Faculty beliefs about the purposes for teaching undergraduate physical chemistry courses
Michael R.
Mack
and
Marcy H.
Towns
*
Department of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: mack3@purdue.edu; mtowns@purdue.edu
First published on 12th October 2015
Abstract
We report the results of a phenomenographic analysis of faculty beliefs about the purposes for teaching upper-division physical chemistry courses in the undergraduate curriculum. A purposeful sampling strategy was used to recruit a diverse group of faculty for interviews. Collectively, the participating faculty regularly teach or have taught physical chemistry courses in 16 different chemistry departments in the United States. While faculty agreed that the goal of teaching physical chemistry was to help students develop robust conceptual knowledge of the subject matter within thermodynamics, statistical mechanics, quantum mechanics, spectroscopy, chemical kinetics, and other major topics, some articulated strong beliefs about epistemic and social learning goals. An understanding of the relations between different ways of thinking about teaching upper-division physical chemistry courses offers practitioners with alternative perspectives that may help them expand their awareness of the purposes for teaching physical chemistry in the undergraduate curriculum. Furthermore, knowledge of faculty beliefs about their teaching provides educational researchers and curriculum developers with an understanding about the potential opportunities or barriers for helping faculty align their beliefs and goals for teaching with research-based instructional strategies. We discuss our findings with the intention to expand faculty awareness of the discourse on physical chemistry education to include various perspectives of the purpose for teaching upper-division physical chemistry courses.
Introduction
The increased scrutiny of undergraduate science, technology, engineering, and mathematics (STEM) education in recent years by high profile reports (President's Council of Advisors on Science and Technology, 2012), national associations (Bransford et al., 2000; Committee on Prospering in the Global Economy of the 21st Century: An Agenda for American Science and Technology, 2007; Association of American Universities, 2011; National Research Council, 2012a, 2012b), educational policy and research organizations (Boyer Commission on Educating Undergraduates in the Research University, 1998), and researchers of higher education, faculty development, and discipline-based education (Seymour and Hewitt, 1997; Goodyear and Hativa, 2002; Fairweather, 2008; Austin, 2011; Henderson et al., 2011, 2012) has urgent implications for the teaching responsibilities of individual faculty. These developments have argued that faculty need to become more responsible for being aware and knowledgeable of theories of learning, knowledge of student learning experiences, and research-based instructional strategies (Boyer Commission on Educating Undergraduates in the Research University, 1998; Goodyear and Hativa, 2002; Fairweather, 2008; Austin, 2011; Henderson et al., 2012; President's Council of Advisors on Science and Technology, 2012). The efforts made by educational researchers and curriculum developers when helping faculty expand their awareness of the research on teaching and learning in higher education must carefully coordinate both the values and norms related to discipline-specific subject matter and practices as well as the situational characteristics that influence faculty thought and action in relation to their teaching responsibilities (Gess-Newsome et al., 2003; Henderson and Dancy, 2007, 2011; National Research Council, 2012).One avenue of educational research that supports the goal of improving teaching in discipline-based educational settings is research on what faculty think about teaching in general and about their own teaching in particular (National Research Council, 2012). A guiding assumption of this research program is that faculty adoption and persistence with research-based instructional strategies will help improve the quality of teaching and learning in undergraduate STEM education. Research on faculty thinking about teaching in disciplinary settings support discipline-based education researchers in understanding the factors, barriers, and potential opportunities that exist for helping faculty adopt research-based instructional strategies (Henderson and Dancy, 2007, 2009; Henderson et al., 2012).
This study investigated faculty thinking about teaching in the context of upper-division physical chemistry courses in order to build an understanding of the beliefs faculty reflect on as being important aspects of their experience. For the purposes of this study, we generally defined beliefs as “psychologically held understandings, premises, or propositions about the world that are felt to be true,” (Richardson, 1996) which are “accepted as guides for assessing the future, are cited in support of decisions, or are referenced to in the passing of judgement on the behavior of others” (Goodenough, 1963, p. 151, as cited in Richardson, 1996). In contrast with knowledge, beliefs do not require a truth condition that gives a claim validity among members of a community (Green, 1971). When faculty members think about their teaching they may draw upon their beliefs about higher education, teaching, and learning (Entwistle and Walker, 2002), which have been shaped by their previous education and training (Austin, 2011) and the normative practices of the culture in which they work (Tobias, 1990; Seymour and Hewitt, 1997; Austin, 2011). It is likely that varied experiences lead to differences in the beliefs faculty construct about teaching physical chemistry at the undergraduate level. Precise knowledge of what those differences are may guide the development of instructional and curricular resources and faculty professional development opportunities that are specific to the interests of physical chemistry educators.
The guiding research question that will be addressed in this paper is: What are the similarities and differences in faculty beliefs about the purposes for teaching physical chemistry? The purpose of this study was to develop a rich description of the beliefs that faculty described as relevant and important when talking about teaching physical chemistry at the upper-division level. Research of this nature begins with the assumptions that faculty thinking about their teaching governs their teaching behavior (Shavelson and Stern, 1981; Shulman, 1986; Goodyear and Hativa, 2002; Dancy and Henderson, 2007). However, it was beyond the scope of this study to investigate the correspondence of beliefs and actual classroom practice. We believe a rich description of the former provides descriptive knowledge that supports further research on faculty beliefs about teaching and its correspondence with actual classroom practice. Research on faculty beliefs about teaching should be judiciously re-examined in light of new research on the actual classroom practices of physical chemistry educators, chemistry educators in general, or discipline-based STEM educators overall.
The choice to study physical chemistry education at the undergraduate level was purposeful. It is the authors' understanding of the practitioner literature that faculty – as a group – hold varied philosophies about teaching physical chemistry (e.g., Moore and Schwenz, 1992; Schwenz and Moore, 1993; Zielinski and Schwenz, 2004; Ellison and Schoolcraft, 2008). On the one hand, practitioners of physical chemistry education have called for overhauls of the curriculum in pursuit of a better one (Moore and Schwenz, 1992). The tacit assumption supporting these calls for reform was the belief that students' difficulties could be overcome by finding more effective ways to select, organize, and present the subject matter. On the other hand, practitioners have also argued that faculty ought to seriously consider more student-centered views of teaching and learning (Zielinski and Schwenz, 2004; Moog et al., 2006). Based on this observation, we became interested in learning about the different beliefs guiding faculty in their thinking about teaching.
In this paper we describe selected literature that supported this study, the theoretical lens guiding our understanding about the nature of faculty beliefs, the methodological choices we made throughout the study, and then we present the findings. But first, we briefly describe an initial framework to think about physical chemistry in the context of undergraduate chemistry education programs in the United States.
A framework for physical chemistry education
The American Chemical Society's Committee on Professional Training (ACS CPT) guidelines are an initial framework to situate ideas about teaching physical chemistry in a wider context of chemistry education at the college and university level in the United States (Committee on Professional Training, 2015). The CPT develops and administers guidelines for programs supporting ACS-certified degrees in chemistry. One way the guidelines served as a resource to situate this study was in its articulation of the nature of physical chemistry as a discipline, as described in the following excerpt from the supplementary materials regarding physical chemistry education (Committee on Professional Training, 2008):Physical chemistry provides the fundamental concepts and organizing principles that are applied in all aspects of chemistry and related fields. It develops rigorous and detailed explanations of central, unifying concepts in chemistry and contains mathematical models that provide quantitative predictions. (p. 1)
Physical chemistry as a discipline is described as a body of knowledge that consists of major facts, concepts, and the relationships among them. There are canons of evidence that constitute knowledge as part of physical chemistry, such as developing and using mathematical models. In those two ways physical chemistry is distinguished from other traditional branches of chemistry.
Another way the CPT guidelines served as a resource to situate this study was in its translation of the discipline into part of the undergraduate curriculum:
Physical chemistry should emphasize the connection between microscopic models and macroscopic phenomena. Courses should develop both qualitative and quantitative models of physical properties and chemical change, and students should critically apply them to deepen their understanding of chemical phenomenon. Problem solving is a key activity in learning physical chemistry. (p. 1)
Physical chemistry as a course follows from the structure of the discipline. The CPT promoted the idea that coursework should emphasize the content of the field and the relationships between mathematical, molecular, and macroscopic models of matter. Further reading of the document suggests that problem solving in physical chemistry involves working with mathematical models and connecting them to physical chemistry concepts, evaluating the assumptions, limitations, and the ability of mathematical models to predict observed chemical phenomena at some level of accuracy (Committee on Professional Training, 2008).
The CPT guidelines provided this study with initial ideas about the beliefs that a faculty member may incorporate into their philosophy for teaching physical chemistry at the undergraduate level, regardless of it contributing to a program's ACS accreditation (e.g. a physical chemistry course for STEM non-majors). It is imperative to understand that the CPT guidelines are not a standard to compare and contrast individual faculty beliefs with, but rather they will help situate the contents of faculty beliefs that emerged during this study in the wider context of undergraduate chemistry education and science education in the United States.
Literature review
Research on teacher thinking
For over four decades education researchers have focused a great deal of attention on teacher thinking in order to construct an understanding of how teaching occurs for use by educational theorists, researchers, policy-makers, curriculum designers, teacher educators, administrators, and teachers themselves (Clark and Peterson, 1986; Clark and Yinger, 1987; Calderhead, 1996; Goodyear and Hativa, 2002). The guiding assumption of this research program is “teachers' thoughts, judgments and decisions guide their teaching behavior” (Shavelson and Stern, 1981, p. 470). Therefore, researchers who study teacher thinking are interested in questions such as: What is it that teachers know about teaching? How is that knowledge organized? And how does it inform their actions?Decades of phenomenographic research has contributed descriptive accounts of teacher thinking in higher education. The goal of many of these studies was to identify and describe qualitative differences in the ways faculty think about their teaching and to understand the relationships between those different ways. One emergent model is a hierarchical relationship between teacher-centered and student-centered conceptions of teaching (Åkerlind, 2008). At the lowest level of the hierarchy is a view of teaching that focuses primarily on presenting information. This conception guides faculty to craft course materials and lecture presentations in optimal ways so that the information is retained by students. At a higher level in the hierarchy is the view of teaching that focuses primarily on facilitating student learning and the belief that students construct their knowledge based on prior experiences. Therefore, students' roles are viewed as active participants in their own learning. Student-centered understandings of teaching are generally believed to be a more sophisticated than teacher-centered views because they “focus on what is happening for both teachers and students in a teaching–learning situation” (Åkerlind, 2008, p. 634). In contrast, “a teacher-centred understanding shows a focus only on what is happening for teachers, with students' reactions taken-for-granted” (p. 634). For example, in an interview study with 24 chemistry and physics faculty from Australian universities, Prosser et al. (1994) identified six different conceptions of teaching within the hierarchy described above. The conceptions of teaching were listed in order of increasing sophistication, as follows:
1. Teaching as transmitting concepts of the syllabus. The responsibility of the teacher is to present information according to the conceptual topics in the textbook or syllabus. Not much attention is given to the relation between concepts and students' prior knowledge.
2. Teaching as transmitting the teacher's knowledge. The responsibility of the teacher is to present information according to their own understanding of the ideas and concepts. Not much attention is given to the relation between concepts and students' prior knowledge.
3. Teaching as helping students acquire concepts of the syllabus. Prior knowledge is considered to play an important role in the learning process. Teachers help students develop knowledge of conceptual topics as outlined in the textbook or syllabus.
4. Teaching as helping students acquire teacher knowledge. Prior knowledge is considered to play an important role in the learning process. Teachers help students develop knowledge of the conceptual topics that reflect the teacher's own understanding.
