A novel rosamine-based fluorescent probe for bisulfite in aqueous solution†
Di Zhang*a,
Liu Wenyab,
Keke Chenb,
Junye Chengb,
Yufen Zhaobc and
Yong Ye*bc
aInstitute of Agricultural Quality Standards and Testing Technology, Henan Academy of Agricultural Sciences, Zhengzhou, China. E-mail: pandy811@163.com
bPhosphorus Chemical Engineering Research Center of Henan Province, The College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, China. E-mail: yeyong03@tsinghua.org.cn
cKey Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing, China
First published on 26th October 2016
Abstract
A new rosamine-based fluorescent probe (RosCHO) for bisulfite was obtained by one pot reaction of p-phthalaldehyde and 3-(diethylamino)phenol, which showed excellent selectivity, high sensitivity and a rapid response toward bisulfite in aqueous solution. Upon the addition of HSO3−, the aldehyde moiety in probe RosCHO formed a hydrogen sulfite-dependent adduct product, leading to fluorescence enhancement due to the different ICT process. The detection limit for bisulfite was as low as 7 × 10−8 M, and fluorescence imaging of bisulfite in MCF-7 cells demonstrated its value to practical application in biological systems.
Introduction
Bisulfite, as a main form of SO2 in physiological systems, has been widely used as food preservative, anti-oxidant and antibacterial agents in the food industry.1,2 Moreover, hydrogen sulfites can also be generated endogenously from sulphur-containing amino acids such as cysteine or glutathione in mammals.3 Recent studies have demonstrated that bisulfite at low concentrations (less than 450 μM) has vasodilating, anti-hypertensive and anti-atherogenic effects, and is regarded as a novel messenger in the cardiovascular system.4–9 However, numerous epidemiological studies suggest that abnormal sulfite levels is linked with lung cancer, cardiovascular diseases, and many neurological disorders, such as strokes, migraine headaches, and brain cancer,10–13 even low levels of bisulfite are also harmful to some people.14 Hence, the threshold levels of HSO3− in food and medicine have been strictly controlled in many countries. Therefore, developing effective methods for detecting and monitoring sulfites in environment and biological samples with good selectivity and sensitivity are needed urgently.Nowadays, fluorescent sensing has been developed as an excellent detection technique for monitoring biological species in living systems and environment because of its high selectivity, low detection limit and suitability for real-time monitoring.15–17 Many fluorescent probes for sensing bisulfite have been reported in the past decade, however, most of these needed the participation of an organic cosolvent to guarantee a high probe affinity for HSO3−, while only a few of them can detect bisulfite in 100% aqueous solution.18–28 And some bisulfite probes are not sensitive enough to determine low content of HSO3− and lack living cell imaging.19,29 Therefore, to obtain bisulfite probes with high sensitivity, rapid response, low detection limit in pure aqueous environments and cell imaging is of vital importance.
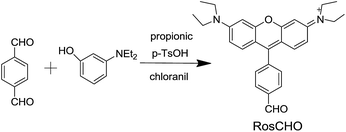
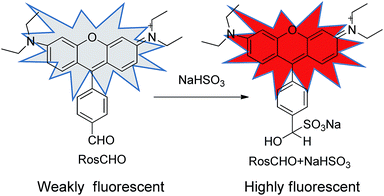
In this paper, we developed a novel rosamine-based fluorescent probe (RosCHO) for sulfites by one-step synthesis (Scheme 1), which can detect HSO3− with excellent selectivity, rapid response, low detection limit in 100% aqueous solution, and exhibit visually determination of HSO3− in living cells. The sensing mechanism of RosCHO toward bisulfite coupled with the fact sulfite can selectively adduct to the aldehyde moiety to induce a significant fluorescence enhancement due to different ICT,22,30 which was illustrated by HR-MS spectrum analysis.
