohols represents a nucleophile. In I9b electrophile was incorrectly defined as a negatively charged atom, group or substance, whilst some of the students from both groups simply wrote that the carbon atom in the carbonyl group of aldehydes and ketones is electrophilic. Within I10 students were expected to apply the knowledge concerning electrophiles and nucleophiles on a carboxylic group in the general formula of the carboxylic acids. Since the number of correct answers on I10a, I10b and I10c was slightly higher than the number of correct answers on I9a and I9b in both groups, it seems that in some cases even such inadequate knowledge concerning electrophiles and nucleophiles was sufficient to solve these items correctly. I10b and I10c were alternative choice items, but their index of difficulty values were 0.43 so it is unlikely that many of the students in both groups actually managed to guess the correct answers on these items. The only discrepancy between the two groups in the pre-test occurred in connection with the notion of esters (I11), of which the students learn in the final year of primary school. In the control group, there was a statistically significant higher value of the chi-square test of independence in this item compared to the experimental group. The index of difficulty for this multiple-choice item was 0.55. About a half of the students in the control group whose answer was coded as incorrect selected answer (c) which stated that esters cannot be found in fats, whilst the rest of them made no attempt to solve this item. When it comes to the students in the experimental group whose answers were coded as incorrect, about 30% of them selected answer (a), 45% selected answer (c), whilst the rest of them made no attempt to solve this item.
Table 4 contains the overall percentage of correct answers pE and pK for both groups and the t(240) test values used to examine the statistical significance of the difference in the overall percentage of correct answers in the experimental and the control group on the post-test. The maximum score on the post-test is 17.
The t(240) test value shows that the students in the experimental group achieved a statistically significant better overall percentage of correct answers than those in the control group.
The results of the chi-square test of independence for each item contained in the pre-test are presented in Table 5. The maximum score on the post-test is 17.
| Items | Number of correct answers in the experimental group | Number of incorrect answers in the experimental group | Number of correct answers in the control group | Number of incorrect answers in the control group | χ 2(1, N = 241) |
|---|---|---|---|---|---|
| a The value of the chi-square test of independence is statistically significant at the level of p < 0.01. b The value of the chi-square test of independence is statistically significant at the level of p < 0.05. | |||||
| I1a | 81 | 37 | 49 | 74 | 20.11a |
| I1eq | 53 | 65 | 44 | 79 | 2.09 |
| I2 la | 87 | 31 | 96 | 27 | 0.615 |
| I2 ca | 74 | 44 | 69 | 54 | 1.09 |
| I2 ta | 62 | 56 | 68 | 55 | 0.18 |
| I2 sa | 85 | 33 | 76 | 47 | 2.85 |
| I2 Marija | 44 | 74 | 29 | 94 | 5.36b |
| I2 Milica | 44 | 74 | 29 | 94 | 5.36b |
| I3 | 74 | 44 | 57 | 66 | 6.50a |
| I4 | 68 | 50 | 51 | 72 | 6.29a |
| I5 | 97 | 21 | 89 | 34 | 3.31 |
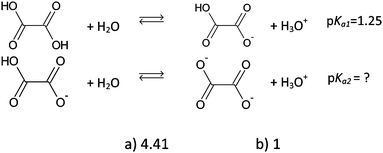
| I6 | 54 | 64 | 20 | 103 | 24.64a |
| I7a | 46 | 72 | 30 | 93 | 5.94b |
| I7b | 61 | 57 | 17 | 106 | 39.46a |
| I8a | 48 | 70 | 26 | 97 | 10.81a |
| I8b | 48 | 70 | 26 | 97 | 10.81a |
| I9 | 57 | 61 | 24 | 99 | 22.37a |
The values of the chi-square test of independence indicate that in 11 out of 17 items of the post-test there were a statistically significant higher number of correct answers in the experimental group than in the control one.
More specifically, the chi-square test of independence values presented in Table 5 indicate that the students in the experimental group had a statistically significant higher number of correct answers on all items on the post-test that required the application of the knowledge in authentic situations, with the exception of I5. Namely, I1a required the application of the knowledge concerning the acidity of carboxylic acids, I2 Marija and I2 Milica checked the students' ability to link their knowledge of the structure of carboxylic acids to their application, I3 and I4 referred to the application of the knowledge concerning the physical properties of carboxylic acids and their derivatives, whilst I7 and I9 required the application of the knowledge concerning the chemical properties of these substances in authentic contexts. Therefore, the cognitive apprenticeship approach has the potential to promote the transfer of the organic chemistry knowledge to the real-life situations. This potential can primarily be attributed to its focus on knowledge acquisition through presentation of the ways of its implementation in real-life. The traditional approach, on the other hand, primarily focuses on the acquisition of pure academic content. For example, the students in the experimental group learned when and how various carboxylic acids can be applied in real-life, even before they learned their structural chemical formulas. The students in the control group were presented with a list of structural chemical formulas of various carboxylic acids, and only after the presentation of the entire academic content concerning these substances was completed the teacher enlisted where and how some of them can be used in real-life. When the students in the experimental group learned about the physical and chemical properties of carboxylic acids and their derivatives they did so through the elaboration of how these properties affect their application in various real-life situations. For example, esters have a pleasant smell, they evaporate easily and are insoluble in water, which is why they are components of perfumes, or acyl halogenides react with water present on the wet surfaces of the human eye and cause severe irritation, which is why they are categorized as lachrymatory substances. In contrast to this approach, the students in the control group were explained the physical properties of carboxylic acids and their derivatives (boiling point, solubility in water, and smell), and the chemical reactions that they take part in.
