A greener process for flow C–H chlorination of cyclic alkanes using in situ generation and on-site consumption of chlorine gas†
Takahide
Fukuyama
*,
Masashi
Tokizane
,
Akihiro
Matsui
and
Ilhyong
Ryu
*
Department of Chemistry, Graduate School of Science, Osaka Prefecture University, Sakai, Osaka 599-8531, Japan. E-mail: fukuyama@c.s.osakafu-u.ac.jp; ryu@c.s.osakafu-u.ac.jp
First published on 10th October 2016
Abstract
Photo-chlorination of C–H bonds was investigated by using a photo microreactor and gaseous chlorine in situ generated from HCl and NaOCl. Under photoirradiation using a black light (15 W, 352 nm), chlorination of hydrocarbons proceeded smoothly within 1 min residence time to give chlorinated products in good yields.
Chlorinated organic compounds are contained in a variety of natural products and biologically active compounds, and are also frequently employed as basic substrates for C–C bond forming reactions in organic synthesis, which involve Grignard reactions, radical reactions, and cross-coupling reactions, just to name a few.1 Photo-induced C–H chlorination using chlorine gas is among the straightforward and standard methods to prepare organochlorine compounds.2 The use of highly toxic and corrosive chlorine gas requires very careful handling for safety. We and others previously reported microflow photo radical C–H chlorination using chlorine gas,3–6 which allows for the use of the minimum excess amount of chlorine gas compared with batch chlorination because of a little dead volume of the microflow system.7–9 However, even with the flow system, we are still not freed from the use of a chlorine gas cylinder, which requires special caution and is not useful for laboratory experiments.
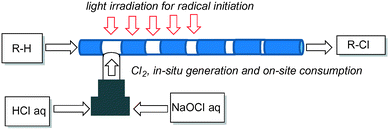
If chlorine gas is generated in situ and consumed quickly on-site, flow chlorination would be much safer as in the case of flow carbonylation using a CO precursor.10 Herein we report on flow C–H chlorination using in situ generation of molecular chlorine from aqueous hydrochloric acid and sodium hypochlorite.11 We found that the flow chlorination proceeded quickly by the use of a glass-made flow reactor and a 15 W black light as a light source (Scheme 1).9 It should be noted that, concurrently with this work, Cantillo, Kappe, and co-workers reported the flow chlorination of hydrosilanes, secondary alcohols, and toluenes based on a similar in situ concept, in which they used a FEP tubing reactor and a 75 W Hg lamp as a light source.12
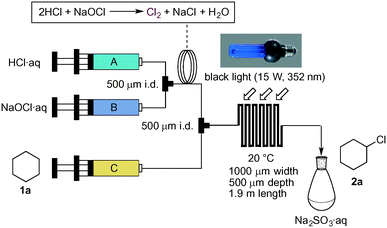
We started to investigate the photochlorination of cyclohexane (1a) with in situ generated Cl2 as a model reaction (Scheme 2). We used a glass-made flow reactor, Mikroglas Dwel Device (Foturan glass, 1000 μm width, 500 μm depth, 1.9 m length, total hold-up volume: 0.95 mL), for this study, which was connected with two PEEK-made T-mixers (500 μm i.d.), one of which is to generate molecular chlorine and the other is to mix the generated chlorine gas with 1a.
Thus, hydrochloric acid (2 M) and sodium hypochlorite solution (1.9 M) were introduced into the first micromixer through gas-tight syringes A and B driven by a syringe pump. Right after mixing, plug flow was observed in the PTFE tube (1 mm i.d. × 1 m), which was a good sign of chlorine gas generation. This plug flow was guided into the second mixer, to mix with cyclohexane coming through syringe C. The resulting mixed solution was introduced into the photo-microreactor and exposed to natural room light or black light (15 W, 352 nm peak wavelength). The reaction mixture, exiting through the PTFE tube, was quenched with 10% aqueous Na2SO3. The organic phase could be easily separated from the aqueous phase, and the product yield was determined by GC analysis. When a mixed solution of cyclohexane (1a)/HCl/NaOCl (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was exposed to natural room light in flow with a residence time of 19 min, 74% yield of chlorocyclohexane (2a) was obtained (Table 1, entry 1). With a highly shortened residence time of 1 min, the power of the room light of the ceiling was totally insufficient (entry 2); however irradiation using a 15 W black light gave 2a in 94% yield (entry 3). The reaction with 1 equiv. of HCl resulted in the formation of lower yields of 2a (entries 4 and 5).