Results and discussion
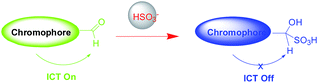
The structure of target compound RosCHO was well characterized by 1H NMR, 13C NMR, and HRMS (ESI, Fig. S1–S3†). In this work, p-phthalaldehyde and 3-(diethylamino) phenol were used as the raw material to get the target probe directly, the aldehyde group act as the NaHSO3 recognition unit. The probe (RosCHO) showed very weak fluorescence signal, however, upon the addition of HSO3−, the solution of RosCHO and HSO3− displayed strong fluorescence increase due to the different intramolecular charge transfer (ICT) efficiency.The process of nucleophilic addition reaction between aldehyde and NaHSO3 was shown in Scheme 2. The conversion of the aldehyde into the hydrogen sulfite adduct bring about a change in the electron acceptor strength and the concomitant ICT efficiency. Intramolecular charge transfer (ICT) molecules are sensitive to changes in the external environment, and their quantum yields generally increase as the environment becomes hydrophobic, which produces the dramatic fluorescence spectral changes.31,32 The fluorescence quantum yields of RosCHO were 0.15957 (cacld according to Yu = Ys × Fu/Fs × Fs/Au). After 10 equiv. HSO3− was introduced into the solution of RosCHO, the fluorescence quantum yields became 0.68171. That means it is the ICT sensing process.
 | ||
| Scheme 2 The process of nucleophilic addition reaction between aldehyde and NaHSO3 and the resulting changed ICT efficiency and optical properties. | ||
Sensing properties
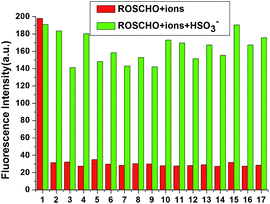
With this probe in hand, we first tested its fluorescent spectral changes upon the addition of HSO3− and other analytes in aqueous buffers. The Tris–HCl buffer (10 mM, pH = 5) system was found to give the best results, so this system was used in all the experiments described here. In the fluorescent emission studies, as shown in Fig. 1, the free probe RosCHO exhibited a weak fluorescence at about 590 nm. When 10 equiv. HSO3− was introduced in a 10 μM solution of RosCHO in Tris–HCl (10 mM, pH = 5), a remarkably enhancement of fluorescence spectra at 580 nm was observed. Other biologically relevant analytes such as H2O2, S2O32−, SCN−, PO43−, HCO3−, NO2−, ClO−, H2S, AcO−, F−, CO32−, Br−, I−, Cys, GSH and N3− almost could not lead to any notably changes under the same test condition.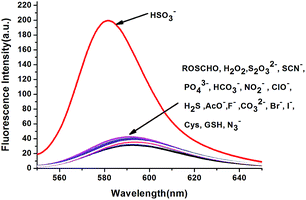 | ||
| Fig. 1 Fluorescent spectra of RosCHO (10 μM) with the presence of 10 equiv. various species in Tris–HCl (10 mM, pH = 5) at room temperature (λex = 520 nm, slits: 5.0 nm). | ||
For practical applications it is often necessary to detect an analyte in the presence of competing substrates. The competition experiment was also conducted. As shown in Fig. 2, after adding 10 equiv. of HSO3− into other analytes, respectively, no significant variation in fluorescence intensity was found. It is gratifying to note that all the tested anions almost have no interference with the fluorescence response of probe RosCHO toward HSO3−. These results indicated that RosCHO was characteristic of high selectivity toward HSO3− over other competitive analytes. The ICT efficiency of RosCHO in the presence of HSO3− also was found by the UV spectral change. As shown in Fig. 3, with the increasing concentrations of HSO3−, the maximum absorption wavelength gradually shifted from 568 to 558 nm.
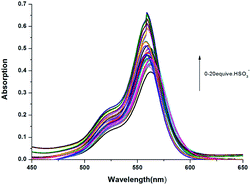 | ||
| Fig. 3 UV-vis spectra of RosCHO (10 mM Tris–HCl, pH = 5) in the presence of different concentrations of HSO3−. | ||
Time dependence
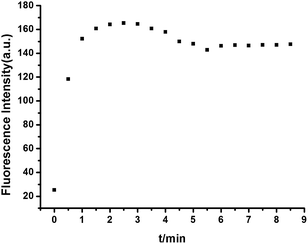