The chi-square test of independence values presented in Table 5 further indicate that the students in the experimental group had a statistically significant higher number of correct answers on all items on the post-test that checked their understanding of the acquired knowledge, with the exception of I5. Namely, I1a and I6 checked the students' understanding of the acidity of carboxylic acids. Within I6 students were asked to select an appropriate value of the pKa2 of oxalic acid, and based on their knowledge concerning the factors that influence the acidity of carboxylic acids, provide an explanation for their choice. Students in the experimental group significantly outperformed the students in the control group on this item. At the same time, answers that were coded as incorrect did not differ greatly between the two groups, as in most of these cases students made no attempt to solve the item. Within the framework of I1a students had to determine which of the three substances (vinegar, aceton and alcohol for medicinal purposes) can be used for removal of limescale and on the basis of their knowledge concerning the acidity strength of these substances provide an explanation for their choice. Students in the experimental group significantly outperformed the students in the control group on these items, whilst the answers that were coded as incorrect also differed between the two groups. Of the 37 students in the experimental group whose answers were coded as incorrect, about a half selected vinegar as an appropriate substance for removal of limescale but offered no explanation for such a choice, whilst the rest of them made no attempt to solve this item. On the other hand, about 80% of the students in the control group whose answer was coded as incorrect made no attempt to solve this item, but the rest of them selected alcohol stating that as a relatively polar substance it can be used to dissolve limescale.
I3 and I4 checked students understanding of the knowledge concerning the physical properties of carboxylic acids and their derivatives. Within the framework of I3 students had to explain how urea (both groups were introduced to the structure as well as IUPAC and common name of this substance during the elaboration of the teaching topic Carboxylic acid and their derivatives) can help to retain the water on the surface of human skin. Within the framework of I4 the students had to determine which of the three given substances (methyl ethanoate, propanamide, or ethanoyl chloride) has the highest boiling point and explain why it is so. Students in the experimental group significantly outperformed the students in the control group on both items, but answers that were coded as incorrect also differed between the two groups. In majority of these cases in the experimental group students made no attempt to solve I3, whilst on I4 most of them selected propanamide but provided no explanation for their choice. On the other hand, nearly a half of these students in the the control group stated that urea reacts with water, and presented a chemical equation of the reaction of hydrolysis in which one or both –NH2 groups of urea were replaced by the –OH group. When it comes to I4, about one third of the students in the control group whose answers were coded as incorrect made no attempt to solve the item, the second third selected propanamide but provided no explanation for such a choice, whilst the rest of them selected ethanoil chloride and stated that it has the highest boiling point because it is the most reactive.
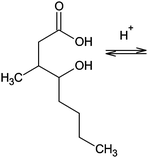
Students in the experimental group also significantly outperformed the students in the control group on all three items (I7, I8 and I9) that required a deep understanding of the chemical properties of carboxylic acid derivatives while the answers that were coded as incorrect differed between the two groups on I7a and I8. Within the framework of I7 students had to select an appropriate substance for the synthesis of aspirin in the given circumstances. When it comes to I7b, most of the students in both groups whose answers were coded as incorrect selected ethanoyl chloride as a substance of choice for the synthesis of aspirin in the presence of a powerful ventilation system, but failed to provide an explanation for such a choice. When it comes to I7a, most of the students in the control group whose answers were coded as incorrect made no attempt to solve this item. The same can be said for about 55% of such students in the experimental group, whilst the rest of them selected ethanamide but provided no explanation for their choice. Within the framework of I8, by applying the knowledge of the mechanism of the esterification reaction students had to derive the formula of whiskey lactone. Whilst the students in the experimental group whose answers were coded as incorrect either made no attempt to solve this item or presented a lactone ring that had four or six angles instead of five, nearly 45% of these students in the control group stated that this is actually a dehydration reaction as its end product presented a carboxylic acid with a double bond between C3 and C4 atoms. I9 basically checked students' understanding of the factors that influence the equilibrium of the reaction of esterification. In this instance, both the students in the control and the experimental group whose answers were coded as incorrect either made no attempt to try to solve this item or selected one of the two given options but provided no explanation for their choice.
