A promising screw-extrusion steam explosion pretreatment process: effects on the morphological and structural features of Eucalyptus woodchips†
Yong Liang,
Bo Lei,
Hui-Ting Zhong,
Yan-Hong Feng* and
Jin-Ping Qu
National Engineering Research Center of Novel Equipment for Polymer Processing, Key Laboratory of Polymer Processing Engineering, Ministry of Education, South China University of Technology, Guangzhou 510641, P. R. China. E-mail: yhfeng@scut.edu.cn; Fax: +86-20-87112503; Tel: +86-20-87111349
First published on 11th November 2016
Abstract
The effects of continuous screw-extrusion steam explosion (SESE) pretreatment on the morphology of Eucalyptus woodchip fibers and the structures of Eucalyptus lignins were investigated. The morphologies of untreated and SESE pretreated fibers were investigated by scanning electron and optical microscopies. The resulting enzymatic mild acidolysis lignins were evaluated by nuclear magnetic resonance spectroscopy, pyrolysis-gas chromatography/mass spectrometry, thermogravimetric analysis, and gel permeation chromatography. The surface of the SESE pretreated fiber was strongly disrupted, leading to a significant decrease in fiber size. The total lignin content of the Eucalyptus fibers decreased from 31.1 to 15.1% after four SESE pretreatment cycles. Lignin molecules were subjected to competitive depolymerization and repolymerization during the SESE pretreatment. This yielded lignin macromolecules with a complex heterogeneous structure and high molecular weight. The thermal stability and char conversion rate characteristics of the lignin increased with the increasing number of SESE pretreatment cycles. SESE pretreatment is a continuous high-efficiency process. These results demonstrate that SESE pretreatment can achieve similar effects to traditional steam explosion techniques, for breaking the structural recalcitrance of lignocelluloses.
1 Introduction
Lignocellulosic biomass is a sustainable and renewable source of value-added biomaterials and chemicals.1–3 However, lignocellulosic materials are naturally resistant to biological and chemical deconstruction.4 The complex structure of lignin makes it difficult for enzymes to access its core structure and cleave individual components, leading to biomass recalcitrance.5 Lignin contains varying types of interunit-linked phenylpropanoid units,6 which consist of lignin structures linked by various linkages. The most common linkage is β-O-4′ ether bonds, with other C–O–C and C–C linkages including β-5′, β-β′, and β-1′.7,8 Differences in the linkages between monomeric lignin units and the nature of the functional groups result in complex lignin macromolecules.Various pretreatment methods have been developed to overcome lignocellulosic recalcitrance, and expand the application of this valuable resource. These include acid hydrolysis, alkaline treatment, hydrothermal pretreatment, autohydrolysis, and steam explosion (SE).9–11 The effects of biomass pretreatments include (1) disrupting the structure of the plant cell wall, (2) increasing the pore size of cellulose substrates, and (3) eliminating lignin.12 SE is the most well-established pretreatment method, and is traditionally used to enhance the accessibility, reactivity, and surface area of lignocellulosic biomass.13 During SE pretreatment, the biomass is exposed to highly pressurized steam followed by rapid decompression, which disrupts the internal structure of lignocellulose.14 Lignin, hemicellulose, and cellulose networks are disrupted in this pretreatment, resulting in the solubilization of a large proportion of the hemicellulose. The lignin and cellulose components remain as residues. SE is therefore an effective pretreatment for disrupting the structure of biomass materials, and increasing their chemical reactivity for further operations.15
High-throughput pretreatment methods suitable for large-scale processing are desirable for the industrial production of value-added materials from lignocellulosic biomass.16 SE processes are poorly suited to industrial application, because they are non-continuous, and involve non-recyclable chemical reagents and high production costs. Screw extrusion processes are widely used in polymer and food processing, to mix and transport raw materials.17 Screw extrusion is a high-throughput technique suitable for large-scale production. Screw-extrusion steam explosion (SESE) pretreatments have been developed for the continuous pretreatment of various biomass materials. However, most SESE technologies require heating systems and steam generators.16,17 We previously reported an alternative continuous SESE equipment, which allowed for high production capacity.18 It featured a purpose-designed screw system, with a high compression ratio. This generated a high shearing force and high friction compared with those of traditional SE processes, which provided heat to the raw material during pretreatment. This SESE process therefore required no steam generator or additional heating system. The intensity of the SESE pretreatment process could be adjusted by increasing/decreasing the number of pretreatment cycles for different biomass feedstocks, depending on application requirements. SESE pretreatment can therefore pretreat biomass materials at lower temperatures, and provide higher material throughputs than traditional SE pretreatments.