5. Teaching as helping students develop conceptions. The focus is primarily on students' conceptions of the subject matter. Teachers help students elaborate or extend their prior knowledge of conceptual topics.
6. Teaching as helping students change conceptions. The focus is primarily on students' conceptions of the subject matter. Teachers facilitate the process of conceptual change toward more scientifically accurate knowledge of the conceptual topics.
The strength of this research program emerges from the agreement among findings across several studies (Samuelowicz and Bain, 1992; Prosser et al., 1994; Kember, 1997; Martin et al., 2000; Åkerlind, 2004; González, 2011). These ways of thinking about teaching exist across location, time, and institutional context, which lends to a general belief in the external validity of the results.
Research on teacher thinking has also focused on the nature of teachers' knowledge of teaching specific subject matters. Shulman (1987, p. 8) described pedagogical content knowledge (PCK) as a blend of “content and pedagogy into an understanding of how particular topics, problems, or issues are organized, represented, and adapted to the diverse interests and abilities of learners, and presented for instruction.” The essential features of his model of teacher thinking include: (1) knowledge of diverse representations of the subject matter, (2) an understanding of specific learning difficulties, and (3) students' conceptions of the subject matter. In her cross-case analysis of teaching English in high schools in the United States, Grossman (1990, p. 8) described how “[t]eachers must draw upon both their knowledge of subject matter to select appropriate topics and their knowledge of students' prior knowledge and conceptions to formulate appropriate and provocative representations of the content to be learned.” She delineated four distinct components of PCK: (1) knowledge and beliefs about the purposes for teaching a subject, (2) knowledge of students' understandings, conceptions, and misconceptions of particular topics in a subject matter, (3) knowledge about curricular resources available for teaching particular subject matter, and (4) knowledge of instructional strategies that are particularly effective for teaching a subject matter. An important finding from Grossman's work was that teachers who exhibited robust PCK tended to deal well and reflect on situations that required complex and idiosyncratic solutions. Those individuals had experienced more professional training than those who did not. Furthermore, individuals with less PCK often left the teaching profession after a few years on the job (Grossman, 1990).
Working off of Grossman's model, Magnusson et al. (1999) conceptualized PCK for science teaching based on the following components: (1) orientations toward science teaching, (2) knowledge and beliefs about instructional strategies, (3) knowledge and beliefs about science curriculum, (4) knowledge and beliefs about students' understanding of science concepts, and (5) knowledge and beliefs about assessment in science education. The relationship between these components of teacher knowledge are illustrated in Fig. 1. The bi-directional arrows imply a reciprocal relationship between components of PCK. According to Magnusson et al., “[a]n orientation represents a general way of viewing or conceptualizing science teaching” and these orientations influence instructional planning, decision making, and reflecting. For example, a teacher may have the goal for her students to acquire content knowledge about a subject matter. One way in which the teacher might choose to accomplish her goal would be through a clear and accurate presentation of that knowledge and information using lecture-based instructional strategies.
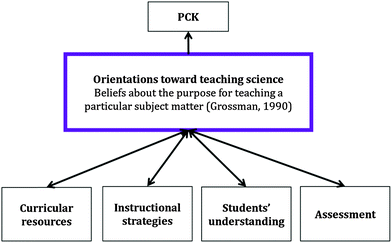 | ||
| Fig. 1 A model of PCK adapted from Magnusson et al. (1999). Faculty beliefs about the purposes for teaching physical chemistry are modeled as one dimension of orientations toward teaching science. Bi-directional arrows imply a reciprocal relationship between components of PCK. | ||
Teacher knowledge and beliefs about science curriculum encompass the goals and objectives mandated by a particular curriculum as well as specific curricular resources available for teaching (Grossman, 1990; Magnusson et al., 1999). Although the curricula in upper-division physical chemistry courses are not mandated, there is a general belief about the topics that are traditionally included in the curriculum (Committee on Professional Training, 2008). Chemistry faculty members' subject matter knowledge is likely to inform their curricular selections, organizations, and critiques (e.g., Moore and Schwenz, 1992; Zielinski and Schwenz, 2004; Mortimer, 2008; Van Hecke, 2008).
Teachers make decisions about what to teach and how to teach it based on their knowledge and beliefs about students' understanding of specific topics (Grossman, 1990; Magnusson et al., 1999). This component of PCK encompasses knowledge about students' prior coursework, topics that students typically find difficult to learn, as well as alternative conceptions about a topic. For example, chemistry education research has demonstrated that students often exhibit conceptions of entropy as a measure of disorder in terms of the physical motions of particles as opposed to the scientifically accepted definition of entropy as a measure of the different ways that energy can be distributed throughout a system (Sözbilir and Bennett, 2007). Faculty may have their own experiential knowledge about this phenomenon or have accommodated that knowledge from the literature – in either case, this knowledge is available as a resource to inform instructional and curricular planning and decision making.
Finally, Magnusson et al. (1999) included knowledge and beliefs about assessment as a crucial component of a teacher's PCK. This component of PCK encompasses teachers' knowledge of what to assess and how to assess it. For example, chemistry faculty may choose to focus their assessment on conceptual learning over mathematical methods or they may consider take-home examinations as an alternative to in-class examinations in the context of physical chemistry education (Zielinski and Schwenz, 2004).
Research on teacher thinking suggests that different chemistry faculty may exhibit different conceptions of teaching. Furthermore, their subject matter knowledge will play a crucial role in articulating knowledge and beliefs specific to teaching upper-division physical chemistry courses. Thus, one of the goals of this study was to understand how faculty coordinate both their disciplinary expertise and pedagogical knowledge when describing their beliefs about the purposes for teaching physical chemistry at the undergraduate level.
Teacher thinking about undergraduate physical chemistry education
Few studies on faculty thinking in the context of upper-division physical chemistry courses exist to date (Sözbilir, 2004; Padilla and Van Driel, 2011; Fox and Roehrig, 2015). As part of a larger study that investigated the alignment of student and faculty perceptions of physical chemistry education at two Turkish universities, Sözbilir (2004) found that two lecturers perceived systemic factors, such as overcrowded classes, lack of resources and staff, and students' academic background and socio-economic conditions to be the leading problems affecting students' learning in physical chemistry. An important finding was that these lecturers did not give sufficient thought to contemporary views of how people learn.Padilla and Van Driel (2011) interviewed six instructors at different universities in the Netherlands about their PCK for teaching quantum chemistry. Across all six participants, the authors found that the instructors used their disciplinary expertise fluidly in planning and making decisions about what curricular topics are important at the advanced level. Furthermore, they described a general awareness of students' conceptual and mathematical difficulties with the subject matter, but the interview data suggests that a general awareness was not sufficient to inform instructors about how to adjust their instruction to help students overcome those difficulties.
In the United States, Fox and Roehrig (2015) recently conducted a national survey of physical chemistry courses across 331 ACS accredited chemistry programs to assess several aspects of teacher thinking about physical chemistry education. Of their many findings was the majority of faculty (79%) reported using instructor-centered methods to deliver content, such as lecture-based instructional styles. Furthermore, this category of instructional strategies was commonly reported by faculty from large doctoral granting institutions. The few faculty reporting student-centered instructional strategies (8 of 331) were from baccalaureate and master's granting institutions. Fox and Roehrig also found that the majority of faculty reported goals for students to develop either conceptual or mathematical understandings of the subject matter, or solely conceptual learning. However, the nature of both “types” of understanding were not clearly articulated in the study. Precisely what faculty believe are the nature of conceptual and mathematical understandings of physical chemistry subject matters is further explored in this study.
While chemistry education researchers have made initial strides in understanding what faculty think about their teaching in the context of physical chemistry education, the existing research is limited in depth. Additional insights into what faculty think about teaching physical chemistry at the upper-division level may be found in the practitioner literature. While these communications were intended to serve as resources for helping faculty make decisions about selecting and organizing their curriculum, we may think of them as a collection of teacher thinking about undergraduate physical chemistry courses because they provide rich descriptions about faculty curricular and instructional planning.
In 1973, the ACS Division of Chemical Education report of the Physical Chemistry Subcommittee (1973) described physical chemistry as a field of study “not as a branch of chemistry with a particular collection of subject matter, but rather as a set of characteristically quantitative approaches to the solution of chemical problems.” It was the position of the subcommittee that the skills necessary for this kind of quantitative thinking in chemistry included not only strong foundational knowledge of physics and mathematics, but a conceptual understanding of the particulate nature of matter. One common critique of physical chemistry education is the overreliance on mathematical techniques (Society Committee on Education, 1984). In 1984, a group of chemists and chemical engineers convened as part of the ACS Society Committee on Education (SOCED) and recommended that physical chemistry courses focus less on mathematical derivations and more on the knowledge and skills necessary to produce more qualified chemists and engineers for graduate studies and employment in industrial settings (Society Committee on Education, 1984). Another recommendation made by the committee was that physical chemistry curricula should shift the subject matter away from outdated technical chemical processes and more on applications to new industrial processes and modern research in the field. A product of these recommendations was the book Essays in Physical Chemistry, which was designed to support chemistry faculty in selecting and organizing the curriculum based on recommendations made by SOCED (Lippincott, 1988). The contents of this resource outlined the views of several chemists' and chemical engineers' beliefs and knowledge about particular topics, problems, and laboratory activities to support teachers in planning physical chemistry curricula.
A few years later Moore and Schwenz (1992) described their transcendental philosophy of physical chemistry in the undergraduate curriculum, which is described in the following text:
It is… incumbent on the physical chemistry instructor to present this material in a manner that excites students, illustrates the usefulness of the material, and generates an understanding of the chemistry, rather than as a series of dull mathematical abstractions upon which the foundations of chemistry are laid. (p. 1001)
The purpose of their provocative opinion was to provide possible explanations for students' apparent lack of motivation for studying physical chemistry and to offer curricular solutions to address the problem. Among their solutions, they made the following suggestions: (1) reorganize the curriculum to focus on the study of quantum mechanics first and (2) laboratories should be modernized. By including quantum mechanics earlier, they believed the curriculum would better address students' interests in topics such as chemical bonding, intermolecular interactions, and spectroscopy. Similarly, by changing the laboratory curriculum and instrumentation they believed students would be more interested in studying physical chemistry. Their philosophy was one the first calls for educational changes to undergraduate physical chemistry courses that addressed affective dimensions of student learning and experience. However, their solutions to these problems focused exclusively on new ways to select and organize the curriculum. One facet of teacher-centered ways of thinking is a curriculum-oriented focus. In other words, faculty have a strong belief in a relation between the structure and organization of subject matter and the quality of student learning. This focus can have limitations when more attention is given to the nature of the subject matter and its presentation and not enough attention is given to the nature of how people learn (Åkerlind, 2008).
Once a physical chemistry curriculum is organized with adequate connections to other chemistry courses and has sufficient interdisciplinary applications, Zielinski and Schwenz (2004) argued that the goals of instruction should center on facilitating the understanding and use of mathematical models in science and developing students' discipline-based ways of thinking about chemical information so that students can develop more of an appreciation for what physical chemists actually do. Others believe that the goals of instruction should center on creating learning environments that are conducive for students to construct their own knowledge of the subject matter (Spencer and Moog, 2008). For example, the Process-Oriented Guided Inquiry Learning (POGIL) approach to teaching and learning physical chemistry adopts cooperative learning strategies that are designed to guide students through cycles of data analysis, model development, and applications of concepts to a problem (Spencer and Moog, 2008). Furthermore, the POGIL approach to teaching emphasizes both knowledge and science process skill development (Moog et al., 2006). Faculty who adopt more student-centered understandings of teaching may hold different beliefs about physical chemistry education relative to faculty who approach their teaching with more curriculum-oriented, teacher-centered understandings.