On the basis of these results, we can say that the cognitive apprenticeship approach has the potential to promote students' better understanding of the organic chemistry content. This can be attributed to several factors. Within the framework of the cognitive apprenticeship approach the students were given the opportunity to acquire new knowledge through examples of its application in solving real-life problems and were constantly encouraged to link the new knowledge with the knowledge that they already possessed, all of which promotes conceptual understanding. Additionally, when faced with various types of academic content, various teaching methods and principles of the cognitive apprenticeship approach can be applied and combined in order to ensure that all types of content are presented to the students in the most appropriate way. On the other hand, the traditional teaching approach makes little reference to the use of academic content in real-life and most of it is presented to the students in the same way.
Within the framework of the first four segments of item two, the students were expected to write the structural chemical formulas of the given carboxylic acids, whilst within the framework of the second half of item one the students were required to write the equation of the chemical reaction between acetic acid and sodium bicarbonate. Concerning these requirements, the difference in the number of correct answers of the students from the two groups is not statistically significant. Therefore, the cognitive apprenticeship approach did not prove to have more potential than the traditional approach when it comes to promoting students writing the chemical formulas of organic compounds or writing equations of organic chemistry reactions. Compared to the requirements for understanding and application of the acquired knowledge in real-life situations of most of the other items of the post-test, these requirements were relatively simple. Therefore, the reason for the lack of a statistically significant difference in the effectiveness of this approach compared to the traditional approach probably does not lie in the complexity of the requirements of these items but, once again, in the very nature of the two approaches. As previously stated, the traditional teaching approach encourages rote learning of the chemical formulas of as many carboxylic acids as possible. On the other hand, teaching based on the cognitive apprenticeship model encourages the students to perceive the characteristics of the structure of a given carboxylic acid (for example, how many carboxylic groups there are, whether there is a nucleophilic core in the vicinity of a carboxyl group, etc.) and to understand how the structure influences their physical and chemical properties and their application in real life. In accordance with this, most of the students in the experimental group who tried to write the structural formula of lactic acid were correct about the existence of a hydroxyl group on the α carbon atom, but replaced the methyl group with a hydrogen atom or with some other alkyl group. In the case of citric acid, several students in the experimental group noted only verbally that this carboxylic acid contains three carboxyl groups. When it comes to chemical reactions, the cognitive apprenticeship approach insists on understanding their mechanisms and their application in real life, whilst the traditional model insists on their correct presentation in the form of chemical equations. Therefore, even though a large number of the students from the experimental group wrote the correct chemical formulas of the reactants and the products in the chemical equation representing the reaction between acetic acid and sodium bicarbonate in item I1b, some of them forgot to write the corresponding coefficients in the equation. Consequently, their answers were coded as incorrect and on account of this the number of correct answers in the two groups does not differ in a statistically significant degree for this item.
Within the framework of I5, the students were expected to propose a way of distinguishing which of the given substances is hexanoic acid and which is ethyl butanoate. This is a relatively complex item which requires a thorough understanding and the application of knowledge concerning the physical and the chemical properties of carboxylic acids and their derivatives, and the cognitive apprenticeship approach had proved to have the potential to be effective for these purposes. However, the difference in the number of correct answers of the students from the two groups is not statistically significant, so the cognitive apprenticeship approach did not prove to be more effective than the traditional approach when it came to the requirements of this particular item. A lack of any statistically significant difference in achievement concerning I5 could be linked with the results of I11 from the pre-test, where, before the intervention, a statistically significant difference was established in favour of the control group. When solving I5 in the post-test, the students in the experimental group offered a number of different solutions (distinguishing the given substances on the basis of smell, solubility in water, the boiling point, reaction with sodium bicarbonate), which indicates that the cognitive apprenticeship model has the potential to enable students to approach solving any given problem from a number of diverse angles. Meanwhile, almost all the students from the control group proposed only distinguishing on the basis of smell. This, however, is a characteristic of which they already demonstrated a statistically significant better knowledge in I11 in the pre-test. It is possible that this initial advantage that the students in the control group had, managed to neutralize the advantage that the cognitive apprenticeship approach provided for the students in the experimental group when it comes to understanding and the functionalization of the newly acquired knowledge about carboxylic acids and their derivatives, so that in the end the achievement of the students from the two groups on I5 in the post-test turned out to be essentially equal.
Finally, as can be seen in Tables 2 and 4, the overall percentage of correct answers in the experimental group went from a 51% on the pre-test to a 54% on the post-test while the control group actually declined from a 51% on the pre-test to a 38% on the post-test. As previously explained, the pre-test and the post-test were two different tests and we believe that one of the causes of such results could lay in the way in which the items on the post-test were conceived. The items on the pre-test, as previously discussed, resembled the items in the chemistry textbook, i.e. they were conceived as straightforward academic questions or requests, and the students from both groups were well used to dealing with them. On the other hand, in order to obtain answers to our research questions, the majority of the items in the post-test were conceived so as to require the application of the acquired knowledge in solving real-life problems. This was the first time that the students from both groups encountered test items conceived in such a way. It is possible that being faced with an unfamiliar form of the test items in the post-test for the first time made it more difficult for students in both groups to demonstrate efficient and consistent use of the newly acquired knowledge and skills.