Lignin undergoes considerable structural changes in conditions typically used in SE processes.13,19,20 SE pretreatment can lead to a decrease in the number of β-O-4′ units, and an increase in the number of C–C condensed structures. The formation of lignins with higher molecular weights than those of the starting material has been observed, and new linkages can be formed via radical coupling reactions (e.g. interunit β-1′ and β-5′). These phenomena arise as a result of competing depolymerization and repolymerization reactions. A high-efficiency method based on a combination of enzymatic hydrolysis and mild acidolysis was recently reported, and the resulting lignin was defined as enzymatic mild acidolysis lignin (EMAL).21 In this procedure, most carbohydrates were removed during the initial enzymatic hydrolysis of cellulose, and residual lignin–carbohydrate bonds were cleaved in the subsequent mild acidolysis. This procedure liberated lignin in high purity and yield, making it more representative of whole lignin present in natural wood.22
In the current study, Eucalyptus fibers were pretreated with a continuous SESE process. Changes in fiber morphology upon pretreatment were investigated by optical and electron microscopies. Nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) were used to characterize changes in the linkages and molecular weight distributions of the lignins. Thermogravimetric analysis (TGA) was used to evaluate the effect of the SESE process on the thermal degradation of the lignins. The results of these analyses are used to infer structural differences, to investigate how the SESE pretreatment intensity changes the structure and morphology of the fibers.
2 Results and discussion
2.1 Analysis of lignin content
SE is an effective pretreatment method for disrupting the structural recalcitrance of lignocelluloses.23 The effects of different pretreatments can be estimated from the percentages of different components, which in turn can be estimated by component analysis. The lignin contents of Eucalyptus samples were determined using TAPPI methods,24 and the results are shown in Fig. 1. The lignin content decreased with increasing number of SESE pretreatment cycles. The total lignin content of the Eucalyptus fibers decreased from 31.1% (untreated, denoted EP-0) to 15.1% (pretreated with four SESE cycles, denoted EP-4). Hemicellulose was degraded during the SESE pretreatment, thereby breaking the restriction of lignin. Plant cells were readily deformed during the SESE pretreatment because of the shearing force and heat. This resulted in the migration and redistribution of lignin in the fibers. The shear force of the screw can assist in the peeling of the middle lamella and lignin particles,18 allowing fibers and lignin-rich particles to be separated after pretreatment, using a simple sieve. Friction generated between the material, screw, and interior wall of the SESE equipment provides extra heat and energy, which promotes lignin degradation.17 The current SESE pretreatment of Eucalyptus fibers gave similar results to those pretreated by traditional SE processes, but the current process was more efficient. EP-4 exhibited the highest reduction in lignin content among the tested samples, so was used as a representative for the SESE process, and was subsequently investigated in greater detail.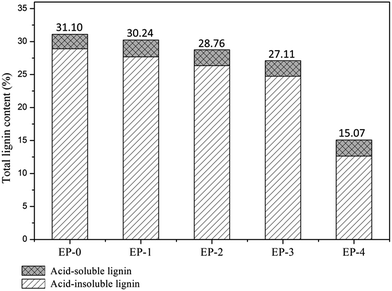 | ||
| Fig. 1 Lignin contents of Eucalyptus woodchips before and after increasing numbers of SESE pretreatment cycles. | ||
2.2 Morphology of Eucalyptus fibers