The time dependence of the response of RosCHO to HSO3− was also investigated. As shown in Fig. 4, the kinetics of fluorescence enhancement at 580 nm by the new developed fluorescent probe was recorded. It can be seen that the fluorescence intensity of RosCHO with HSO3− increased for a few seconds, and leveled off as the time continues. The time-dependent change plot demonstrated the reaction could complete in about 2 min, which indicated the probe RosCHO had a fast response for HSO3−. Therefore, a 2 min reaction time was selected in subsequent experiments in order to make the metal ions chelate with the sensors sufficiently.Fluorescence titration
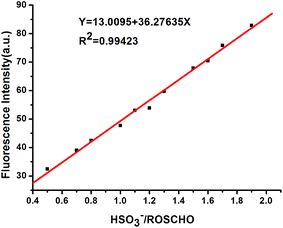
To further conform the capability of RosCHO for sensing of HSO3−, we successively evaluated its performance on quantitative detection of HSO3− in Tris–HCl buffer (10 mM, pH = 5) solution (Fig. S4†). With the continuous addition of HSO3− (0 to 100 μM) leads to a gradual increase of emission intensity at about 580 nm. Generally, one of the most important and useful applications for a fluorescent sensor is the detection of HSO3−. To investigate the practical applicability of RosCHO, the detection limit of this new chemodosimeter system was evaluated (Fig. 5). An excellent linear relationship between fluorescence intensity and HSO3− concentration (0–20 μM) was found with a correlation coefficient as high as 0.9942. The detection limit (3σ/slope)33,34 of probe RosCHO for the determination of HSO3− was estimated to be 0.07 μM. This result was far below the 2 mg kg−1 (1.25 μM) limit in final food products required by European Union regulation.35 These results suggested that probe RosCHO can detect HSO3− both qualitatively and quantitatively, and could be used in the detection of HSO3− in food or beverages for quality control.Effect of pH
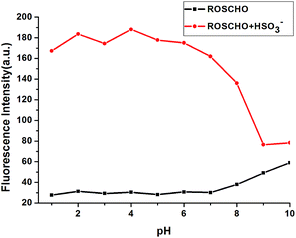
To evaluate the potential applications of RosCHO in different biological environments, the changes in fluorescence intensity under various pH values were evaluated. As shown in Fig. 6, the fluorescence intensity of free RosCHO and RosCHO + HSO3− in Tris–HCl buffer solution (10 mM) did not show significant change between pH 1.0 and 8.0. Experimental results shown that both RosCHO and RosCHO + HSO3− were pH insensitive in a biologically relevant pH range and could meet the selective requirements for bioimaging applications.Response mechanism
To further investigate the binding stoichiometry of RosCHO and HSO3−, the reaction mixture of RosCHO and NaHSO3 was characterized by HR-MS. A major ion peak was founded at m/z 531.1926 (Fig. S5†), which correspond to the resulting compound RosCHO + NaHSO3. Herein, according to our knowledge,26,36,37 we broached a conceivable mechanism of HSO3− complex with RosCHO. As shown in Scheme 3, HSO3− attacked the aldehyde moiety in RosCHO to form the aldehyde–hydrogen sulfite adduct, which deconjugated from the xanthene moiety, leading to the dramatic fluorescent increase due to the different ICT.Bioimaging applications
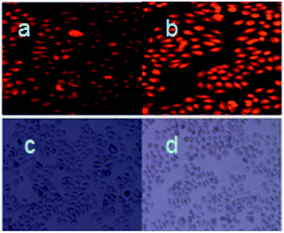
Bioimaging applications of compound RosCHO for monitoring of HSO3− in living cells were then carried out.38,39 MCF-7 cells were used for this experiment. Fluorescent imaging inside MCF-7 cells was monitored by fluorescence microscopy. As shown in Fig. 7, weakly fluorescence of RosCHO inside the living MCF-7 cells was observed (Fig. 7a). After washing with water twice, 100 μM of NaHSO3 were then supplemented to the cells. After incubated at 37 °C for another 5 min, a significant enhancement in the fluorescence from the intracellular area was observed (Fig. 7b). A bright field transmission image of cells with RosCHO and RosCHO with NaHSO3 confirmed that the cells were viable throughout the imaging experiments (Fig. 7c and d). Therefore, these results demonstrated that probe RosCHO was cell membrane permeable and capable of fluorescence imaging of HSO3− in biological samples.Conclusions