Prior research on teacher thinking about physical chemistry education at the undergraduate level is limited, but the available literature suggests that many faculty exhibit teacher-centered understandings about the teaching–learning situation (Sözbilir, 2004; Padilla and Van Driel, 2011; Fox and Roehrig, 2015). Interview and survey-based studies found that: (1) faculty have a general awareness of student difficulties, but self-report data suggests that this awareness does not always guide faculty to adjust their pedagogy, (2) faculty may rationalize student difficulties based on factors that they believe are beyond their control, and (3) the majority of faculty at ACS-accredited departments in the United States reported using instructor-centered pedagogical strategies. The existing practitioner literature offered additional insights into teacher planning and philosophies for teaching physical chemistry. Much of the discourse focused on beliefs about the structure and organization of the curriculum, but it also addressed issues of emerging theories of learning and student-centered instructional strategies in the context of physical chemistry education. Taken as a whole, the literature suggests that different faculty work with varied beliefs about physical chemistry education. One way to improve our understanding of these beliefs, their nuances, and how they are related is to construct rich descriptive knowledge based on faculty reports of their experience teaching physical chemistry.
Theoretical framework
Phenomenography
Teaching physical chemistry is the experience of an instructor in a physical chemistry course setting communicating with students about fundamental unifying concepts of chemistry and physics and engaging them in practices that are intended to model what physical chemists do. As such, faculty construct knowledge and beliefs about teaching physical chemistry based on their experiences in physical chemistry education, including their own experience as a student. We chose phenomenography as a theoretical framework for this study because our cumulative experience as students, teaching assistants, and as an instructor of physical chemistry led us to believe that different faculty construct diverse knowledge and beliefs about teaching physical chemistry courses. Phenomenography is an empirical research tradition that seeks to describe the different ways in which people experience a certain phenomenon (Marton, 1981, 1986). As a theoretical framework, phenomenography provided several assumptions about the nature of faculty knowledge and beliefs about teaching that helped guide this study.Individuals discern various aspects of a phenomenon in different ways (Marton, 1986; Åkerlind, 2008; Orgill et al., 2015). Phenomenography assumes that no individual has the complete experience of any phenomenon because one's experience is related to how they perceive their interaction with the external world (Orgill, 2007). Different people have different perceptions and it is the collective sum of those perceptions that constitute a phenomenon. The commonalities and differences across faculty perceptions' of their experience will lead to a finite number of discernable features of teaching physical chemistry (Marton, 1986). An understanding of the variation in those perceptions leads education researchers to a better understanding of the phenomenon that is teaching physical chemistry.
The epistemological assumption about the nature of faculty beliefs (often called conceptions in phenomenographic research) is that “different conceptions of teaching are seen as representing different breadths of awareness of the phenomenon of teaching, constituted as an experiential relationship between the teacher and the phenomenon” (Åkerlind, 2008, p. 634). For example, a student-centered understanding of teaching covers a larger breadth of views about teaching and learning relative to a teacher-centered understanding because it guides the teacher to focus on both the students' and their own experience in an educational situation (Prosser et al., 1994; Åkerlind, 2004). A teacher-centered understanding is narrower in the sense that the teacher focuses primarily on their own experience while making general assumptions about student learning. Conceptual development regarding one's teaching experience is described as an expanded awareness of a potential for variation in the different aspects of teaching that are recognized by the individual. For example, as teachers develop a student-centered understanding of teaching they expand their awareness of the role of students' characteristics and experience in the teaching and learning process. Teacher-centered understandings of teaching are not wrong, but they lack awareness of key aspects of teaching and learning that are central to our contemporary views about how people learn, such as the active participation of the student in the learning process (Bransford et al., 2000). The development of teacher thinking from teacher-centered to student-centered is a matter of conceptual expansion (Åkerlind, 2008).
The epistemological assumptions about phenomenography guided this research with a broad view of faculty beliefs about teaching in general and about their own teaching in the context of undergraduate physical chemistry courses. At the same time, we applied a model of pedagogical content knowledge as a second theoretical framework to understand faculty thinking about teaching because it offered additional assumptions about an individual faculty member's knowledge and beliefs about teaching a specific subject matter at a particular level. Furthermore, the additional theoretical layer to this study helped us recognize and understand discipline-specific nuances in faculty member's knowledge and beliefs about teaching physical chemistry because PCK gives considerable attention to the nature of subject matter knowledge when thinking about teaching (Shulman, 1986; Gess-Newsome, 1999).
Pedagogical content knowledge
As a theoretical framework, PCK offers several assumptions about the nature of faculty knowledge and beliefs about teaching. First, it classifies the blending of subject matter knowledge with pedagogical knowledge as a separate, but related body of knowledge for teachers to refer to when planning, making decisions, and reflecting on their teaching (Miller, 2007). Second, as a model of individual faculty member's thinking about teaching, PCK is constructed based on one's prior experience and knowledge related to teaching specific subject matter at a particular level. Finally, PCK consists of several key concepts of teaching that are common to many teachers. We adopted the model of PCK described by Magnusson et al. (1999), as described earlier, because it was useful to help us categorize faculty knowledge and beliefs about teaching and learning. In this paper, we explore one category of faculty PCK for teaching physical chemistry: beliefs about the purposes for teaching physical chemistry. In this study, we conceptualized orientations toward science teaching to consist, in part, of beliefs about the purposes for teaching the subject matter in order to provide a better theoretical basis of the construct in the research on teacher thinking (see Fig. 1) (Friedrichsen et al., 2011). This was an appropriate use of concepts of teacher thinking because Grossman (1990) described that “[t]eachers' conceptions of the purposes for teaching particular subject matter influence their choices both of particular content to teach and of instructional activities with which to teach that content” (p. 86). We explore the relationship between faculty members' orientations toward science teaching and other dimensions of their PCK in a future manuscript.Methods
The research methods employed in this study were qualitative in nature. We conducted interviews with faculty who teach or have taught physical chemistry because “interview data can help illuminate not only actions and beliefs, but also the reasons behind the actions and beliefs” (AAAS, 2013). Furthermore, interview-based methodologies allow the investigator to adapt to the unique and idiosyncratic features of a participant's experience (King and Horrocks, 2010).Sampling strategy
Participants were purposefully sampled such that it was likely they could offer contrasting evidence and views (Kuzel, 1992; Åkerlind, 2004). For example, one sampling strategy that we believed offered contrasting views was to recruit participants across varying academic ranks because faculty hold different teaching, research, and administration responsibilities during different stages of their career (Austin, 2011). Participants' academic ranks ranged from Lecturer to Full Professor. In the United States, the title Lecturer is given to faculty who assume a non-tenured track position that focuses mainly on teaching responsibilities and little or no research responsibilities, although a lecturer's responsibilities may vary from institution to institution. The title Assistant Professor is traditionally given to junior faculty who enter a tenure-track position. Promotion then leads to the rank of Associate Professor and eventually Full Professor. Another sampling strategy that we believed would offer contrasting views was to recruit participants across different institution types because institutional structures and cultural norms of academic departments can influence teaching practices and beliefs (Gess-Newsome et al., 2003; Fairweather, 2008; Austin, 2011). Based on this purposeful sampling criteria, a diverse range of physical chemistry courses and class sizes emerged as additional dimensions of variation in participants' experiences. Participating faculty tended to teach in at least one of three different kinds of physical chemistry courses: courses intended for chemistry majors, courses intended for chemistry majors with a professional emphasis (e.g., secondary education), and courses intended for STEM non-majors (e.g., biology). Some of the courses that participants taught contributed to their department's ACS-certified degree, while some did not. Depending on the type of institution, class sizes also ranged from less than 15 students to more than 60.We solicited attendees who gave a presentation about research in a physical chemistry related field or about physical chemistry education at two conferences: the 2014 Biennial Conference on Chemical Education and the 248th American Chemical Society National Meeting. Participants' demographic information are presented in Table 1. All participant names are pseudonyms. Some participants were also recruited through snowball sampling. Overall, 78 faculty were invited to participate in this research. Twenty-four agreed to either a face-to-face or remote interview. Permission to conduct this research was granted by the Purdue University Institutional Review Board and informed verbal consent was obtained from the participants at the time of the interview.
| Participants (pseudonym) | Career stagea | Institution typeb | Class size |
|---|---|---|---|
| a Based on information about promotional status made available through department websites at the time of data collection. b Based on the Carnegie Classifications of Institutions of Higher Education (http://carnegieclassifications.iu.edu/). c Participant volunteered course syllabus/syllabi as part of the analysis for this study. | |||
| Dr Gennac | Associate professor | Baccalaureate colleges | <15 |
| Dr Rosalinda | Professor | Baccalaureate colleges | <15 |
| Dr Thaddeus | Professor | Baccalaureate colleges | <15 |
| Dr Stephen | Associate professor | Doctoral university | 15–30 |
| Dr Aidenc | Professor | Master's colleges and universities – large | <15 |
| Dr Craig | Assistant professor | Master's colleges and universities – large | 15–30 |
| Dr Liamc | Professor | Master's colleges and universities – large | 15–30 |
| Dr Nevaeh | Professor | Master's colleges and universities – large | <15 |
| Dr Renata | Professor | Master's colleges and universities – large | 15–30 |
| Dr Jacobc | Associate professor | Master's colleges and universities – medium | 15–30 |
| Dr Amos | Professor | University with very high research activity | >60 |
| Dr Elisec | Associate professor | University with very high research activity | >60 |
| Dr Elliot | Professor | University with very high research activity | >60 |
| Dr Holly | Associate professor | University with very high research activity | <15 |
| Dr Melanie | Lecturer | University with very high research activity | 31–45 |
| Dr Patrickc | Associate professor | University with very high research activity | 31–45 |
| Dr Rikuc | Assistant professor | University with very high research activity | >60 |
| Dr Xic | Associate professor | University with very high research activity | >60 |
Interviews
In-depth semi-structured interviews with 24 participants lasting between 45 and 100 minutes were collected. Most interviews lasted over one hour. The protocol is provided in Appendix 1. The focus of the protocol was the faculty member's beliefs and self-reported practices that were salient to his or her account of their experience teaching physical chemistry courses at the undergraduate level. During each interview, the first author invited participants to reflect on the “grand tour” question, “How would you describe your approach to teaching physical chemistry?” This opened the discussion to beliefs, goals, strategies, and practices, among other things, that faculty chose to introduce without explicit prompting. This prompt was generated based on our analysis of the practitioner literature related to teaching physical chemistry (e.g., Moore and Schwenz, 1992; Zielinski and Schwenz, 2004; Committee on Professional Training, 2008). During the interviews, faculty made specific references to a particular physical chemistry course, for example, an ACS-accredited course for chemistry majors that focused on quantum mechanics and spectroscopy. This narrowed our conversation to specific lesson plans, course goals, or instructional strategies for one particular course. Beliefs about the purposes for teaching physical chemistry were targeted through multiple aspects of teaching, including goal statements, planning and decision making strategies, rationalizing instructional practices, beliefs about student learning, commenting on colleagues' approaches to teaching physical chemistry, and the future role of physical chemistry in the undergraduate curriculum in order to gain as full an understanding about faculty beliefs as possible. Literature on teacher thinking in higher education (AAAS, 2013) and discipline-based education research (Dancy and Henderson, 2007) also helped to guide the development of the interview protocol.Eighteen interviews were selected for the complete analysis based on the amount of reflection participants contributed. Some participants offered short responses or an unwillingness to articulate ideas when prompted, so in these cases we did not include the data in our complete analysis. Audio recordings from the interviews were transcribed verbatim in order to create a written text of the participants' experience (King and Horrocks, 2010). Analytic memos were composed and refined throughout the analysis as a way to reflect on the data collection and analysis, including initial impressions and emergent patterns (Strauss and Corbin, 1998; Saldaña, 2009). Follow-up emails were sent to participants in order to request clarification and/or elaboration on specific statements in the transcripts. Transcripts and analytic memos were imported into NVivo 10 for coding and analysis (QSR International Pty Ltd, 2012).