Scanning electron microscopy (SEM) and optical microscopy images of Eucalyptus fibers before and after SESE pretreatment are shown in Fig. 2. Untreated Eucalyptus fibers exhibited smooth surfaces and regular shapes. The fibers exploded into fibrils and bundles, after subjecting to four SESE pretreatment cycles. The middle lamella were peeled away from the fibers, which was consistent with the results from component analysis and previous reports.18 The surfaces of the pretreated materials were strongly disrupted. SESE pretreatment significantly reduced the fiber size from hundreds to several micrometers, yielding a material with a very large surface-to-volume ratio. Fiber size is an important parameter for determining the efficiency of SESE processes and enzymatic hydrolyses,25 and can also affect the compatibility of biocomposites.26 The reduction in fiber size led to an increase in specific surface area. This has previously been shown to increase the hydrolysis efficiency of cellulose fibrils in biofuels, and the strength of interfacial bonding interactions in the biocomposites.272.3 2D-HSQC NMR spectroscopy
Lignin consists of three basic components with diverse linkages, such as β-O-4′, β-β′, and β-5′. Two-dimensional heteronuclear single quantum coherence (2D-HSQC) analyses of the EP-0 and EP-4 samples indicated that SESE pretreatment resulted in changes to the lignin structure. The amounts of lignin units and interunit linkages are shown in the 2D-HSQC NMR spectra in Fig. 3. These spectra were characterized by comparing their peak assignments with reported data (Table S1†).28–30 | ||
| Fig. 3 2D-HSQC NMR spectra of side-chain and aromatic regions of lignins in untreated and SESE pretreated Eucalyptus fibers. | ||
The aryl ether (β-O-4′, A), resinol (β-β′, B) and phenylcoumaran (β-5′, C) interunit regions of the lignin samples were assigned based on previous reports. All of the 2D HSQC spectra contained signals corresponding to β-O-4′ linkage units (A). The Cα–Hα correlations assigned to β-O-4′ linkages were observed at δC/δH = 72.2/4.85. The Cβ–Hβ correlations of β-4′ linkages belonging to syringyl and guaiacyl units were observed at δC/δH = 84.1/4.31 and 86.2/4.12, respectively. The Cγ–Hγ correlations of β-O-4′ linkages were observed at δC/δH = 59.7/3.20–3.75. As well as a large number of β-O-4′ linkages, numerous β-β′ linkages were observed in the spectra. The Cα–Hα, Cβ–Hβ, and Cγ–Hγ correlations of β-β′ linkages were observed at δC/δH = 84.8/4.66, 53.9/3.05, and 71.2/3.82–4.18, respectively. The signal at δC/δH = 86.8/5.45 was assigned to the Cα–Hα correlation of the phenylcoumaran unit (β-5′, C).
Signals corresponding to syringyl- and guaiacyl-type units were observed in the lignin unit regions of the 2D-HSQC spectra. The C2,6–H2,6 correlation observed at δC/δH = 104.5/6.70 was assigned to syringyl-type units. A correlation was observed at δC/δH = 106.8/7.22, which was attributed to the oxidation of syringyl-type units (S′2,6). Cross signals corresponding to guaiacyl-type units were assigned to three different correlations (G2, G5, and G6), including C2–H2 (δC/δH = 111.0/6.90), C5–H5 (δC/δH = 115.2/6.70), and C6–H6 (δC/δH = 119.0/6.75).
The different interunit linkages observed between the lignin samples were semiquantitatively investigated.31 The integration of (G2 + S2,6/2) was used as an internal standard. The quantitation process assumed that the C2 position of guaiacyl-type units and the C2 and C6 positions of syringyl-type units were not substituted.11 The percentages of the major interunit linkages, and the relative abundances of the different substructures, are shown in Table 1. As expected, EP-O and EP-4 predominantly consisted of β-O-4′ aryl ether bonds (A, 73.0–74.5% of the total number of interunit bonds), a lesser proportion of β-β′ resinol-type bonds (B, 21.1–22.0%), and a small proportion of β-5′ phenylcoumaran-type bonds (C, 4.42–4.99%). A small number of different structural interunit linkages were cleaved, in accordance with the literature.13
| Sample | β-O-4′ | β-β′ | β-5′ | S/G |
|---|---|---|---|---|
| EP-0 | 55.22 (72.97) | 16.68 (22.04) | 3.78 (4.99) | 1.57 |
| EP-4 | 54.22 (74.47) | 15.37 (21.11) | 3.22 (4.42) | 1.49 |
The S/G ratio can provide important information about chemical processes affecting lignins, especially in terms of the pretreatment chemical requirements and sugar release efficiency.32 Very little difference was observed between the S/G ratios of EP-0 and EP-4. The total lignin content of the pretreated Eucalyptus fibers decreased significantly after SESE pretreatment, which resulted in minimal chemical requirements and a high sugar release efficiency.