Conclusions
The pedagogical experiment presented in this paper was conducted in order to determine the effectiveness of the cognitive apprenticeship model in organic chemistry teaching in secondary schools, to what extent this approach contributes to a better understanding of the organic chemistry content and to the students being better equipped to apply knowledge acquired in this way in real-life, compared to the traditional approach. The experiment was conducted within the framework of dealing with the teaching topic Carboxylic acids and their derivatives. On the basis of the results obtained and concerning the two research hypotheses that we posed, the following conclusions can be made:• Concerning the first research hypothesis we can conclude that the cognitive apprenticeship approach has the potential to contribute to a better students' understanding of the organic chemistry concepts. This corresponds to the results of the study conducted by Roth and Bowen (1995), which indicated that the cognitive apprenticeship model contributes to a better understanding of scientific concepts in comparison with the traditional teaching. It is also consistent with the related literature indicating that learning based on problem solving in the framework of authentic learning situations promotes deeper understanding of the acquired knowledge (Winther and Volk, 1994; Newmann et al., 1995, Demircioğlu et al., 2009; Schwartz-Bloom et al., 2011; Godin et al., 2014). Within the framework of the intervention presented in this paper, every learning situation was developed taking into account all four dimensions (content, teaching methods, sequencing, and sociology) of the cognitive apprenticeship model. Various teaching methods and principles of approach were combined in order to ensure that all types of content were presented to the students in the most appropriate way. Students were given the opportunity to acquire new knowledge through examples of its application in real-life contexts and were constantly encouraged to link this new knowledge with the knowledge that they already possessed. The post-test results indicated that this approach can contribute to the students' better understanding of the elaborated concepts. However, the overall improvement of the achievement of the students in the experimental group after the applied intervention was very slight and therefore the first research hypothesis cannot be fully confirmed.
• Concerning the second research hypothesis we can conclude that the cognitive apprenticeship approach has the potential to improve the secondary school students' ability to apply the knowledge about organic chemistry concepts on the examples from real-life. This finding is inconsistent with the results of the only other study (Hendricks, 2001) that checked the effectiveness of the cognitive apprenticeship approach when it comes to promoting the transfer of acquired knowledge to real-life, but supports the claim of Dennen and Bruner (2008) that learning within the context of application contributes to improved practice. The potential of the cognitive apprenticeship approach when it comes to promoting knowledge transfer to authentic situations primarily lies in its focus on knowledge acquisition through presentation of the ways of its implementation in real-life, which stands in marked contrast to the traditional approach's focus on acquisition of pure academic knowledge. However, the overall improvement of the achievement of the students in the experimental group after the applied intervention was very slight and therefore the second research hypothesis cannot be fully confirmed.
The results of our study further indicate that whilst the cognitive apprenticeship approach has the potential to be more effective than the traditional approach when it comes to relatively complex requirements for deep understanding and transfer of acquired organic chemistry knowledge to real-life, the same cannot be said for the requirements such as writing structural chemical formulas and the equations of chemical reactions of organic compounds. The reason for this could be found in the nature of the two approaches. When it comes to the structural chemical formulas of organic compounds, the traditional approach presupposes memorising as many of them as possible, whilst teaching based on the cognitive apprenticeship model encourages the students to perceive the characteristics of the structure of a given substance and to understand how the structure influences its physical and chemical properties and corresponding application in real-life. When it comes to chemical reactions, the cognitive apprenticeship approach is focused on understanding the reactions' mechanisms and corresponding application in real-life, whilst the traditional model is focused on the correct presentation of chemical equations. Therefore, in the future it would be of interest to consider the ways in which the application of the cognitive apprenticeship approach could be modified in order to make it more effective with regard to these requirements.
Nevertheless, the results of our study indicate that the intervention based on the cognitive apprenticeship model that we applied has a potential to contribute to the improvement of the teaching about carboxylic acids and their derivatives in secondary schools. Specifically, the results of our study indicate that teaching based on the global to local skill principle within the framework of which the teacher first elaborated the general mechanism of the nucleophilic acyl substitution reaction and then encouraged the students to apply this knowledge to the concrete examples of the reaction of esterification or the reactions of carboxylic acid derivatives with various nucleophiles facilitated learning about the chemical properties of these substances. The teaching based on the increasing difficulty principle that encompassed the application of the modelling teaching method and the Itakamura method of articulation facilitated the acquisition of knowledge concerning the acidity of carboxylic acids. Furthermore, the teaching based on the global to local skill principle that encouraged the students to derive most of the physical properties of carboxylic acids and their derivatives on their own, on the basis of the previously observed relationship between the structure and physical properties of already elaborated-upon classes of organic compounds, facilitated the acquisition of knowledge concerning these properties. Acquisition of all knowledge was further facilitated by the general organization of the lessons that featured cooperative problem solving in small groups with the teacher's constant support in the form of coaching and scaffolding, and a presentation of knowledge in real-life contexts that are relevant to the students, which in turn promoted their intrinsic motivation.