Course artifacts
Eight participating faculty volunteered course syllabi as artifacts to further explore faculty beliefs about the purposes for teaching physical chemistry at the upper-division level. In total, eleven syllabi were collected. One participant offered two syllabi from two different semesters of teaching physical chemistry because he approached his curriculum selection and organization in a markedly different way than what he believed was the “traditional” approach. In another case, a participant volunteered three different syllabi because he taught three different physical chemistry courses: thermodynamics, quantum mechanics and chemical kinetics, and an introductory physical chemistry course for chemistry majors with professional emphases. These artifacts were collected and reviewed for information that supported or contradicted the ideas discussed during the interviews within each case. In addition, we looked for instances where the reflections in the interview transcripts aligned or contrasted with statement made about the nature of physical chemistry as a discipline or as part of an undergraduate chemistry education in the course syllabi. Typically, sections of course syllabi titled “Course Description” or “Course Objectives” included statements that provided triangulating evidence of faculty beliefs about the purposes for teaching physical chemistry. Course syllabi were imported into NVivo 10 for coding and analysis (QSR International Pty Ltd, 2012).Coding
Data analysis followed a variable-oriented approach (Miles and Huberman, 1994) where the focus was on developing an understanding of the similarities and differences in faculty beliefs about the purposes for teaching physical chemistry that emerged from comparing and contrasting cases. In order to manage the complex network of knowledge and beliefs about teaching in the data, concepts of pedagogical content knowledge were applied as a coding scheme in order to systematically analyze faculty knowledge and beliefs about teaching physical chemistry. This offered the analysis a structure to classify and organize “types” of knowledge and beliefs, as well as relationships between the different aspects of an individual faculty member's PCK. Within this coding scheme was the concept that faculty hold beliefs about the purposes for teaching physical chemistry at the upper-division level (Grossman, 1990; Magnusson et al., 1999). Participant responses to prompts in the interview transcripts were examined for beliefs in the form of propositional statements that were cited in support of various decision-making processes in regards to teaching physical chemistry.Coding for beliefs about the purposes for teaching physical chemistry began by examining participants' responses to prompts in the interview protocol. We became aware of selected excerpts that stood out most based on either commonalities across cases or uniqueness of the contents within a particular case. These excerpts were typically related to an individual participant's reflections on their approach to teaching physical chemistry, their awareness of similarities and differences between their own and colleagues' philosophical and pedagogical approaches, or their views about the present and future roles of physical chemistry in the upper-division chemistry curriculum (see Appendix 1, prompts 1, 4, 5, and 6 in the interview protocol). We initially coded these excerpts with descriptive codes (Strauss and Corbin, 1998; Saldaña, 2009). Saldaña (2009) described initial coding as a form of open coding where the researcher breaks down larger units (e.g. a whole transcript) “into discrete parts, closely examining them, and comparing them for similarities and differences” (p. 81). This approach to data analysis helped us to avoid presuppositions about participants' teaching experiences by remaining open to many philosophical stances about physical chemistry education indicated by the close reading of the data. Matrix coding querying capabilities in NVivo 10 were used to constantly compare coded excerpts across cases and to refine and elaborate the operational definitions of the codes for this study (Strauss and Corbin, 1998). The codes and concepts that emerged from the interview data were subsequently applied to the course syllabi data set. A listing and description of codes for the different beliefs about the purposes for teaching physical chemistry can be found in Table 2.
| Code name | Code note (analytic memo) | Example from the data |
|---|---|---|
| Concepts and connections | The purpose of teaching undergraduate physical chemistry courses is to help students identify fundamental concepts of chemical sciences and the relationships between them. Use concepts and connections when faculty talk about presenting topics or helping students develop an understanding of topics and their relationships within and beyond the curriculum, i.e. topics in other courses, current scientific issues, theory and experiment, “real world” applications, problem solving, macroscopic-particulate nature of matter connection, or students' interest in a particular subject matter. | Traditionally, physical chemistry has been divided into six subareas, and this course will provide an overview and introduction to all six subareas: classical thermodynamics, statistical mechanics/thermodynamics, kinetics, dynamics, quantum chemistry, and spectroscopy. The division of the field in this way is somewhat arbitrary in modern physical chemistry; in part, these divisions are historical. Connections and overlaps between the subareas are emphasized in this course. (Course syllabus, Dr Aiden) |
| Develop understanding | A key feature of helping students develop an understanding of the subject matter is to use students' prior knowledge of chemistry, physics, and mathematics as a foundation for further learning. Use develop understanding when participants talk about the role of students' prior knowledge or active participation in the learning process. |
Interviewer: can you describe to me the model of student learning that you use when teaching this physical chemistry course?
Dr Stephen: Well I intend that it's based on connecting to students' prior knowledge… What I want them to walk away with is a more in-depth explanation of whatever that thing is. That their explanation can either be in the algorithmic mathematical sense and that they can do some of the calculations that they were never shown, or that they can have more conceptual understanding of whatever the content is for the topic that they're covering. (Interview, Dr Stephen) |
| Models and modeling | Modeling is a central practice that physical chemists engage in to investigate chemical and physical phenomena. This is a process including cycles through the stages of model development, use, evaluation, and refinement. Use models and modeling whenever participants talk about their beliefs regarding the nature of models and modeling as part of their goals or beliefs about the purposes for teaching physical chemistry courses in the undergraduate curriculum. | Dr Elise: …my course goals with physical chemistry is this idea that we use mathematical models to describe chemical phenomena and the natural world thinking in terms of atoms and molecules, but also the more bulk systems. So this idea that we are using mathematical models to describe chemistry. That's kind of the big one. (Interview, Dr Elise) |
| Problem solving | Problem solving is a key activity in physical chemistry and science education in general. Successful problem solving skills require the individual to access, organize, and apply their existing knowledge to the task at hand. Use problem solving when faculty talk about the role of problem tasks in the development of students' understanding of the subject matter; students make connections by doing exercises or solving problems. | Dr Amos: …what I can do to best serve these students in understanding these things is to try to figure out as clear a way explaining this stuff. Then give them a homework problem so let them work with it so they get a better feel for how it really works. (Interview, Dr Amos) |
| Professional training | Undergraduate coursework in chemistry is part of students' professional training as a chemist, scientist, or citizen. Students have several different goals for pursuing a degree in the chemical sciences. Some students may plan to go to graduate school in a chemical sciences related field or they may enter a field not part of the chemical sciences. Some may plan to enter an industry related to the chemical sciences. Use professional training when participants talk about helping students prepare for life and work beyond their chemistry education in terms of content knowledge only. | Dr Elliot: …my goal is to introduce at a rigorous level of detail the major concepts of physical chemistry. And this is both to train students who may not have another physical chemistry course who will be practicing chemists as well as to- prepare students for graduate school if they are going to pursue further studying chemistry and therefore to cover the major topics in physical chemistry. (Interview, Dr Elliot) |
| Transfer knowledge | The purpose of teaching physical chemistry curricula is to transfer knowledge and information about core concepts, examples, and problems to students, which, in turn, will be applied to solving specific problems (e.g. on problem sets, exams, etc.). Use transfer knowledge when participants talk about their responsibility to provide a comprehensive treatment of topics through an adequate presentation of subject matters and the conceptual links between them. | Dr Amos: … you know… subjects like thermodynamics there is an awful lot of stuff that has been figured out over hundreds of years… Like I have a really hard time imagining how students could… you know, you could set up a situation where they are going to figure out on their own because they took these brilliant people a hundred years to figure out. So I feel like my job, what I can do to best serve these students in understanding these things is to try to figure out as clear a way explaining this stuff. (Interview, Dr Amos) |
| Process skills | Faculty held beliefs about helping students develop domain-general skill sets that are important for graduate school and professional work. Use process skills when participants talk about goals for their physical chemistry courses that go beyond the development of subject-matter knowledge or problem solving skills to include other process skills – e.g. written and oral communication or team skills – that can be applied to future learning experiences or professional settings. | Dr Aiden: …I've also come to realize it is not only about content… there's also skills that they're hopefully developing that are really important and I think POGIL addresses many of those skills-information processing, critical thinking, teamwork… It's transferable practices that they can use in other settings besides chemistry. (Interview, Dr Aiden) |
The analysis of interview transcripts and course syllabi led to a set of qualitatively different beliefs about the purposes for teaching of physical chemistry. These categories are the most important products of phenomenographic research because they describe the contents of faculty experiences (Marton, 1986). An understanding of faculty beliefs about the purposes for teaching physical chemistry are available through the rich descriptions of their accounts of their experience.
Findings
We identified three qualitatively different beliefs about the purposes for teaching physical chemistry based on the contents of faculty reflections on their experience teaching. The different categories build upon one another, such that some are inclusive of multiple beliefs while others are not. Each category is presented with a rich description supported by evidence from the data.Concepts, connections, and a general belief in conceptual learning
By far the most common belief about the purpose for teaching physical chemistry courses in the undergraduate curriculum was to help students develop their knowledge of fundamental concepts, which typically included topics from thermodynamics, statistical mechanics, chemical kinetics, quantum mechanics, and spectroscopy. This was shaped, in part, by beliefs about the nature of physical chemistry as a discipline. For example, Dr Amos described how the relationship between physical chemistry and other sub-disciplines of chemistry made physical chemistry education an integral part of the undergraduate curriculum.Interviewer: So my final question would be what do you think the role of physical chemistry courses in the undergrad curriculum are going to be in the near future, maybe 10 years from now?
Dr Amos: It will all still be there. I mean unless people just don't want to understand chemistry. It's like Ostwald founded the field of physical chemistry because it was the discipline intended to understand how all the other disciplines of chemistry work. That's what physical chemistry is. It's the theoretical underpinnings of how chemistry works.
While physical chemistry as a sub-discipline of chemistry provides the other traditional branches of chemistry with predictive understandings of chemical phenomena, faculty understand the subject matter to be abstract and difficult for undergraduate students. In the case of Dr Amos, this perspective guided his teacher-centered thinking about transferring knowledge as clearly as possible to students using lecture-based instructional strategies, as is described in the following expert from the interview transcript:
Dr Amos: …you know… subjects like thermodynamics there is an awful lot of stuff that has been figured out over hundreds of years… Like I have a really hard time imagining how students could… you know, you could set up a situation where they are going to figure out on their own because they took these brilliant people a hundred years to figure out. So I feel like my job, what I can do to best serve these students in understanding these things is to try to figure out as clear a way explaining this stuff.
Similar beliefs guided faculty to clearly communicate content knowledge to students, but with the goal to prepare them for professional work in industry or graduate school.
Dr Elliot: …my goal is to introduce at a rigorous level of detail the major concepts of physical chemistry. And this is both to train students who may not have another physical chemistry course who will be practicing chemists as well as to- prepare students for graduate school if they are going to pursue further studying chemistry and therefore to cover the major topics in physical chemistry.
Conceptual understandings are supported by a rich network of concepts; facts and ideas are connected by causal explanations, descriptive relationships, and ways of thinking across mathematical, molecular, and macroscopic models of matter. These features of conceptual knowledge were central to what faculty meant by a “deep” understanding of thermodynamics, quantum mechanics, or other major topics in the curriculum.