2.4 Molecular weight distributions
The effects of the SESE pretreatment process on the molecular weight distribution of the different lignin samples were evaluated by GPC analysis. The molecular weights (Mw, Mn) and the polydispersity indices (Mw/Mn) of the lignin samples obtained from the Eucalyptus fibers are shown in Fig. 4. The results revealed that the Mw of EP-4 was twice as high as that of EP-0. The polydispersity of the EP-4 lignin (Mw/Mn = 2.694) was also higher than that of EP-0 (Mw/Mn = 1.535). The cleavage of β-O-4′ linkages was expected to significantly decrease the molecular weight of the lignin. However, the molecular weight gradually increased with increasing number of SESE pretreatment cycles. This suggested that cleavage of the β-O-4′ linkages in the Eucalyptus lignin during SESE pretreatment was accompanied by significant depolymerization and repolymerization, which increased the molecular weight and complexity of the heterogeneous lignin structure.15,20 Some of these changes occurred during the rearrangement of lignin macromolecular chains. Repolymerization became the dominant process with increasing number of SESE pretreatment cycles. | ||
| Fig. 4 Molecular weight distributions of lignins in untreated and SESE pretreated Eucalyptus fibers. | ||
2.5 Thermogravimetric analysis
The thermal degradation of lignin is complicated by the differing thermal stabilities of its functional groups, which degrade over a wide temperature range.33,34 The thermal stability of lignin from Eucalyptus fibers was evaluated by TGA, and the results are shown in Fig. 5. The thermal decomposition of EP-0 proceeded rapidly upon increasing from 200 to 450 °C, with a pyrolytic residue of 29.7% at 800 °C. The major weight loss at 200–450 °C was attributed to cleavage of lignin interunit linkages, and volatilization of the resulting monomeric phenols. The weight loss at >450 °C was attributed to the degradation of aromatic rings. The thermal degradation of EP-4 consisted of two separate pyrolysis steps at 317 and 382 °C, as shown in the first-derivative TGA curve in Fig. 5b. The thermal decomposition of EP-4 predominantly occurred at 200–450 °C, and was followed by degradation and char formation at >450 °C. This result could be attributed to the depolymerization and repolymerization of lignin during SESE pretreatment, which increased the molecular weight and polydispersity.20 These results demonstrate that the current SESE pretreatment significantly affected the thermal stability of lignin, by increasing the char conversion rate. | ||
| Fig. 5 (a) Thermogravimetric and (b) first-derivative thermogravimetric curves of lignin from untreated and SESE pretreated Eucalyptus fibers. | ||
2.6 Pyrolysis-gas chromatography/mass spectrometry
Py-GC/MS is useful for analyzing the composition of lignins, and involves heating lignin samples until they undergo thermal pyrolysis to smaller molecular fragments that can be detected by GC/MS.34 Py-GC/MS has been used to determine the relative number of H/G/S units in lignin samples derived from different types of hardwood.28,29,35 Py-GC/MS chromatograms of EMAL samples isolated from Eucalyptus fibers are shown in Fig. 6. The identities and relative abundances of molecular fragments released from the lignin during this process are listed in Table S2.† All of detected lignin-degradation products are shown in Fig. S2.† Several guaiacyl-type (G) and syringyl-type (S) phenol products were detected. The main ones are phenol, 2-methoxy-4-methyl (peak 2), phenol,2,6-dimethoxy- (peak 5), phenol,4-methoxy-3-(methoxymethyl)- (peak 8), phenol,2-methoxy-4-(1-propenyl)- (peak 9), 4-methyl-2,5-dimethoxybenzaldehyde (peak 13), benzaldehyde,4-hydroxy-3,5-dimethoxy- (peak 17), phenol,2,6-dimethoxy-4-(2-propenyl)- (peak 20), and 3,5-dimethoxy-4-hydroxycinnam-aldehyde (peak 28). | ||