In summary, a new chemodosimeter (RosCHO) based on special nucleophilic addition reaction of aldehydes with hydrogen sulfite was designed and synthesized. Probe RosCHO exhibited high sensitivity and selectivity toward HSO3− over other anions and biothiols (Cys and GSH) in pure aqueous solution. And bioimaging results demonstrated that probe RosCHO showed visually determination of HSO3− in living cells.Experimental section
General information and methods
All chemicals were obtained from commercial suppliers and used without further purification. MCF-7 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Water used in all experiments was doubly distilled and purified by a Milli-Q system. Fluorescence spectra measurements were performed on a Hitachi F-4500 Fluorescence Spectrometer equipped with a xenon lamp, and the excitation and emission wavelength band passes were both set at 5.0 nm. Absorption spectra were measured on a UV-2102 double-beam UV/vis spectrometer, Perkin Elmer precisely. NMR spectra were recorded on a Bruker DTX-400 spectrometer in CDCl3, using TMS as internal standard. Mass spectral determination was carried on a HPLC Q-T of HR-MS. Stock solution (1 mM) of the probe (RosCHO) was prepared by dissolving the requisite amount it in methanol. NaHSO3 was dissolved in water at a concentration of 10 mM. Stock solutions (10 mM) of other anions were prepared from the corresponding inorganic salts.Synthesis of RosCHO
According to the reported methods,40 title compound RosCHO was synthesized from 3-(diethylamino) phenol and p-phthalaldehyde as shown in Scheme 1. Compound RosCHO was a bluish violet solid in 10% isolated yield. Mp: 189–190 °C. 1H NMR (400 MHz, CDCl3, ppm): 1.35 (t, 12H, J = 6.6 Hz), 3.65 (q, 8H, J = 6.3 Hz), 6.9 (s, 2H), 6.98 (d, 2H, J = 9.24 Hz), 7.27 (d, 2H, J = 9.4 Hz), 7.40 (d, 2H, J = 7.4 Hz), 8.18 (d, 2H, J = 7.52 Hz), 10.19 (s, 1H); 13C NMR (100 MHz, CDCl3, δ ppm): 12.70, 46.30, 96.63, 112.90, 144.62, 130.13, 130.26, 131.57, 137.37, 137.73, 155.22, 155.62, 157.84, 191.51. HR-MS (ESI): [C28H31N2O2]+, calcd for 423.2386. Found: 423.2387.Acknowledgements
This work was supported by the National Science Foundation of China (No. 21572209), Program for Innovative Research Team (in Science and Technology) in University of Henan Province (No. 17IRTSTHN002), and Science-Technology Foundation for Outstanding Young Scientists of Henan Academy of Agricultural Sciences (Grant No. 2016YQ22).Notes and references
- D. H. Daniels, F. L. Joe Jr, C. R. Warner, S. D. Longfellow, T. Fazio and G. W. Diachenko, Food Addit. Contam., 1992, 9, 283–289 CrossRef CAS PubMed.
- T. Fazio and C. R. Warner, Food Addit. Contam., 1990, 7, 433–454 CrossRef CAS PubMed.
- M. H. Stipanuk and I. Ueki, J. Inherited Metab. Dis., 2011, 34, 17–32 CrossRef CAS PubMed.
- X. B. Wang, H. F. Jin, C. S. Tang and J. B. Du, Clin. Exp. Pharmacol. Physiol., 2010, 37, 745–752 CrossRef CAS PubMed.
- Y. Liang, D. Liu, T. Ochs, C. Tang, S. Chen, S. Zhang, B. Geng, H. Jin and J. Du, Lab. Invest., 2011, 91, 12–23 CrossRef CAS PubMed.
- J. Li, R. Li and Z. Meng, Eur. J. Pharmacol., 2010, 645, 143–150 CrossRef CAS PubMed.
- H. F. Jin, Y. Wang, X. B. Wang, Y. Sun, C. S. Tang and J. B. Du, Nitric Oxide, 2013, 32, 56–61 CrossRef CAS PubMed.
- X. B. Wang, H. F. Jin, C. S. Tang and J. B. Du, Eur. J. Pharmacol., 2011, 670, 1–6 CrossRef CAS PubMed.
- L. M. Luo, S. Chen, H. F. Jin, C. S. Tang and J. B. Du, Biochem. Biophys. Res. Commun., 2011, 415, 61–67 CrossRef CAS PubMed.
- N. Sang, Y. Yun, H. Li, L. Hou, M. Han and G. Li, Toxicol. Sci., 2010, 114, 226–236 CrossRef CAS PubMed.
- T. M. Chen, J. Gokhale, S. Shofer and W. G. Kuschner, Am. J. Med. Sci., 2007, 333, 249–256 CrossRef PubMed.
- D. Q. Rich, J. Schwartz, M. A. Mittleman, M. Link, H. LuttmannGibson, P. J. Catalano, F. E. Speizer and D. W. Dockery, Am. J. Epidemiol., 2005, 161, 1123–1132 CrossRef PubMed.
- M. Reist, P. Jenner and B. Halliwell, FEBS Lett., 1998, 423, 231–234 CrossRef CAS PubMed.