Dr Aiden: …there's real depth to this stuff. And in my view, and I hope I convince some students of this, there's just a few ideas, and yeah, there's some complicated math, but if you can get at even a conceptual understanding of those few ideas you can understand lots and lots of stuff about chemistry and biology.
In Dr Aiden's course syllabus, he described how a focus on atomic and molecular energies, interactions, and the link between microscopic properties and macroscopic behavior will give one a predictive understanding of chemical change. Furthermore, he stated, “All of chemistry, and by extension nearly all of biology, is within our grasp.” Precisely how students develop those connections is a matter of the instructor providing clear and explicit materials and presentations about those connections across the curriculum, as was described by Dr Aiden in the following excerpt from the interview transcript:
Interviewer: So by reorganizing the curriculum you're drawing more connections. How are students drawing those connections?
Dr Aiden: I think by doing things in a different order I am almost forcing them to think about it in a slightly different way.
Other participants described similar goals for their physical chemistry courses:
Dr Genna: …my ultimate goal is that I want students to see what I see and what many of my colleagues see, which is that there is p chem everywhere in everything that you learn in chemistry… I think the role of p chem in the next ten years is still to allow students to explain and analyze and predict phenomena at a more fundamental level.
Dr Holly: I hope [students] get a really fundamental understanding of how things work, even on the microscale.
Faculty thought that the subject matter should be useful to students. And to make it useful they believe the subject matter should have connections to current scientific issues or context-rich applications. It was often the case that faculty believed it was their role to identify those connections and provide sufficient examples, as was described by Dr Patrick in the following excerpt from the interview transcript.
Dr Patrick: …my goal in this course I think is to convey to the students that physical chemistry is useful to them regardless of the kind of chemistry they're interested in…
Interviewer: Can you maybe give me an example of something that you would consider some motivation for your students to be interested in?
Dr Patrick: So a lot of this comes from my background and my research interests. I tend to focus on… energy science… and also because usually half the class is biochemists I try to incorporate a lot of examples from biochemistry to the best of my ability. Again, taking the material and contextualizing it towards broad scientific concepts, ideas that people may be familiar with or interested in.
Students do not walk into the physical chemistry classroom as blank slates. They have years of experience in STEM education that they can apply to the learning of topics in physical chemistry. Several faculty considered more student-centered conceptions that incorporated students' prior knowledge as a resource for learning the subject matter.
Dr Xi: I want to use [quantum chemistry] concepts to push the chemistry understanding of my students to a new level. This is in the context that they all have taken general chemistry. For example, they all understand 1s22s22p3 for nitrogen atom electronic configuration. So why is that the rule they have to follow? They might not fully appreciate that point. Or they only know that reason from a qualitative way, but not quantitative way. So when they are done with my class they should gain a much more analytical or quantitative way and deeper understanding on the topics they thought they already knew from general chemistry.
Dr Stephen: I try to give the students in that course a sense of how the things we are going to cover in that physical chemistry class both connect back to things that they have learned starting from general chemistry and other chemistry courses and how we build on the models that we start with and then how we can use that to answer more in-depth, more detailed questions about things that they have already been introduced to in the however many years of chemistry courses that they've had.
Conceptual knowledge is a valuable resource for strategically solving domain-specific problems (Larkin et al., 1980; Chi et al., 1981). Students with weak conceptual knowledge of thermodynamics or quantum mechanics tend to use unproductive strategies for solving problems in undergraduate physical chemistry courses, which reinforces their weak understanding of the subject matter (Patron, 1997; Gardner and Bodner, 2007). Faculty described problem solving as an opportunity for students to develop connections between concepts, which in turn get applied to future problem-solving experiences. In other words, faculty described how learning concepts and problem solving in physical chemistry go hand-in-hand.
Interviewer: To begin, how would describe your approach to teaching physical chemistry.
Dr Xi: There are two philosophies I try to pay attention to. One is… an analytical approach for quantum mechanics… in the sense that I require my students not only to understand the concepts not only from qualitative way, but using basic derivation and understand the result from the quantitative analysis and understand the implication of that and how to connect that to the basic concept.
The connection between qualitative and quantitative reasoning was Dr Xi's way talking about making connections between topics through mathematical problem solving. The goal of developing conceptual knowledge through problem solving was stated concisely in her course syllabus for the quantum mechanics and molecular spectroscopy: “There is no better way to master Physical Chemistry than by solving problems. The essence of this subject demands linking abstract mathematical ideas with the experimentally observed behavior of chemical systems.” Continual engagement in problem solving tasks was one way faculty believed students would develop their problem solving skills and conceptual knowledge of topics in physical chemistry, as described by Dr Patrick during the interview:
Dr Patrick: I think at some very philosophical level that scientists need to be good problem solvers. And so that's why essentially most science classes incorporate problems of some kind that the students have to work through out of class. And it's just a continual process of learning to become a better and better problem solver.
Dr Holly: …I'm just hoping by doing enough difficult challenging problems [students] start to make those connections.
Faculty who described problem solving as a means of constructing conceptual knowledge of the subject matter often talked about it in the sense that in general more problem solving leads to more connections, which means a more robust network of concepts that can be applied to future problem solving tasks. This understanding of the learning process was nearly isomorphic with their conception of the development of problem solving skills. Faculty believed students develop along a trajectory from novice problem-solving skills to more expert-like skills by solving more and more problems. In other words, some faculty believed the raw experience of problem solving promoted learning in undergraduate physical chemistry courses.
Several codes from our coding scheme were combined to inform this more general category about the purpose for teaching physical chemistry: concepts and connections, develop understanding, problem solving, professional training, and transfer knowledge (see Table 2). Beliefs about conceptual learning for teaching upper-division physical chemistry courses were supported with different approaches that faculty believed were useful to help students developed that knowledge, for example, by transferring faculty knowledge to students, by making the subject matter relevant to students' interests, by activating students' prior knowledge, or by engaging students in problem solving experiences. Some of these different ways of supporting students in developing conceptual knowledge can be classified as teacher-centered thinking while other beliefs can be classified as student-centered thinking. So the teacher-centered/student-centered paradigm of teacher thinking was not necessarily useful to discern logical patterns in faculty beliefs about conceptual learning or in general. Instead, we interpreted different conceptual boundaries between categories describing the purposes for teaching physical chemistry. The next section describes a belief about the purpose for teaching physical chemistry that is inclusive of conceptual learning beliefs, but focuses on teaching about the nature of models and modeling in science, especially the nature of mathematical models in physical chemistry.
Models, modeling, and a belief in epistemological learning
All the faculty in this study believed that well-crafted problem-solving situations provided students with opportunities to practice their ability to apply or extend their knowledge of the subject matter; however, a few faculty reflected on the limitations of traditional problem-solving assessments to help students develop conceptual knowledge of the topics in physical chemistry. For example, Dr Renata described her awareness of students' unproductive problem-solving strategies when working on traditional problem-solving assessments out of a textbook.Dr Renata: …[students] see “here's the problem: I have heat capacity, I have temperature, I should just look over all of the equations in the book in the section covered by whatever timespan this is and see if I can find some sort of equation that might actually have these kinds of symbols in it and then I will just use it and see if it sort of kind of works.” And they don't really understand what's going on.
Dr Renata was primarily concerned that traditional problem-solving assessments allow students to solve problems with strategies that do not rely on conceptual knowledge, a phenomena which has been demonstrated previously in the literature on student learning in undergraduate physical chemistry courses (Gardner and Bodner, 2007). In order to overcome the limitations of traditional problem-solving assessments some faculty described a models and modeling perspective for teaching physical chemistry. This perspective explicitly addresses the nature of modeling as a key processes in building knowledge about thermodynamics, quantum mechanics, and other major topics in the curriculum.
Dr Renata: …so my goal for [students] is to understand what physical chemists do… and it, after all, is a modeling of real phenomena… we first look at heat capacity as a function as temperature and they actually model this… I just import the data from NIST. And they get a polynomial out of Excel. And then I make them calculate four functions of heat capacity as a function of temperature… And they actually program that into Excel. And then I hand them a data set and say here is the heat content of CO2 as a function of temperature. What functional form of heat capacity as a function of temperature is it? And they discover quickly that it's the integral of CpdT.
Dr Elise described mathematical modeling as a primary focus in her physical chemistry courses because the development and use of these models are the means of generating and validating knowledge claims in the community.
Interviewer: So what are your goals for the overall course? What are your expectations of students by the end of the semester?
Dr Elise: I should pull out the syllabus. I have course objectives… so my course goals with physical chemistry is this idea that we use mathematical models to describe chemical phenomena and the natural world thinking in terms of atoms and molecules, but also the more bulk systems. So this idea that we are using mathematical models to describe chemistry. That's kind of the big one… More concretely, I do a lot with graphing. A lot of looking at graphs and figures and using graphs to understand those mathematical models.
She described this course goal in her syllabus for quantum mechanics with the following statement, “Students will, in words and mathematically, define the most important physical quantities that characterize the atomic and molecular properties of matter and the relationships between these quantities based on quantum mechanics.” She further articulated in her syllabus course goals that students should develop the skill to create, use, and analyze mathematical models to interpret chemical information: “Students will develop… proficiency in information processing by generating and interpreting data presented in tables, graphs, drawings, and models…“
Other faculty articulated similar beliefs about the role of models and modeling in generating and evaluating knowledge claims in the context of physical chemistry subject matters. Dr Rosalinda reflected on her understanding of the structure of Gas Laws as series of models that are generated and then applied to predict or explain phenomena that physical chemists are interested in. Her goal was to communicate that understanding of modeling to her students, as described in the following excerpt from the interview transcript.
Dr Rosalinda: I really try to work with [students] from a goals perspective of where do we make fundamental simplifying assumptions and why do we make them. So why is it that we start out with the concept of an ideal gas or an ideal solution and then look to deviations of that ideal behavior and how you can kind of simplify and work with sort of a simple model and build up from there? … So really trying to get them to have a sense that every model carries with it a set of assumptions and how important it is as a course goal to know what those assumptions are and know therefore how to assess what the limitations of those assumptions are in terms of the predictability of whatever your model is for whatever your system is that you are taking a look at.
Dr Craig also described a more teacher-centered perspective of helping students develop an understanding of the role of models and modeling in physical chemistry.
Dr Craig: I try really hard to instill in them this idea that the goal in a lot of physical chemistry is to define the model that you want to work on that best represents the thing you want to study. So if what you want to learn about is a gas that expands under constant pressure, well we can create a set of rules based on physics or chemistry, basic laws of motion, we can develop a model and then all of our answers have to exist within that model, they have to follow the rules of the model we built. So if you can define your model well enough then the answers sort of come from that. But the challenge for the student is to realize what goes into a good model. You know, what are the parameters that are important here, and what do I not really care about? Because every model has its limitations. Every model can only focus on a certain number of aspects. And so if we can identify what are the important aspects and build our model aligned with those… then we can get some solutions, keeping in mind that those solutions are only good in the context of the model that you've built.
Some faculty described their beliefs about the purpose for teaching physical chemistry at the undergraduate level in terms of helping students understand the nature of models in science and modeling as a science practice. What makes this perspective different from the focus on conceptual learning is the belief that students often do not recognize and comprehend the modeling nature of physical chemistry subject matter when faculty do not explicitly instruct them on the modeling nature of science. The belief that physical chemistry education should explicitly address the modeling nature of science made this perspective unique with respect to the data as a whole. At the same time, it is inclusive of other beliefs about helping students develop conceptual knowledge of fundamental and unifying concepts of chemistry because accurately modeling chemical phenomena requires a conceptual understanding of the phenomena to be studied.