| Fig. 6 Py-GC/MS chromatograms of EMAL untreated and SESE pretreated Eucalyptus fiber samples. Identities and relative abundances of compounds released from samples are listed in Table S2.† All of detected lignin-degradation products are shown in Fig. S2.† | ||
The relative S/G ratios of the lignins were determined by Py-GC/MS, and the results are shown in Table S2.† The S/G ratios of EP-0 and EP-4 were comparable, in agreement with the above 2D-HSQC results. The S/G ratios of EP-0 and EP-4 obtained from thermal decomposition were lower than those obtained from 2D-HSQC results. However, the two analytical methods gave results showing the same overall trend. Demethoxylation occurring during thermal pyrolysis could account for the differing S/G ratios obtained using the two methods. Furthermore, demethoxylation observed for syringyl- and guaiacyl-type units exhibited different reactivity patterns. Syringyl units initiated demethoxylation more readily than guaiacyl units during thermolysis. This promoted the demethoxylation degradation of syringyl units, and led to a decrease in the S/G ratio.28
3 Experimental
3.1 Materials
Eucalyptus woodchips were kindly provided by Guangdong Dingfeng Paper Co., Ltd. (Guangzhou, China). Steam-exploded Eucalyptus fibers were produced using a custom-built continuous SESE equipment, according to our previously reported procedure.18 A schematic diagram of the SESE equipment is shown in Fig. S1.† The pretreatment pressure and temperature were approximately 1.5 MPa and 150 °C, respectively. The residence time was approximately 15 seconds per SESE pretreatment cycle. Eucalyptus samples were ground, sieved through 60–80 mesh, and stored at −4 °C prior to use. Cellulase and hemicellulase enzymes used in enzymatic hydrolysis experiments were supplied by Jiangsu Ruiyang Biotech Co., Ltd. (Wuxi, China). All other chemicals were purchased from Sinopharm (Shanghai, China) and used as received.3.2 Preparation of EMAL samples
EMAL samples were isolated from untreated and SESE pretreated Eucalyptus fibers, according to reported procedures.21,22,36,37 Briefly, ball-milled Eucalyptus fibers were treated with given amounts of cellulase and hemicellulase. Enzymatic hydrolyses were carried out at 50 °C for 48 h at pH 4.8, which was adjusted using a citrate buffer. The solid residue was collected by filtration, washed with acidified water (pH 2), and lyophilized to give crude lignin. The crude lignin was subsequently hydrolyzed using 0.01 mol L−1 HCl in aqueous dioxane (dioxane/water 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) under a nitrogen atmosphere for 2 h. The resulting suspension was centrifuged, and the supernatant was neutralized with 0.1 mol L−1 NaOH before being added dropwise to 1 L of acidified water (pH 2). The resulting mixture was held at room temperature overnight, and the precipitated lignin was collected by centrifugation (12
4, v/v) under a nitrogen atmosphere for 2 h. The resulting suspension was centrifuged, and the supernatant was neutralized with 0.1 mol L−1 NaOH before being added dropwise to 1 L of acidified water (pH 2). The resulting mixture was held at room temperature overnight, and the precipitated lignin was collected by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g), washed with water, and freeze-dried for further use.
000 × g), washed with water, and freeze-dried for further use.