To end with, it is important to note that, in order to successfully implement the cognitive apprenticeship approach in the classroom, the teacher must possess both a deep understanding of the model's principles and methods and the ability to combine them in different ways in order to provide effective presentation of the various types of academic content. Developing these skills requires a lot of time and work which is why the teachers who took part in our study, already heavily burdened by the substantial workload of their regular lessons, showed very little enthusiasm when they were asked to implement it in their teaching. In order to make the effort that is necessary for overcoming the obstacles that stand in the way of introducing a major change in their teaching practice, the teachers must be fully aware of the potential benefits of that change (Greenberg and Baron, 2000). But, it turned out that although all of the teachers who participated in our study had more than two decades of teaching experience, they heard of the cognitive apprenticeship approach for the first time. Therefore, we find it important that in the future teachers get the chance to learn about the cognitive apprenticeship approach and its benefits and develop skills necessary for its implementation in the classroom as a part of their initial teacher training.
Limitations
It is important to note that despite the measures taken to reduce Hawthorne effect, its impact caused by both the intervention and the fact that the students in the experimental group worked with one of the research studies instead of their regular teacher, who taught only the students in the control group, may still be responsible for a part of the gains observed. The potential of the cognitive apprenticeship approach to contribute to better understanding and knowledge transfer to real-life situations was established within the framework of dealing with the teaching topic Carboxylic acids and their derivatives. Further studies confirming our findings in situations where this approach is applied in teaching various other organic chemistry topics are necessary in order to make these findings more readily generalizable to the entire field of organic chemistry teaching in secondary schools.Appendix 1
The pre-test| Item 7 | Item 8 |
|---|---|
| Answer the following questions: | Answer the following questions: |
| (a) How are acids defined according to the proteolytic theory? | (a) The pKa value of HA acid is 6, and the pKa value of HX acid is 8. Which of the two acids is stronger? |
| (b) Write the equation of the chemical reaction of the dissociation of the hypothetical acid HA in a water solution: | (b) If the pKa value of HA acid is 6, and the pKa value of HX acid is 8, does the following chemical reaction occur? |
| (c) Write the expression for the equilibrium constant of the preceding reaction: | HX + NaA → NaX + HA |
| (d) What is the mathematical ratio of the equilibrium constant of the reaction of the dissociation of HA acid and the pKa value of this acid? | Provide a justification for your answer. |
| Item 11 |
|---|
| Encircle the letter in front of the statement that is correct: |
| (a) Esters are exceptionally unstable compounds, which is why they do not exist independently in nature. |
| (b) The smell of fruit and vegetables originates from compounds that belong to the class of esters. |
| (c) Esters are compounds that are not found as components of fats. |
Appendix 2
The post-test| Item 1 | Item 2 |
|---|---|
|
(a) You can often hear complaints that, due to cooking in hard water, layers of limescale accumulate in kitchen dishes over time. Encircle the number in front of a substance that you would recommend for removing limescale from dishes.
(1) acetone (2) vinegar (3) alcohol for medicinal purposes Provide a justification for your answer. (b) Write the equation of the chemical reaction that occurs during the removal of limescale. |
(a) Write the structural formula of lactic, citric, tartaric and salicylic acid. Salicylic acid is the trivial name of o-hydroxybenzoic acid, derived from the Latin term for the plant from which this acid was derived for the first time (Lat. Salyx = willow).
(b) All the carboxylic acids whose formulas you wrote are components of hair shampoos and substances used for washing and cleaning the skin. However, these substances are not suited to all types of skin. In people with oily skin, a relatively thick layer of oily sebum covers the skin surface, blocking the pores, through which the skin breathes and receives moisture, thus preventing the removal of dead cells from the skin surface. Through the accumulation of dead cells in the pores of facial skin, blackheads are created, whereas the dead cells of the skin of the head, accumulated and glued together, thus trapped in an oily layer on the skin surface, constitute dandruff. If you know that β-hydroxy carboxylic acids are liposoluble, and α-hydroxy carboxylic acids are hydrosoluble, which of the substances mentioned above would you recommend to Marija, who has oily skin, and which ones to Milica, who has a normal skin type? Write the number of the substance next to the name of the person to whom you recommend it. (1) shampoo X with a willow extract, (2) shampoo Y with a lemon extract, (3) a substance for washing facial skin containing tartaric acid, (4) yoghurt as a substance for washing facial skin. Marija: Milica: |
| Item 3 | Item 4 |
|---|---|
|
Cosmetic substances that contain urea are used for hydrating the skin. Thus substances with an exceptionally high content of urea (40%) can moisturise even thoroughly hardened skin, like the skin of the heels.
Explain how urea contributes to retaining water in the skin. |
Only one of the three substances given below is in a liquid aggregate state at the temperature of 220 °C, while the other two are in a gaseous aggregate state at that temperature. Encircle the letter in front of the name of the substance which is in a liquid aggregate state at the said temperature.