Process skills through social interactions
While faculty generally believed that learning the subject matter, i.e. conceptual topics, problems, and models, was the most substantive goal for teaching physical chemistry, some held strong beliefs about helping students develop process skills. The CPT (2008) described process skills as “generic and transferable, are marketable and lifelong, and have wide applications that go beyond course content alone.” For example, Dr Aiden described that he supported students' development of process skills because he believed they provided students with additional preparation for professional work.Dr Aiden: I've also come to realize it is not only about content… there's also skills that they're hopefully developing that are really important and I think POGIL addresses many of those skills – information processing, critical thinking, teamwork. You can call them soft skills, you can call them lifelong learning skills. Its transferable practices that they can use in other settings besides chemistry. I mean most of those skills should be applicable to almost anything they are going to do in the world of work.
Dr Aiden included statements about this dimension of learning in his physical chemistry courses in his syllabus. He provided the following learning objectives to support students' development of process skills in his courses: Students will be able (a) to effectively communicate ideas in both oral and written form, (b) to collaborate with other students in class group work and in lab, (c) to work safely in lab, and (d) to do all the above while demonstrating respect for others and their ideas, both formally (e.g., proper citations) and informally (e.g., not talking over each other in groups). Not only do students develop communication and team skills through group learning during class time, but Dr Aiden also described more student-centered beliefs about creating environments for students to articulate and discuss their own knowledge of the material, as described in the following excerpt from the interview transcript.
Interviewer: My next question is how do you think students are learning differently in this POGIL curriculum or this POGIL approach versus the way you did it more traditionally like with lectures?
Dr Aiden: I think they are learning through communication with others much more so than in lecture. I think learning can happen in both ways… I think they learn more in the groups than they do from me in lecture… The content for the most part is being delivered through those group activities… But in terms of how they learn I really think they're learning by discussing the material… They are doing something that's guided inquiry and that's forcing them ideally to learn through each other… They learn through the discussions, through the oral communication. And sometimes written communication like working out a derivative or something like that. That's what I think.
Dr Thaddeus held similar beliefs about teaching physical chemistry. He described physical chemistry education as a place in the undergraduate chemistry curriculum where students not only learn content, but process skills that apply to industry and future learning.
Interviewer: What do you think the role of p chem is in the near future, like 5 to 10 years from now?
Dr Thaddeus: So the students are tending towards many things in the health sciences, which tend to use more of the organic and biochemistry… the ones who move immediately into the chemical industry tend to use a little more analytical chemistry and things like that. So I think physical chemistry we ought to be cognizant of the fact that we are probably teaching some things like critical thinking, and team building, and communication. As well as providing a kind of a basis for understanding some of those other areas. But I think we'd probably be best served if we realized that we have other things to offer other than just teaching people how to calculate expectation values… I think it is important to realize that physical chemistry might be… I don't want to privilege it over others, but it might be a good way to think about things like critical thinking, communication, skills that serve people as scientists generally.
Dr Elise passionately defended her beliefs about specific subject matters in the physical chemistry curriculum and how students ought to approach their learning of the subject matter. The following excerpt is in the context of using the POGIL approach in her physical chemistry courses.
Dr Elise: They don't need to know the derivation of the equations that describe the hydrogen atom. They don't! And I tell them that. That's not what's important. What's important to me is that you can take something that you haven't seen before, and with facilitation, and reading, and guidance, you can extract the important concepts from that… it is much more important that they learn how to think, and that's what I really want them to do.
This excerpt was particularly interesting because Dr Elise rejected the goal of covering a certain amount of content. Whereas some faculty believed that more problem solving contributed to better quality conceptual knowledge, Dr Elise was focused on the quality of the learning activity; she believed that creating a learning environment that engaged students in critical readings of the materials and discussions was more important than depth in some content areas.
When faculty held beliefs about helping students develop process skills they often described these skills not just as outcomes, but also as the process by which students learned the subject matter. Besides developing skill sets in addition to content knowledge, faculty firmly believed that working in groups, communicating clearly and effectively, and actively participating in activities facilitated student learning of thermodynamics, quantum mechanics, and other major topics in the physical chemistry curriculum. In other words, process skills were not secondary goals to content knowledge, but rather faculty viewed them as mediating the process by which students developed their conceptual understandings of the subject matter and therefore, they were important dimensions of their goals for teaching. These faculty believed the purpose for teaching physical chemistry was to model science as inquiry, a process by which knowledge is socially constructed.
Discussion
Faculty demonstrated different beliefs about the purposes for teaching physical chemistry at the upper-division level. In some cases, faculty worked with more than one of these beliefs simultaneously (e.g., Dr Aiden). In many cases, it was possible to describe these belief statements as teacher-centered or student-centered. For example, Dr Amos described his beliefs about helping students develop conceptual knowledge of physical chemistry subject matter, but since the subject matter is quite abstract he believed it was his role to clearly communicate that knowledge to his students. The concept of transmitting information is a useful metaphor to describe this perspective and such interpretations of teacher thinking have previously been characterized as “teacher-centered” because it demonstrates a “focus only on what is happening for teachers, with students' reactions taken-for-granted” (Åkerlind, 2008, p. 634). Other faculty described more student-centered conceptions of teaching when they articulated ideas about the role of students' prior knowledge or active participation in the learning process. However, when we compared and contrasted faculty beliefs about the purposes for teaching physical chemistry within the teacher-centered/student-centered paradigm, we did not find logical patterns among the various beliefs. For example, faculty beliefs about helping students develop knowledge and skills regarding mathematical modeling practices in physical chemistry could be classified in some cases as student-centered while in other cases as teacher-centered. In other words, conceptual, epistemic, and social learning goals do not necessary align with teacher-centered or student-centered conceptions of teaching in any particular logical way. This should not be surprising as there is no theoretical basis for a connection between conceptions of teaching and beliefs about the purposes for teaching physical chemistry. But it is possible to infer conceptions of teaching through faculty statements about their beliefs and experiences related to teaching physical chemistry.Our interpretation of the similarities and differences between faculty beliefs about the purposes for teaching physical chemistry led us to conceptualize an emergent hierarchical model, as shown in Fig. 2, consisting of beliefs about conceptual, epistemic, and social learning goals. This model places beliefs about conceptual learning at the “lowest” level of the hierarchy. This should not be thought of as an unsophisticated belief about the purpose for teaching physical chemistry, but rather as the common denominator among the faculty who participated in this study. In other words, we consider it is as a baseline belief about the purpose for teaching physical chemistry. At the heart of this belief is the notion that students ought to develop robust conceptual knowledge of physical chemistry subject matters. The focus that faculty placed on helping students develop conceptual knowledge is not unprecedented. For over three decades, researchers and practitioners have been calling for a stronger focus on conceptual learning in the undergraduate physical chemistry education (e.g., Physical Chemistry Subcommittee, 1973; Society Committee on Education, 1984; Lippincott, 1988; Moore and Schwenz, 1992; Sözbilir, 2004; Zielinski and Schwenz, 2004; Ellison and Schoolcraft, 2008). These calls have spurred changes to the content and organization of the curriculum (Zielinski and Schwenz, 2004), instructional technologies used to teach the subject matter (Zielinski, 2008), and student-centered instructional strategies for delivering content and practices (Spencer and Moog, 2008). Educational research has demonstrated that many students leave formal education in physical chemistry with alternative conceptions about fundamental concepts (Gardner and Bodner, 2007; Patron, 1997; for reviews see Bain et al., 2014; Bain and Towns, 2015; Tsaparlis, 2007), thereby providing another reason to focus strongly on conceptual learning in the classroom. In fact, a recent national survey of 331 physical chemistry instructors' teaching practices and beliefs suggests that the most prominent faculty goal is to help students develop conceptual knowledge of the subject matter (Fox and Roehrig, 2015). Finally, the focus on conceptual learning is consistent with the traditional approach to science education in general in the United States, which for over half a century has worked with a general belief that the purpose of science education is for students to develop robust conceptual knowledge of science subject matters (Duschl, 2008).
 | ||
| Fig. 2 Hierarchy of beliefs about the purposes for teaching physical chemistry in upper-division courses. | ||
More nuanced beliefs about the purposes for teaching physical chemistry also emerged from our phenomenographic analysis. The next level in the hierarchy describes faculty beliefs about mathematical models and modeling practices in the physical chemistry curriculum (see Fig. 2). Some faculty believed the purpose of teaching physical chemistry in upper-division courses should focus on helping students understand the nature of mathematical modeling practices. What makes this perspective different from the exclusive focus on conceptual learning is the understanding that students experience difficulty learning about the modeling nature of physical chemistry curricula when it is not explicit in instruction. Therefore, faculty believed the purpose of teaching physical chemistry is to instruct students on the nature of mathematical modeling in the chemical sciences. At the same time, this belief is inclusive of conceptual learning goals because mathematical modeling requires one to apply their conceptual knowledge when studying and making knowledge claims about a chemical phenomenon (Gardner and Bodner, 2007). When faculty articulated this kind of focus on mathematical modeling during the interview, we believed they worked with epistemic beliefs for teaching physical chemistry because they focused on helping students understand the process by which chemical knowledge is generated and evaluated within a community.
Finally, we placed beliefs about social aspects of scientific practices at the highest level of the hierarchy because it is inclusive of the other two beliefs (see Fig. 2). Faculty who articulated beliefs about helping students develop scientific communication skills and the ability to work cooperatively in teams believed it was important to model science as inquiry, a process by which knowledge is socially constructed. Faculty described the development of communication and team skills not only as beneficial for future learning or professional development, but also as a productive medium for students to develop conceptual knowledge of the subject matter and to interact with mathematical models. We can consider these as social beliefs for teaching physical chemistry because, again, faculty focused on helping students build skill sets to help them participate in social practices that model the creation and evaluation of knowledge claims within a community.
Beliefs about the purposes for teaching physical chemistry reported in this study spanned conceptual, epistemic, and social domains of learning. Some faculty reported more inclusive beliefs that integrated conceptual, epistemic, and social aspects of science for teaching and learning in upper-division physical chemistry courses. This suggests that different faculty who teach physical chemistry may approach their teaching with different beliefs or goals, which is suggestive evidence that faculty construct different PCK for teaching physical chemistry because “[t]eachers' conceptions of the purposes for teaching particular subject matter influence their choices both of particular content to teach and of instructional activities with which to teach that content” (Grossman, 1990, p. 86). A future manuscript explores the relationship between these different beliefs and other categories of faculty PCK for teaching upper-division physical chemistry courses.
Trustworthiness of findings in qualitative research
To combat threats against the trustworthiness of the findings in this study, we gained access to participants across several educational contexts. A key factor in the transferability of the data is the representativeness of the participants such that the results can be transferable to a particular group (Krefting, 1991). While the demographics of the faculty who participated in this study may not be representative of the demographics of faculty who teach physical chemistry in the United States, faculty from several different educational contexts are represented in the sample. In other words, the results have potential to transfer across multiple educational contexts, including institution type, career stage, and class size.Another strategy to combat threats against the trustworthiness of the findings was to provide a rich description of the experiences reported by faculty. The findings in this study are presented as a description of our interpretations of faculty experiences teaching physical chemistry. Our intention was to allow the reader to come to an understanding of the experiences reported in this study based on the description and supporting data. We believe we provided sufficient data and description for the reader to make comparisons with their own situation or experiences and to make their own judgments about how well the findings fit in other contexts. When a reader is able to recognize or reinterpret the description presented in a research report to their own situation or experience, then the results are deemed trustworthy (Guba, 1981).