3.3 2D HSQC NMR analysis
50 mg of freeze-dried lignin was dissolved in 600 μL of DMSO-d6. 2D HSQC NMR spectra were collected at 25 °C using a Bruker Avance 600 MHz NMR spectrometer. Chemical shifts of the DMSO peak (δC/δH 39.5/2.5 ppm) were used as a reference. The 2D HSQC correlation peaks were processed using Topspin software version 3.1 (Bruker), as previously reported.12,28,29,383.4 GPC analysis
The lignin was acetylated and analyzed according to a previously reported procedure.24,39 Briefly, a small lignin sample (10 mg) was added to a dry reaction vial, followed by anhydrous glacial acetic acid (2.3 mL), and the resulting mixture was stirred at room temperature for 30 min. Acetyl bromide (0.25 mL) was added to the vial, and the mixture was stirred at room temperature for 0.5 h. The mixture was then dried under high vacuum conditions at 30 °C for 45 min, to remove the glacial acetic acid and excess acetyl bromide. The resulting residue was dissolved in THF to a concentration 1 mg mL−1, and then filtered. The molecular weight of the sample was determined using an Agilent 1200 HPLC system (Agilent, USA) equipped with an ultraviolet detector at 280 nm and a PL-gel column (10 × 7.5 mm), which was calibrated with polystyrene standards prior to use. The system was operated at room temperature using THF as a mobile phase, with a flow rate of 1.0 mL min−1.3.5 Thermogravimetric analysis
The thermal stabilities of approximately 10 mg lignin samples were evaluated using a Netzsch Thermo-gravimetric Analyzer (TG 209 F3, Netzsch, Selb, Germany). All measurements were conducted under a nitrogen atmosphere, with a gas flow rate of 50 mL min−1. Each test sample was heated from room temperature to 800 °C, at a heating rate of 10 °C min−1.3.6 Scanning electron microscopy
Fiber surfaces before and after SESE pretreatment were observed using a Quanta FEG 250 field emission scanning electron microscope (FEI, Hillsboro, Oregon, USA) at room temperature. Samples were dried at room temperature, and sputter-coated with gold before observation. Images were collected at an accelerating voltage of 5 kV.3.7 Pyrolysis-gas chromatography/mass spectrometry
Isolated lignin samples were characterized using a Pyroprobe 5200HP pyrolyzer (Chemical Data Systems) connected to a QP2100 GC/MS apparatus (Shimadzu, Japan), which was equipped with a Rxi-1ms capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness). Approximately 100 μg of lignin sample was pyrolyzed at 500 °C for 20 s, at a heating rate of 20 °C ms−1. Mass spectra were obtained for m/z values of 29–400, using EI mode at 70 eV. Helium was used as the carrier gas, with a flow rate of 1 mL min−1 and split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Compounds were identified by comparison with the NIST library and literature reports.28,29,35,40
50. Compounds were identified by comparison with the NIST library and literature reports.28,29,35,40
4 Conclusions
Eucalyptus fibers were pretreated using a continuous screw-extrusion steam explosion equipment. Change in the morphology of the Eucalyptus fibers and changes in the structure of the lignin were investigated using various techniques. The surface of the SESE pretreated fibers was strongly disrupted. SESE pretreatment decreased the fiber size from hundreds to several micrometers, which greatly increased the surface-to-volume ratio. The total lignin content of the Eucalyptus fibers decreased from 31.1 to 15.1%, after four SESE pretreatment cycles. The cleavage of linkages between lignin moieties during SESE pretreatment was accompanied by significant depolymerization and repolymerization, which increased the molecular weight and complexity of the heterogeneous lignin structure. SESE pretreatment significantly affected the thermal stability of the lignin. The char conversion rate of the lignin increased with increasing number of SESE pretreatment cycles. The current SESE pretreatment is a continuous high-efficiency pretreatment that dramatically decreases the fiber size. It could therefore be used to improve accessibility during enzymatic hydrolysis, and to promote the interfacial compatibility of biocomposites.Acknowledgements
The authors acknowledge financial support from The National Natural Science Foundation of China (No. 51373058), the Science and Technology Planning Project of Guangdong Province, China (No. 2014B090921006), Special Support Program of Guangdong Province (No. 2015TX01X151), and the Fundamental Research Funds for the Central Universities (No. 2015ZZ004).References
- H. Tang, N. Butchosa and Q. Zhou, Adv. Mater., 2015, 27, 2070–2076 CrossRef CAS PubMed.
- V. K. Thakur, M. K. Thakur, P. Raghavan and M. R. Kessler, ACS Sustainable Chem. Eng., 2014, 2, 1072–1092 CrossRef CAS.