1) was exposed to natural room light in flow with a residence time of 19 min, 74% yield of chlorocyclohexane (2a) was obtained (Table 1, entry 1). With a highly shortened residence time of 1 min, the power of the room light of the ceiling was totally insufficient (entry 2); however irradiation using a 15 W black light gave 2a in 94% yield (entry 3). The reaction with 1 equiv. of HCl resulted in the formation of lower yields of 2a (entries 4 and 5).
| Entry | Light source | HCl (equiv.) | Residence time (min) | Yieldb |
|---|---|---|---|---|
| a Conditions: 1a (19 equiv.), 2 M HCl (1 or 2 equiv.), and 1.9 M NaOCl (1 equiv.), Mikroglas Dwel Device, natural room light or 15 W black light (peak wavelength: 352 nm). b Yields were determined by GC analysis by comparing the peak area with that of the standard solution containing chlorocyclohexane (2a). | ||||
| 1 | Natural room light | 2 | 19 | 74% |
| 2 | Natural room light | 2 | 1 | 11% |
| 3 | Black light (15 W) | 2 | 1 | 94% |
| 4 | Black light (15 W) | 1 | 1 | 40% |
| 5 | Black light (15 W) | 1 | 3 | 53% |
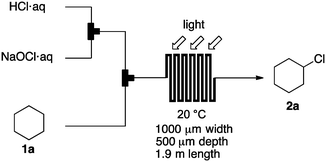
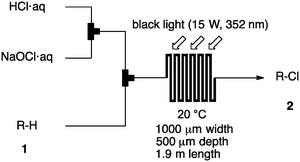
Photochlorination of some other C–H bonds by in situ generated Cl2 was examined using a black light as a light source (Table 2). As in the case of cyclohexane, cycloalkanes having different ring sizes 1b–1d gave the corresponding chlorinated cycloalkanes 2b–2d in good to high yields (entries 2–4). In the flow chlorination of toluene (1e), we confronted irregular plug flow, which often caused an uncontrolled shorter residence time by pushing the solution phase. The use of a back pressure tube (narrow inner diameter tube; 250 μm i.d. × 10 cm length) as an outlet tube circumvented this problem and benzyl chloride (2e) was obtained in 72% yield with 0.5 min residence time (entry 5). In the case of ethylbenzene (1f), chlorination occurred mainly on the benzylic position to give 2f (entry 6). In this case β-phenethyl chloride (2f′) was also formed as a side product (2f/2f′ = 87/13). This ratio corresponds to a result of photo-chlorination of ethylbenzene using sulfuryl chloride as a chlorinating reagent in a batch system (85/15).13 The reaction of cyclohexanone (1g) gave 2-chlorocyclohexanone (2g) in 60% yield (entry 7). Since under the dark conditions the reaction gave a similar result, we think that this chlorination proceeds via an ionic mechanism.