Threats to the validity of interpretations were reduced by triangulating data across interviews and course artifacts (Patton, 2002). The role of course artifacts in this study was important for providing supporting evidence for demarcating the three categories describing faculty beliefs about the purposes for teaching physical chemistry courses at the upper-division level. Analyzing both data sets helped us to make the interpretation that some beliefs are more inclusive than others. Consider the case of Dr Aiden. In his syllabus, he listed several goals (bullet points) related to conceptual learning and process skills with no indication of relative importance besides the relative grade distribution among exams and group work. However, as we demonstrated in the Findings section, we gained insight into the relationship between those two different goals by looking at the interview data.
We did not find any disconfirming evidence across the interview transcripts and course syllabi. We believe one reason to help explain this observation is that two out of the eight participants who volunteered their course syllabi for this analysis did not include statements about course goals or objectives. Instead, these documents consisted mainly of course logistics (i.e. instructor/TA info, lecture times, office hour schedule, required/recommended text, exam dates, grading) and the lecture schedule. This suggests that not all faculty include course objectives or statements of teaching philosophy in their syllabi. Two out of eight participants who volunteered course syllabi included explicit goal statements in their course syllabi. These two participants, plus four others included broader statements of their philosophy for teaching physical chemistry. These were rich sources to infer faculty beliefs about the purposes for teaching physical chemistry, but they did not provide as much depth as the semi-structured interviews.
Limitations
The analytical process of making interpretations of faculty experiences based on what was said during interviews and stated in course artifacts may have generated only a subset of beliefs about the purposes for teaching physical chemistry at the undergraduate level. We believe the interview-based methodology used in this study does not guarantee a full articulation of beliefs about the purposes for teaching physical chemistry at the upper-division level. This does not make the findings less valid, but rather it offers chemistry education researchers a starting point in further exploring faculty beliefs about teaching physical chemistry. This study does not attempt to account for teaching practices, which are the practices faculty actually experience in the classroom, rather than what they say they do in the classroom. The latter data provides a starting point to better understand teacher thinking in the context of upper-division chemistry courses, which can be further articulated in future studies on classroom practices.Implications
One implication of the results of this study for chemistry education at the college and university level is to account for the broadened understanding of what science is, how it is practiced, and how it is learned in formal educational settings because our best understanding of how science works is that it “takes place in complex settings of cognitive, epistemic, and social practices” (Duschl, 2008, p. 270). The implication of this work for the way faculty think about teaching upper-division physical chemistry courses is to expand their awareness for the potential of variation in the purposes for teaching physical chemistry education. If faculty take this line of reasoning seriously, they should conceptualize teaching in terms of three integrated domains: “the conceptual structures and cognitive processes used when reasoning scientifically, the epistemic frameworks used when developing and evaluating scientific knowledge, and the social processes and contexts that shape how knowledge is communicated, represented, argued, and debated” (p. 277). This does not mean that faculty ought to adopt new perspectives for teaching physical chemistry, but rather the chemistry education community benefits from an expanded awareness of the different perspectives, the assumptions guiding each perspective, the implications of those perspectives for student learning and departmental outcomes, and how those beliefs about teaching physical chemistry would be supported or hindered in a particular department or institution. It was our intention to provide a rich description of the variation in beliefs about the purposes for teaching physical chemistry for faculty to use as a resource in that development of their teaching philosophy.One approach for faculty to begin the process of expanding their awareness of different purposes or goals for teaching physical chemistry is to engage in reflective journaling about their beliefs about higher education, teaching in general, teaching upper-division physical chemistry courses specifically, and the relationship between learning and teaching (Entwistle and Walker, 2002). Another way for faculty to expand their awareness of different purposes for teaching physical chemistry is to establish a dialogue with other physical chemistry instructors about their philosophy for teaching physical chemistry. Making philosophies accessible for others in a scholarly setting could be a productive way to refine and expand one's beliefs about teaching and learning. Initiating a dialogue with colleagues within or across institutions, especially colleagues who have dissimilar beliefs, would be a big step in clarifying beliefs about teaching physical chemistry and developing an understanding of alternative perspectives. If faculty are motivated enough to engage in this kind of dialogue, then they may benefit from participating in existing communities that promote advancements in physical chemistry education. Such communities exist and are usually present and organized at the Biennial Conference on Chemical Education (BCCE) and other technical chemistry conferences.
One implication for future research is the continued study of faculty beliefs and teaching practices in upper-division chemistry courses in order to further understand how the teaching and learning of chemistry works in these settings (Towns, 2013). The findings from this study offers chemistry education researchers a starting point to further explore faculty beliefs about teaching physical chemistry and other dimensions of their PCK using alternative methodologies, such as recruiting faculty to participate in reflective tasks including ‘card sorting tasks’ or ‘concept mapping’ their own PCK (Baxter and Lederman, 1999), reflections on a specific lesson (Lee and Luft, 2008), and multi-method evaluations of teacher thinking (Dinham, 2002). For example, comparing and contrasting faculty beliefs-in-action through classroom observations and observation protocol to espoused beliefs would be one way to validate or disconfirm the interpretations arrived at in this study and offer new insights into faculty thinking about teaching physical chemistry.
The findings from this study have further implications for curriculum and pedagogical developments in the context of upper-division physical chemistry courses. The phenomenographic analysis reported here suggests that the artificial demarcations between “conceptual” and “mathematical” learning in physical chemistry does not capture nuances in faculty beliefs about the purposes for teaching physical chemistry. Instead, a new and potentially useful perspective to approach curriculum and pedagogical developments for physical chemistry education would be to focus on conceptual, epistemic, and social learning goals. In other words, research and development should consider faculty beliefs about helping students develop content knowledge, disciplinary practices (e.g., mathematical modeling), and process skills (e.g., scientific communication skills). For example, a research-based assessment instrument that helps faculty to easily and reliably measure students' mathematical modeling practices could be quite useful for some faculty who are interested in teaching and assessing mathematical modeling practices. As another example, an educational workshop that helps faculty develop pedagogical skills to improve the quality of student-driven argumentation in the classroom would be quite useful for some faculty who are interested in teaching and assessing scientific communication practices. The findings from this study suggest that are many opportunities to support faculty in achieving their goals for teaching physical chemistry. At the same time, it suggests there may be potential barriers if new curricular or pedagogical developments do not align with a faculty member's beliefs about conceptual, epistemic, or social learning in physical chemistry.
Conclusions
The phenomenographic analysis reported in this paper provided a rich description of the similarities and differences in beliefs about the purposes for teaching physical chemistry that emerged from interviews with faculty. While prior phenomenographic research on teacher thinking in higher education has found other ways to characterize teacher thinking (and approaches), such as the teacher-centered/student-centered conceptions paradigm, this study found an alternative model to conceptualize differences in teacher thinking about physical chemistry education. We believe this was an artifact of our discipline-based study because discipline-based ideas related to teaching and learning of physical chemistry subject matter was the focus of our conversations with participants during the interviews. For example, discussions about reasoning using the particulate nature of matter dominated faculty beliefs about conceptual learning goals for students, discussions about mathematical modeling practices were a big focus of what we classified as beliefs about epistemic learning, and discussions about scientific communication or working collaboratively were a big focus of what we classified as beliefs about social learning. We believe that it is likely this hierarchical model is useful to conceptualize teacher thinking in other chemistry and STEM contexts as well; however, we only claim to have observed it within a community of faculty who teach or have taught upper-division physical chemistry courses.Appendix 1. Interview protocol
1. How would you describe your approach to teaching [course name]?• What are your goals for the course? Can you give me an example? How do you achieve that goal as an instructor?
• (Use a reported lesson, topic, goal, or instructional practice as an example to contextualize later questions.)
2. What happens during a typical class that you teach?
• What do you do during a typical class?
• What are you trying to achieve? How do you do that?
• (If that does not work try) I'm trying to get a picture of you in the classroom and your actions. What are you doing to…?
• What are students doing? How do you see yourself helping students learn? What do you believe are the roles of students during class time? Outside of class? Why?
3. Ok, we've talked about how you approach your teaching in physical chemistry. Let's switch gears and talk about student learning. I'd like to preface this next question with a statement. As physical chemists, we often work with models to make sense of out things we cannot interact with directly. Can you describe to me the model of student learning that you use when teaching [course name]?
• (If that doesn't work try) How do you believe students are learning in your course?
• Tell me how you see yourself helping students learn the concepts of… in [course name].
• Is there anything else you wish for your students to achieve in your course? Why is that? How do you see yourself helping them achieve that?
4. What changes, if any, have your colleagues made to their physical chemistry courses that you are aware of? What about colleagues at other institutions?
• What effect do you believe these have on student learning?
• How have these changes impacted your approach to teaching physical chemistry, if at all?
5. What changes, if any, have you made to your physical chemistry course in the last five years? Why?
• What effect do you believe these have on student learning? How do you know this?
6. What do you think the role of physical chemistry courses is in the near future? Ten years from now.
References
- AAAS, (2013), Describing and Measuring Undergraduate STEM Teaching Practices: A Report from a National Meeting on the Measurement of Undergraduate Science, Technology, Engineering, and Mathematics (STEM) Teaching, retrieved from http://ccliconference.org/files/2013/11/Measuring-STEM-Teaching-Practices.pdf.
- Åkerlind G. S., (2004), A new dimension to understanding university teaching, Teaching in Higher Education, 9(3), 363–375.
- Åkerlind G. S., (2008), A phenomenographic approach to developing academics' understanding of the nature of teaching and learning, Teaching in Higher Education, 13(6), 633–644.
- Association of American Universities, (2011), Undergraduate STEM Initiative, retrieved from http://www.aau.edu/policy/article.aspx?id=12588.
- Austin A. E., (2011), Promoting evidence-based change in undergraduate science education, paper presented at the Fourth Committee Meeting on Status, Contributions, and Future Directions of Discipline-Based Education Research.
- Bain K. and Towns M., (2015), A review of research on the teaching and learning of chemical kinetics, Chem. Educ. Res. Pract. (submitted).
- Bain K., Moon A., Mack M. and Towns M., (2014), A review of research on the teaching and learning of thermodynamics at the university level, Chem. Educ. Res. Pract., 15(3), 320–335.
- Baxter J. and Lederman N., (1999), Assessment and Measurement of Pedagogical Content Knowledge, in Gess-Newsome J. and Lederman N. (ed.), Examining Pedagogical Content Knowledge, Netherlands: Springer, pp. 147–161.
- Boyer Commission on Educating Undergraduates in the Research University., (1998), Reinventing undergraduate education: a blueprint for America's research universities, Menlo Park, CA: Carnegie Foundation for the Advancement of Teaching.
- Bransford J. D., Brown A. L. and Cocking R. R. (ed.), (2000), How people learn, Washington, DC: National Academy Press.
- Calderhead J., (1996), Teachers: Beliefs and knowledge, in Berliner D. C. and Calfee R. C. (ed.), Handbook of educational psychology, London, England: Prentice Hall International, pp. 709–725.
- Chi M. T. H., Feltovich P. J. and Glaser R., (1981), Categorization and representation of physics problems by experts and novices, Cognitive Sci., 5(2), 121–152.
- Clark C. M. and Peterson P. L., (1986), Teachers' thought processes, in Wittrock M. C. (ed.), Handbook of research on teaching, New York: Macmillan, 3rd edn, pp. 255–296.
- Clark C. M. and Yinger R. J., (1987), Teacher Planning, in Calderhead J. (ed.), Exploring teachers' thinking, London: Cassell, pp. 84–103.
- Committee on Professional Training, (2008), Undergraduate professional education in chemistry: ACS guidelines and evaluation procedures for Bachelor's degree programs [Physical Chemistry Supplement], Washington, DC: American Chemical Society.
- Committee on Professional Training, (2015), Undergraduate Professional Education in Chemistry: ACS Guidelines and Evaluation Procedures for Bachelor's Degree Programs, Washington, DC: American Chemical Society.