(a) methyl ethanoate (b) propanamide (c) ethanoyl chloride Provide a justification for your answer. |
Appendix 3
An outline of the teaching design in the experimental group (Fig. 3).Appendix 4
The principles for coding of open-ended items on the pre-test and the post-test (Tables 6 and 7).| Item | Item was coded as correct if the student |
|---|---|
| I1b | Correctly wrote the chemical formula of benzoic acid |
| I2 H2O | Wrote the chemical formula of water as the reactant in the given reaction |
| I2 H2SO4 | Wrote the chemical formula of sulfuric acid as a catalyst for the given reaction |
| I2 K2Cr2O7 | Wrote the chemical formula of potassium dichromate as an oxidant in the given reaction |
| I5 | Arranged all three compounds in a sequence based on increasing boiling point and provided a full explanation on why he/she arranged the given compounds in such an order |
| I6a | Arranged all three compounds in a sequence based on increasing solubility in water and provided a full explanation on why he/she arranged the given compounds in such an order |
| I6b | Arranged all three compounds in a sequence based on increasing solubility in water and provided a full explanation on why he/she arranged the given compounds in such an order |
| I7a | Correctly defined acids according to the proteolytic theory |
| I7b | Correctly presented the equation of the chemical reaction of the dissociation of the hypothetical acid HA in a water solution |
| I7c | Correctly presented the expression for the equilibrium constant of the preceding reaction |
| I7d | Correctly presented the mathematical ratio of the equilibrium constant of the reaction of the dissociation of HA acid and the pKa value of this acid: pKa = −log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka Ka |
| I8a | Correctly determined which of the two given acids is stronger |
| I8b | Concluded that the given reaction is not possible and based on the pKa values of the given acids provided a full explanation on why such a conclusion was made |
| I9a | Provided a full definition of the term nucleophile |
| I9b | Provided a full definition of the term electrophile |
| I10a | Correctly marked the partial electric charge of the carbon atom in the carboxyl group in the general formula of the carboxylic acids |
| Item | Item was coded as correct if the student |
|---|---|
| I1a | Encircled the number in front of vinegar as a substance that can remove layers of limescale and based on the knowledge concerning the acidity strength of acetic acid, ethanol, acetone and carbonic acid provided a full explanation for such a choice |
| I2 la | Correctly wrote the chemical formula of lactic acid |
| I2 ca | Correctly wrote the chemical formula of citric acid |
| I2 ta | Correctly wrote the chemical formula of tartaric acid |
| I2 sa | Correctly wrote the chemical formula of salicylic acid |
| I2 Marija | In this instance the student had to correctly select all appropriate shampoos for both of the given persons in order to code his answer as correct for each of the persons |
| I2 Milica | |
| I3 | Explained that urea contributes to retaining water in the skin by establishing hydrogen bonds with water molecules |
| I4 | Encircled the letter in front of propanamide and based on the knowledge concerning the physical properties of carboxylic acid derivatives provided a full explanation for such a choice |
| I5 | Based on the knowledge concerning physical and chemical properties of carboxylic acids and esters presented an appropriate procedure for discerning between the two given substances |
| I6 | Encircled the letter (a) that stands in front of the correct numerical value of the pKa2 of oxalic acid and based on the knowledge concerning the factors that influence the acidity of carboxylic acids provided a full explanation for such a choice |
| I7a | Wrote the number in front of the name of the correct substance which should be used as substance X for the synthesis of aspirin under the given conditions, and based on these conditions and the knowledge concerning the differences in the reactivity of various classes of carboxylic acid derivatives provided a full explanation for such a choice |
| I7b | |
| I8a | Recognized that the given reaction is the esterification reaction and correctly wrote the chemical formula of its product-whiskey lactone |
| I8b | Concluded that lactones are esters |
| I9 | Encircled the letter in front of the reaction system B, and based on the knowledge concerning the Le Chatelier's principle provided a full explanation for such a choice |
Acknowledgements
This paper is the result of working on the project “The Theory and Practice of Science in Society: Multidisciplinary, Educational and Intergenerational Perspectives”, no. 179048, the realisation of which is financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia.References
- Aikenhead G. S., (2006), Science education for everyday life – evidence-based practice, New York: Teacher College Press.
- Akkuzu N. and Uyulgan M. A., (2015), An epistemological inquiry into organic chemistry education: exploration of undergraduate students' conceptual understanding of functional groups, Chem. Educ. Res. Pract., DOI: 10.1039/c5rp00128e.
- American Association for the Advancement of Science (AAAS), (1993), Benchmarks for science literacy, New York: Longman.
- Anderson J. P., (2009), Learning the language of organic chemistry: how do students develop reaction mechanism problem solving skills? Doctoral dissertation, Purdue University, West Lafayette, Indiana.
- Anzovino M. E. and Lowery Bretz S., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Bailey Jr. P. S. and Bailey C. A., (1971), A Program for Relevant Organic Chemistry in High School, J. Chem. Educ., 48(4), 263–264.
- Beasly W., (1980), High School Organic Chemistry Studies: Problems and Prospects, J. Chem. Educ., 57(11), 807–809.
- Bojczuk M., (1982), Topic difficulties in O and A level chemistry, Sch. Sci. Rev., 63(224), 545–551.