- H. Vally, N. L. Misso and V. Madan, Clin. Exp. Allergy, 2009, 39, 1643–1651 CrossRef CAS PubMed.
- M. Y. Wu, T. He, K. Li, M. B. Wu, Z. Huang and X. Q. Yu, Analyst, 2013, 138, 3018–3025 RSC.
- N. Bones, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- X. Dai, T. Zhang, Z. F. Du, X. J. Cao, M. Y. Chen, S. W. Hu, J. Y. Miao and B. X. Zhao, Anal. Chim. Acta, 2015, 888, 138–145 CrossRef CAS PubMed.
- Y. Sun, S. W. Fan, S. Zhang, D. Zhao, L. Duan and R. F. Li, Sens. Actuators, B, 2014, 193, 173–177 CrossRef CAS.
- G. Wang, H. Chen, X. L. Chen and Y. M. Xie, RSC Adv., 2016, 6, 18662–18666 RSC.
- Y. Liu, K. Li, K. X. Xie, L. L. Li, K. K. Yu, X. Wang and X. Q. Yu, Chem. Commun., 2016, 52, 3430–3433 RSC.
- X. J. Liu, Q. W. Yang, W. Q. Chen, L. N. Mo, S. Chen, J. Kang and X. Z. Song, Org. Biomol. Chem., 2015, 13, 8663–8668 CAS.
- W. Q. Chen, X. J. Liu, S. Chen, X. Z. Song and J. Kang, RSC Adv., 2015, 5, 25409–25415 RSC.
- Y. J. Zhang, L. M. Guan, H. Yu, Y. H. Yan, L. B. Du, Y. Liu, M. T. Sun, D. J. Huang and S. H. Wang, Anal. Chem., 2016, 88, 4426–4431 CrossRef CAS PubMed.
- X. F. Yang, M. Zhao and G. Wang, Sens. Actuators, B, 2011, 152, 8–13 CrossRef CAS.
- C. M. Yu, M. Luo, F. Zeng and S. Z. Wu, Anal. Methods, 2012, 4, 2638–2640 RSC.
- X. Cheng, H. Jia, J. Feng, J. Qin and Z. Li, J. Mater. Chem. B, 2013, 1, 4110–4114 RSC.
- Y. Q. Sun, P. Wang, J. Liu, J. Y. Zhang and W. Guo, Analyst, 2012, 137, 3430–3433 RSC.
- P. H. Xie, G. Q. Gao, W. J. Zhang, G. Y. Yang and Q. Jin, J. Chem. Sci., 2015, 127, 1267–1273 CrossRef CAS.
- X. H. Cheng, H. Z. Jia, J. Feng, J. G. Qin and Z. Li, Sens. Actuators, B, 2013, 184, 274–280 CrossRef CAS.
- O. Sire, B. Alpert and C. A. Royer, Biophys. J., 1996, 70, 2903–2914 CrossRef CAS PubMed.
- M. Mazumdar, P. K. Parrack and B. Bhattacharyya, Eur. J. Biochem., 1992, 204, 127–132 CrossRef CAS PubMed.
- W. Chen, Z. Li, W. Shi and H. Ma, Chem. Commun., 2012, 48, 2809–2811 RSC.
- Z. Li, X. Li, X. Gao, Y. Zhang, W. Shi and H. Ma, Anal. Chem., 2013, 85, 3926–3932 CrossRef CAS PubMed.
- Commission Regulation (EU) no. 1130/2011, 11 Nov. 2011.
- X. F. Yang, M. Zhao and G. Wang, Sens. Actuators, B, 2011, 152, 8–13 CrossRef CAS.
- C. Yu, M. Luo, F. Zeng and S. Wu, Anal. Methods, 2012, 4, 2638–2640 RSC.
- Y. Zhao, Y. Sun, X. Lv, Y. Liu, M. Chen and W. Guo, Org. Biomol. Chem., 2010, 8, 4143–4147 CAS.
- C. Wang, D. Zhang, X. Huang, P. Ding, Z. Wang, Y. Zhao and Y. Ye, Sens. Actuators, B, 2014, 198, 33–40 CrossRef CAS.
- I. C. S. Cardoso, A. L. Amorim, C. Queirós, S. C. Lopes, P. Gameiro, B. D. Castro, M. Rangel and A. M. Silva, Eur. J. Org. Chem., 2012, 5810–5817 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, HRMS spectra of the probes. See DOI: 10.1039/c6ra24667b |
| This journal is © The Royal Society of Chemistry 2016 |