- Committee on Prospering in the Global Economy of the 21st Century (U.S.), and Committee on Science, Engineering, and Public Policy (U.S.), (2007), Rising Above the Gathering Storm: Energizing and Employing America for a Brighter Economic Future, Washington, DC: The National Academies Press.
- Dancy M. and Henderson C., (2007), Framework for articulating instructional practices and conceptions, Physical Review Special Topics-Physics Education Research, 3, 010103.
- Dinham S., (2002), Use of Multiple Methods in Research on College Teachers, in Hativa N. and Goodyear P. (ed.), Teacher Thinking, Beliefs and Knowledge in Higher Education, Netherlands: Springer, pp. 321–334.
- Duschl R., (2008), Science education in three-part harmony: balancing conceptual, epistemic, and social learning goals, Rev. Res. Educ., 32(1), 268–291.
- Ellison M. and Schoolcraft T. (ed.), (2008), Advances in teaching physical chemistry, Washington, DC: American Chemical Society, vol. 973.
- Entwistle N. and Walker P., (2002), Strategic alertness and expanded awareness within sophisticated conceptions of teaching, Instr. Sci., 28(5), 335–361.
- Fairweather J., (2008), Linking evidence and promising practices in science, technology, engineering, and mathematics (STEM) undergraduate education: a status report for the National Academies National Research Council Board of Science Education.
- Fox L. J. and Roehrig G. H., (2015), Nationwide Survey of the Undergraduate Physical Chemistry Course, J. Chem. Educ., 92(9), 1456–1465.
- Friedrichsen P., Van Driel J. H. and Abell S. K., (2011), Taking a closer look at science teaching orientations, Sci. Educ., 95(2), 358–376.
- Gardner D. E. and Bodner G. M., (2007), The Existence of a Problem-Solving Mindset Among Students Taking Quantum Mechanics and its Implications, in Ellison M. and Schoolcraft T. (ed.), Advances in Teaching Physical Chemistry, Washington, DC: American Chemical Society, pp. 155–173.
- Gess-Newsome J., (1999), Secondary teachers' knowledge and beliefs about subject matter and their impact on instruction, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge, Springer, pp. 51–94.
- Gess-Newsome J., Southerland S. A., Johnston A. and Woodbury S., (2003), Educational reform, personal practical theories, and dissatisfaction: the anatomy of change in college science teaching, Am. Educ. Res. J., 40(3), 731–767.
- González C., (2011), Extending research on ‘conceptions of teaching’: commonalities and differences in recent investigations, Teaching in Higher Education, 16(1), 65–80.
- Goodenough W. H., (1963), Cooperation in change: an anthropological approach to community development, Russell Sage Foundation.
- Goodyear P. and Hativa N., (2002), Introduction, in Hativa N. and Goodyear P. (ed.), Teacher thinking, beliefs and knowledge in higher education, Netherlands: Springer, pp. 1–14.
- Green T. F., (1971), The Activities of Teaching, New York: McGraw-Hill.
- Grossman P. L., (1990), The making of a teacher: teacher knowledge and teacher education, Teachers College Press, Teachers College, Columbia University.
- Guba E. G., (1981), Criteria for assessing the trustworthiness of naturalistic inquiries, ECTJ, 29(2), 75–91.
- Henderson C. and Dancy M., (2007), Barriers to use of research-based instructional strategies: the influence of both individual and situational characteristics, Physical Review Special Topics - Physics Education Research, 3, 020102.
- Henderson C. and Dancy M., (2009), Impact of physics education research on the teaching of introductory quantitative physics in the United States, Physical Review Special Topics-Physics Education Research, 5(2), 020107.
- Henderson C. and Dancy M., (2011), Increasing the impact and diffusion of STEM education innovations, retrieved from http://create4stem.msu.edu/sites/default/files/discussions/attachments/HendersonandDancy10-20-2010.pdf.
- Henderson C., Beach A. and Finkelstein N., (2011), Facilitating change in undergraduate STEM instructional practices: an analytic review of the literature, J. Res. Sci. Teach., 48(8), 952–984.
- Henderson C., Dancy M. and Niewiadomska-Bugaj M., (2012), Use of research-based instructional strategies in introductory physics: where do faculty leave the innovation-decision process? Physical Review Special Topics-Physics Education Research, 8(2), 020104.
- Kember D., (1997), A reconceptualisation of the research into university academics' conceptions of teaching, Learn. Instr., 7(3), 255–275.
- King N. and Horrocks C., (2010), Interviews in qualitative research, Los Angeles: Sage.
- Krefting L., (1991), Rigor in qualitative research: the assessment of trustworthiness, Am. J. Occup. Ther., 45(3), 214–222.
- Kuzel A. J., (1992), Sampling in qualitative inquiry, in Crabtree B. F. and Miller W. L. (ed.), Doing Qualitative Research, Thousand Oaks, CA: Sage Publications, Inc., pp. 31–44.
- Larkin J., McDermott J., Simon D. P. and Simon H. A., (1980), Expert and Novice Performance in Solving Physics Problems, Science, 208(4450), 1335–1342.
- Lee E. and Luft J. A., (2008), Experienced Secondary Science Teachers' Representation of Pedagogical Content Knowledge, Int. J. Sci. Educ., 30(10), 1343–1363.
- Lippincott W. T. (ed.), (1988), Essays in Physical Chemistry, Washington, DC: American Chemical Society.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome, J. and Lederman, N. (ed.), Examining pedagogical content knowledge, Springer, pp. 95–132.
- Martin E., Prosser M., Trigwell K., Ramsden P. and Benjamin J., (2000), What university teachers teach and how they teach it, Instr. Sci., 28(5), 387–412.
- Marton F., (1981), Phenomenography—describing conceptions of the world around us, Instr. Sci., 10(2), 177–200.
- Marton F., (1986), Phenomenography—A Research Approach to Investigating Different Understandings of Reality, Journal of Thought, 21(3), 28–49.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: an expanded sourcebook, Thousand Oaks: Sage.
- Miller M., (2007), Pedagogical Content Knowledge, in Bodner G. and Orgill M. (ed.), Theoretical Frameworks for Research in Chemistry/Science Education, Uper Saddle River, NJ: Pearson, pp. 86–106.
- Moog R. S., Creegan F. J., Hanson D. M., Spencer J. N. and Straumanis A. R., (2006), Process-Oriented Guided Inquiry Learning: POGIL and POGIL Project, Metropolitan Universities, 17(4), 41–52.
- Moore R. J. and Schwenz R. W., (1992), The problem with P. Chem., J. Chem. Educ., 69(12), 1001.
- Mortimer R. G., (2008), Decisions in the Physical Chemistry Course Advances in Teaching Physical Chemistry, J. Am. Chem. Soc., 973, 28–39.
- National Research Council, (2012a), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas, Washington, DC: The National Academies Press.
- National Research Council, (2012b), Discipline-Based Education Research: Understanding and Improving Learning in Undergraduate Science and Engineering, Washington, DC: The National Academies Press.
- Orgill M., (2007), Phenomenography, in Bodner G. and Orgill M. (ed.), Theoretical Frameworks for Research in Chemistry/Science Education, Upper Saddle River, NJ: Pearson, pp. 122–131.
- Orgill M., Bussey T. J. and Bodner G. M., (2015), Biochemistry instructors' perceptions of analogies and their classroom use, Chem. Educ. Res. Pract., 16, 731–746.
- Padilla K. and Van Driel J., (2011), The relationships between PCK components: The case of quantum chemistry professors. Chem. Educ. Res. Pract., 12(3), 367–378.
- Patron F., (1997), Conceptual understanding of thermodynamics: A Study of undergraduate and graduate students (Unpublished doctoral dissertation), Purdue University, West Lafayette, Indiana.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, SAGE Publications.
- Physical Chemistry Subcommittee, (1973), Report of the Physical Chemistry Subcommittee of the Curriculum Committee, J. Chem. Educ., 50(9), 612.
- President's Council of Advisors on Science and Technology, (2012), Engage to Excel: Producing One Million Additional College Graduates with Degrees in Science, Technology, Engineering, and Mathematics, retrieved from http://https://www.whitehouse.gov/sites/default/files/microsites/ostp/pcast-executive-report-final_2-13-12.pdf.
- Prosser M., Trigwell K. and Taylor P., (1994), A phenomenographic study of academics' conceptions of science learning and teaching, Learn. Instr., 4(3), 217–231.
- QSR International Pty Ltd, (2012), NVivo qualitative data analysis software (Version 10, edn).
- Richardson V., (1996), The role of attitudes and beliefs in learning to teach, Handbook of research on teacher education, vol. 2, pp. 102–119.
- Saldaña J., (2009), The coding manual for qualitative researchers, London: Sage.
- Samuelowicz K. and Bain J., (1992), Conceptions of teaching held by academic teachers, Higher Education, 24(1), 93–111.
- Schwenz R. W. and Moore R. J. (ed.), (1993), Physical Chemistry: Developing a Dynamic Curriculum, Washington, DC: American Chemical Society.
- Seymour E. and Hewitt N. M., (1997), Talking about leaving: why undergraduates leave the sciences, Boulder, CO: Westview Press, vol. 12.
- Shavelson R. J. and Stern P., (1981), Research on Teachers' Pedagogical Thoughts, Judgments, Decisions, and Behavior, Rev. Educ. Res., 51(4), 455–498.
- Shulman L. S., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 4–14.
- Shulman L. S., (1987), Knowledge and teaching: foundations of the new reform. Harvard Educational Review, 57(1), 1-23.
- Society Committee on Education, (1984), Content of Undergraduate Physical Chemistry Courses, Washington DC: American Chemical Society.
- Sözbilir M., (2004), What makes physical chemistry difficult? Perceptions of Turkish chemistry undergraduates and lecturers, J. Chem. Educ., 81(4), 573.
- Sözbilir M. and Bennett J. M., (2007), A study of Turkish chemistry undergraduates' understandings of entropy, J. Chem. Educ., 84(7), 1204–1208.
- Spencer J. N. and Moog R. S., (2008), POGIL in the Physical Chemistry Classroom, in Moog R. S. and Spencer J. N. (ed.), Process Oriented Guided Inquiry Learning (POGIL), Washington, DC: American Chemical Society, pp. 148–156.
- Strauss A. and Corbin J., (1998), Basics of qualitative research: procedures and techniques for developing grounded theory, Thousand Oaks, CA: Sage.
- Tobias S., (1990), They're Not Dumb, They're Different: Stalking the Second Tier, Tucson, AZ: Research Corporation.
- Towns M., (2013), New Guidelines for Chemistry Education Research Manuscripts and Future Directions of the Field, J. Chem. Educ., 90(9), 1107–1108.
- Tsaparlis G., (2007), Teaching and learning physical chemistry: A review of educational research, in Ellison M. and Schoolcraft T. (ed.), Advances in Teaching Physical Chemistry, Washington, DC: American Chemical Society, pp. 75–112.
- Van Hecke G. R., (2008), What to teach in physical chemistry: is there a single answer? in Ellison M. D. and Schoolcraft T. A. (ed.), Advances in Teaching Physical Chemistry, Washington, DC: American Chemical Society, vol. 973, pp. 11–27.
- Zielinski T. J., (2008), Physical Chemistry Curriculum: Into the Future with Digital Technology, in Ellison M. and Schoolcraft T. (ed.), Advances in Teaching Physical Chemistry, Washington, DC: American Chemical Society, vol. 973, pp. 177–193.
- Zielinski T. J. and Schwenz R. W., (2004), Physical chemistry: a curriculum for 2004 and beyond, Chem. Educ., 9, 108–121.
| This journal is © The Royal Society of Chemistry 2016 |