| a Conditions: 1 (19 equiv.), 2 M HCl (1 or 2 equiv.), and 1.9 M NaOCl (1 equiv.), Mikroglas Dwel Device, 15 W black light (peak wavelength: 352 nm). b Yields were determined by GC analysis by comparing the peak area with that of the standard solution containing an authentic sample. c A back pressure tube (0.25 mm i.d. × 10 cm length) was employed. | ||||
|---|---|---|---|---|
| Entry | Substrate | Residence time (min) | Product | Yieldb |
| 1 |
 1a
1a
|
1 |
 2a
2a
|
94% |
| 2 |
 1b
1b
|
1 |
 2b
2b
|
76% |
| 3 |
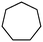 1c
1c
|
1 |
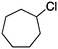 2c
2c
|
87% |
| 4 |
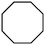 1d
1d
|
1 |
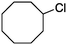 2c
2c
|
69% |
| 5c |
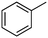 1e
1e
|
0.5 |
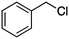 2d
2d
|
72% |
| 6c |
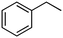 1f
1f
|
0.5 |
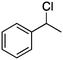 2f
2f
|
59% |
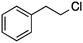 2f′
2f′
|
9% | |||
| 7 |
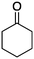 1g
1g
|
1 |
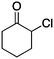 2g
2g
|
60% |
Conclusions
In this work we have reported that C–H chlorination of cyclic alkanes, alkylbenzenes, and cyclohexanone is successfully carried out by a consecutive flow protocol consisting of in situ generation of chlorine gas and on-site reaction of chlorine gas. The flow chlorination was completed within 1 min, when we used a combination of a glass-plate reactor and a 15 W black light as a light source. This work and the preceding Kappe's work published in this journal12 demonstrate well that the flow chlorination protocol is now freed of the use of a chlorine gas cylinder and the safe execution of chlorination by molecular chlorine is possible in laboratories.Acknowledgements
This work was supported by the Grant-in-Aid for Scientific Research (A) (no. 26248031) from the JSPS and Scientific Research on Innovative Areas 2707 Middle molecular strategy (no. 15H05850) from the MEXT.Notes and references
-
Halides, Pseudo-Halides and Azides in PATAI'S Chemistry of Functional Group, ed. S. Patai and Z. Rappoport, John Wiely & Sons Inc, Chichester, 1983, vol. 1 and 2 Search PubMed
.
-
(a) K. U. Ingold, J. Lusztyk and K. D. Raner, Acc. Chem. Res., 1990, 23, 219 CrossRef CAS
; (b) B. Bletcher, N. K. Suleman and J. M. Tanko, J. Am. Chem. Soc., 1998, 120, 11839 CrossRef
; (c) N. Sun and K. J. Klabunde, J. Am. Chem. Soc., 1999, 121, 5587 CrossRef CAS
.
- H. Matsubara, Y. Hino, M. Tokizane and I. Ryu, Chem. Eng. J., 2011, 167, 567 CrossRef CAS
.
- H. Ehrich, D. Linke, K. Morgenschweis, M. Baerns and K. Jahnisch, Chimia, 2002, 56, 647 CrossRef CAS
.
- For a review on halogenation using a flow microreactor, see: P. Löb, H. Löwe and V. Hessel, J. Fluorine Chem., 2004, 125, 167 CrossRef
.
- For a review on radical reaction using a flow microreactor, see: T. Fukuyama and I. Ryu, Radical Chemistry by Using Flow Microreactor Technology, in Encyclopedia of Radicals in Chemistry, Biology, and Materials, ed. A. Studer and C. Chatgilialoglu, Wiley, Chichester, 2012, vol. 2, pp. 1243–1258 Search PubMed
.
- For selected reviews on microflow chemistry, see:
(a)
T. Wirth, Microreactors in Organic Synthesis and Catalysis, Wiley-VCH, Weinheim, 2013 Search PubMed
; (b) V. Hessel, A. Renken, J. C. Schouten and J. Yoshida, Micro Process Engineering, Wiley-VCH, Weinheim, 2009 Search PubMed
; (c) S. Born, E. O'Neal and K. F. Jensen, Organic Synthesis in Small Scale Continuous Flow: Flow Chemistry, In Comprehensive Organic Synthesis, ed. P. Knochel and G. A. Molander, Elsevier, Oxford, 2nd edn, 2014, vol. 9, pp. 54–93 Search PubMed
; (d) B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef CAS PubMed
; (e) D. Webb and T. F. Jamison, Chem. Sci., 2010, 1, 675 RSC
; (f) S. Suga, D. Yamada and J. Yoshida, Chem. Lett., 2010, 39, 404 CrossRef CAS
; (g) J. Wegner, S. Ceylan and A. Kirschning, Adv. Synth. Catal., 2012, 354, 17 CrossRef CAS
; (h) S. V. Ley, Chem. Rec., 2012, 12, 378 CrossRef CAS PubMed
; (i) C. Wiles and P. Watts, Green Chem., 2012, 14, 38 RSC
; (j) D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384 CrossRef CAS PubMed
; (k) S. G. Newman and K. F. Jensen, Green Chem., 2013, 15, 1456 RSC
; (l) T. Fukuyama, M. T. Rahman, M. Sato and I. Ryu, Synlett, 2008, 151 CAS
; (m) T. Fukuyama, T. Totoki and I. Ryu, Green Chem., 2014, 16, 2042 RSC
; (n) B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688 CrossRef CAS PubMed
; (o) R. Porta, M. Benaglia and A. Puglisi, Org. Process Res. Dev., 2016, 20, 2 CrossRef CAS
.