- Bretz S. L., (2001), Novak's theory of education: human constructivism and meaningful learning, J. Chem. Educ., 78(8), 1107.
- Brown J. S., Collins A. and Duguid P., (1989), Situated cognition and the culture of learning, Educ. Res., 18(1), 32–42.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12, 29–39.
- Cave A., (2010), Learning Math in Second Grade: An Application of Cognitive Apprenticeship, National Forum of Applied Educational Research Journal, 23(3), 1–16.
- Charney J., Hmelo-Silver C. E., Sofer W., Neigeborn L., Coletta S. and Nemeroff M., (2007), Cognitive Apprenticeship in Science through Immersion in Laboratory Practices, Int. J. Sci. Educ., 29(2), 195–213.
- Childs P. E. and Sheehan M., (2009), What's difficult about chemistry? An Irish perspective, Chem. Educ. Res. Pract., 10, 204–218.
- Cohen L., Manion L. and Morrison K., (2007), Research Methods in Education, 6th edn, New York: Routledge.
- Collins A., (2006), Cognitive Apprenticeship, in Sawyer R. K. (ed.) The Cambridge Book of the Learning Science, New York: Cambridge University Press, pp. 47–60.
- Collins A., Brown J. S. and Newman S. E., (1989), Cognitive apprenticeship: teaching the crafts of reading, writing, and mathematics, in Resnick L. B. (ed.), Knowing, Learning and Instruction: Essays in Honor of Robert Glaser, Hillsdale, NJ: Lawrence Erlbaum Associates, pp. 453–494.
- Cook D., (1967), The impact of the Hawthorne effect in experimental designs in educational research, Report No. 0726, Washington, DC: U.S Office of Education.
- Cruz-Ramirez de Arellano D. and Towns M. H., (2014), Students' understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- Davis D. D., (1999), The research apprenticeship program: Promoting careers in biomedical sciences and the health professions for minority populations, paper presented at the Annual Meeting of the American Educational Research Association, Montreal, Ontario, April 19–23.
- Davis E. A., (2003), Prompting middle school science students for productive reflection: generic and directed prompts, J. Learn. Sci., 12(1), 91–142.
- Davis, E. A. and Linn M. C., (2000), Scaffolding students' knowledge integration: prompts for reflection in KIE, Int. J. Sci. Educ., 22(8), 819–837.
- Demircioğlu H., Demircioğlu G. and Çalik, M., (2009), Investigating the effectiveness of storylines embedded within a context-based approach: the case for the Periodic Table, Chem. Educ. Res. Pract., 10, 241–249.
- Dennen V. P. and Bruner, K. J. (2008). The cognitive apprenticeship model in educational practice, in Spector J. M., Merrill M. D., van Merriënboer J. J. G. and Driscoll M. P. (ed.), Handbook of research on educational communications and technology, 3rd edn, Mahwah, NJ: Erlbaum, pp. 425–439.
- Duffy A. M., (2006), Students' ways of understanding aromaticity and electrophilic aromatic substitution reactions, Doctoral dissertation, Mathematics and Science Education, University of California, San Diego.
- Ebenezer J. V. and Zoller U., (1993), Grade 10 Students' Perceptions of and Attitudes toward Science Teaching and School Science, J. Res. Sci. Teach., 30(2), 175–186.
- EURYDICE, (2011), Science education in Europe: National policies, practices and research, Brussels: EURYDICE.
- Furio-Mas C., Calatayud M.-L. and Barcenas S. L., (2007), Surveying Students' Conceptual and Procedural Knowledge of Acid–Base Behavior of Substances, J. Chem. Educ., 84(10), 1717–1724.
- Godin E. A., Kwiek N., Sikes S. S., Halpin M. J., Weinbaum C. A., Burgette L. F., Reiter J. P. and Schwartz-Bloom, R. D., (2014), Alcohol Pharmacology Education Partnership: Using Chemistry and Biology Concepts To Educate High School Students about Alcohol, J. Chem. Educ., 91, 165–172.
- Greenberg J. and Baron R. A., (2000), Behavior in organizations, 7th edn, Upper Saddle River, NJ: Prentice Hall.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13(3), 201.
- Grove N. P., Cooper M. M. and Cox E. L., (2012), Does Mechanistic Thinking Improve Student Success in Organic Chemistry?, J. Chem. Educ., 89(7), 850–853.
- Hargreaves A., (2005), Educational change takes ages: life, career and generational factors in teachers' emotional responses to educational change, Teach. Teach. Educ., 21, 967–983.
- Hendricks C. C., (2001), Teaching Causal Reasoning Through Cognitive Apprenticeship: What Are Results From Situated Learning, J. Educ. Res., 94(5), 302–311.
- Hwang G. J., Yang T. C., Tsai C. C. and Yang Stephen, J. H., (2009), A context-aware ubiquitous learning environment for conducting complex science experiments, Comput. Educ., 53(2), 402–413.
- Jimoh A. J., (2005), Perception of difficult topics in chemistry curriculum by students in Nigeria secondary schools, Ilorin J. Educ., 24, 71–78.