- For recent examples of photoreactions using microreactors, see:
(a) S. Aida, K. Terao, Y. Nishiyama, K. Kakiuchi and M. Oelgemöller, Tetrahedron Lett., 2012, 53, 5578 CrossRef CAS
; (b) R. Telmesani, S. H. Park, T. Lynch-Colameta and A. B. Beeler, Angew. Chem., Int. Ed., 2015, 54, 11521 CrossRef CAS PubMed
; (c) M. Bumann and I. R. Baxendale, React. Chem. Eng., 2016, 1, 147 RSC
; (d) Y. Su, K. P. L. Kuijpers, N. König, M. Shang, V. Hessel and T. Noël, Chem. – Eur. J., 2016, 22, 12295 CrossRef CAS PubMed
; (e) D. Chandrasekhar, S. Borra, J. B. Nanubolu and R. A. Maurya, Org. Lett., 2016, 18, 2974 CrossRef CAS PubMed
; (f) M. Bergami, S. Protti, D. Ravelli and M. Fagnoni, Adv. Synth. Catal., 2016, 358, 1164 CrossRef CAS
; (g) Y. Nishiyama, H. Tanimoto, T. Morimoto and K. Kakiuchi, Org. Process Res. Dev., 2016, 20, 1626 CrossRef
; (h) For reviews, see: Y. Matsushita, T. Ichimura, N. Ohba, S. Kumada, K. Sakeda, T. Suzuki, H. Tanibata and T. Murata, Pure Appl. Chem., 2007, 79, 1959 CrossRef CAS
; (i) M. Oelgemöller, Chem. Eng. Technol., 2012, 35, 1144 CrossRef
; (j) D. Cambié, C. Battecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276 CrossRef PubMed
.
- For flow photoreactions irradiated using a black light, see:
(a) A. Sugimoto, T. Fukuyama, Y. Sumino, M. Takagi and I. Ryu, Tetrahedron Lett., 2006, 47, 6197 CrossRef CAS
; (b) A. Sugimoto, T. Fukuyama, Y. Sumino, M. Takagi and I. Ryu, Tetrahedron, 2009, 65, 1593 CrossRef CAS
; (c) T. Fukuyama, Y. Kajihara, Y. Hino and I. Ryu, J. Flow Chem., 2011, 1, 40 CrossRef CAS
; (d) Y. Manabe, Y. Kitawaki, M. Nagasaki, K. Fukase, H. Matsubara, Y. Hino, T. Fukuyama and I. Ryu, Chem. – Eur. J., 2014, 20, 12750 CrossRef CAS PubMed
. Also see: ref. 3.
- For microflow carbonylation with in situ generated CO, see: C. Brancour, T. Fukuyama, Y. Mukai, T. Skrydstrup and I. Ryu, Org. Lett., 2013, 15, 2794 CrossRef CAS PubMed
. Also see a review 7m.
-
(a) For reactions using Cl2 gas generated from NaOCl and HCl in a batch reaction system, see: S. W. Wright and K. N. Hallstrom, J. Org. Chem., 2006, 71, 1080 CrossRef CAS PubMed
; (b) X.-F. Zhao and C. Zhang, Synthesis, 2007, 4, 551 Search PubMed
.
- F. J. Strauss, D. Cantillo, J. Guerra and C. O. Kappe, React. Chem. Eng., 2016, 1, 472 CAS
.
- L. Delaude and P. Laszlo, J. Org. Chem., 1990, 55, 5260 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedure. See DOI: 10.1039/c6re00159a |
| This journal is © The Royal Society of Chemistry 2016 |