- Kolikant Y. B. D., Gatchell D. W., Hirsch P. L. and Linsenmeier R. A., (2006), A Cognitive-Apprenticeship-Inspired Instructional Approach for Teaching Scientific Reading and Writing, J. Coll. Sci. Teach., November/December, 20–25.
- Lewis S. E. and Lewis J. E., (2005), The Same or Not the Same: Equivalence as an Issue in Educational Research, J. Chem. Educ., 82(9), 1408–1412.
- Linnenbrink-Garcia L., Pattal, E. A. and Messersmith, E. E., (2013), Antecedents and consequences of situational interest, Brit. J. Educ. Psychol., 83(4), 591–614.
- Mabrouk P. A., (2007), Introducing Summer High School Student–Researchers to Ethics in Scientific Research, J. Chem. Educ., 84(6), 952–954.
- Martella R. C., Nelson J. R., Morgan R. L. and Marchand-Martella N. E., (2013), Understanding and Interpreting Educational Research, New York: The Guilford Press.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual students in general chemistry, J. Chem. Educ., 70(1), 52–55.
- Newmann F. M., Marks H. M. and Gamoran A., (1995), Authentic pedagogy: standards that boost student performance, Issues in Restructuring Schools, 8, 1–4.
- Novak J. D., (2002), Meaningful learning: the essential factor for conceptual change in limited or inappropriate propositional hierarchies leading to empowerment of learners, Sci. Educ., 86(4), 548–571.
- Nunnally J. C., (1978), Psychometric theory, 2nd edn, New York: McGraw-Hill.
- O'Dwyer A. and Childs P. E., (2011), Second-level Irish pupils' and teachers' view of difficulties in organic chemistry, http://lsg.ucy.ac.cy/esera/e_book/base/ebook/strand10/ebookesera2011_ODWYER-10.pdf.
- O'Dwyer A. and Childs P., (2014), Organic Chemistry an Action! Developing an intervention program for Introductory Organic Chemistry to improve learner's Understanding, Interest and Attitudes, J. Chem. Educ., 91, 987–993.
- OECD, (2009), PISA 2009 assessment framework—key competencies in reading, mathematics and science, Paris: OECD Publishing.
- Osborne J., Simon S. and Collins S., (2003), Attitudes towards science: a review of the literature and its implications, Int. J. Sci. Educ., 25(9), 1049–1079.
- Özmen H., (2008), Determination of students' alternative conceptions about chemical equilibrium: a review of research and the case of Turkey, Chem. Educ. Res. Pract., 9, 225–233.
- Peterson R., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26, 301–314.
- Ratcliffe M., (2002), What's difficult about A-level Chemistry? Educ. Chem., 39(3), 76–80.
- Roth W. M. and Bowen G. M., (1995), Knowing and interacting: a study of culture, practices, and resources in a grade 8 open-inquiry science classroom guided by a cognitive apprenticeship metaphor, Cognition Instruct., 13, 73–128.
- Sanger M. J., (2005), Evaluating students' conceptual understanding of balanced equations and stoichiometric ratios using a particulate drawing, J. Chem. Educ., 82(1), 131–134.
- Schoenfeld A. H., (1985), Mathematical problem solving, New York, NY: Academic Press.
- Schwartz-Bloom R. D., Halpin M. J. and Reiter J. P., (2011), Teaching High School Chemistry in the Context of Pharmacology Helps Both Teachers and Students Learn, J. Chem. Educ., 88, 744–750.
- Shadish W. R., Cook T. D. and Campbell D. T., (2002), Experimental and quasi-experimental designs for generalized causal inference, Boston: Houghton Mifflin.
- Stockhausen L. J., and Zimitat C. (2002), New learning: Re-apprenticing the learner, Educational Media International, 39(3/4), 331–338.
- Taber, K. S. (2014). Methodological issues in science education research: a perspective from the philosophy of science, in Matthews M. R. (ed.), International Handbook of Research in History, Philosophy and Science Teaching, Netherlands: Springer, vol. 3, pp. 1839–1893.
- Vaino K., Holbrook J. and Rannikmae M., (2012), Stimulating students' intrinsic motivation for learning chemistry through the use of context-based learning module, Chem. Educ. Res. Pract., 13, 410–419.
- Van Berkel B., Pilot A. and Bulte A., (2009), Micro-macro thinking in chemical education: why and how to escape, in Gilbert J. and Treagust D. (ed.), Multiple Representations in Chemical Education, Springer Science + Business Media B.V., vol. 4, pp. 31–54.
- Winther A. A. and Volk T. L., (1994), Comparing achievement of inner-city high school students in traditional versus STS-based chemistry courses, J. Chem. Educ., 71, 501–505.
- Zhou Q., Wang T. and Zheng Q., (2015), Probing high school students' cognitive structures and key areas of learning difficulties on ethanoic acid using the flow map method, Chem. Educ. Res. Pract., 2015, 16, 589–602.
| This journal is © The Royal Society of Chemistry 2016 |