- Y. Z. Wang, S. De and N. Yan, Chem. Commun., 2016, 52, 6210–6224 RSC.
- Q. Sun, Y. Pu, X. Meng, T. Wells and A. J. Ragauskas, ACS Sustainable Chem. Eng., 2015, 3, 2203–2210 CrossRef CAS.
- M. Huron, D. Hudebine, N. L. Ferreira and D. Lachenal, Ind. Crops Prod., 2016, 79, 104–109 CrossRef CAS.
- P. Wang, Y. Fu, Z. Shao, F. Zhang and M. Qin, BioResources, 2016, 11, 4086–4103 CAS.
- O. Gordobil, R. Moriana, L. Zhang, J. Labidi and O. Sevastyanova, Ind. Crops Prod., 2016, 83, 155–165 CrossRef CAS.
- T.-Q. Yuan, S.-N. Sun, F. Xu and R.-C. Sun, J. Agric. Food Chem., 2011, 59, 10604–10614 CrossRef CAS PubMed.
- H. Rabemanolontsoa and S. Saka, Bioresour. Technol., 2016, 199, 83–91 CrossRef CAS PubMed.
- S.-L. Sun, J.-L. Wen, M.-G. Ma, R.-C. Sun and G. L. Jones, Ind. Crops Prod., 2014, 56, 128–136 CrossRef CAS.
- X. Chen, Y.-J. Gao, L. Wang, H.-Z. Chen and N. Yan, ChemPlusChem, 2015, 80, 1565–1572 CrossRef CAS.
- J.-L. Wen, T.-Q. Yuan, S.-L. Sun, F. Xu and R.-C. Sun, Green Chem., 2014, 16, 181–190 RSC.
- H. Heikkinen, T. Elder, H. Maaheimo, S. Rovio, J. Rahikainen, K. Kruus and T. Tamminen, J. Agric. Food Chem., 2014, 62, 10437–10444 CrossRef CAS PubMed.
- B. Deepa, E. Abraham, B. M. Cherian, A. Bismarck, J. J. Blaker, L. A. Pothan, A. L. Leao, S. F. de Souza and M. Kottaisamy, Bioresour. Technol., 2011, 102, 1988–1997 CrossRef CAS PubMed.
- R. Martin-Sampedro, E. A. Capanema, I. Hoeger, J. C. Villar and O. J. Rojas, J. Agric. Food Chem., 2011, 59, 8761–8769 CrossRef CAS PubMed.
- H.-J. Zhang, X.-G. Fan, X.-L. Qiu, Q.-X. Zhang, W.-Y. Wang, S.-X. Li, L.-H. Deng, M. A. Koffas, D.-S. Wei and Q.-P. Yuan, Bioprocess Biosyst. Eng., 2014, 37, 2425–2436 CrossRef CAS PubMed.
- J. Chen, W. Zhang, H. Zhang, Q. Zhang and H. Huang, Bioresour. Technol., 2014, 161, 230–235 CrossRef CAS PubMed.
- P. Ma, J. Lan, Y. Feng, R. Liu, J. Qu and H. He, BioResources, 2016, 11, 1417–1431 CAS.
- R. Martin-Sampedro, J. A. Martin, M. E. Eugenio, E. Revilla and J. C. Villar, BioResources, 2011, 6, 4922–4935 CAS.
- J. Li, G. Henriksson and G. Gellerstedt, Bioresour. Technol., 2007, 98, 3061–3068 CrossRef CAS PubMed.
- I. F. Anderson Guerra, L. A. Lucia, C. Saquing, S. Baumberger and D. S. Argyropoulos, J. Agric. Food Chem., 2006, 54, 5939–5947 CrossRef PubMed.
- S. Wu and D. Argyropoulos, J. Pulp Pap. Sci., 2003, 29, 235–240 CAS.
- X. Yang, X. Zhang and S. Chen, Wood Sci. Technol., 2012, 46, 515–527 CrossRef CAS.
- X. Yang, Y. Zeng and X. Zhang, BioResources, 2010, 5, 488–498 CAS.
- J.-L. Wen, S.-L. Sun, T.-Q. Yuan and R.-C. Sun, Green Chem., 2015, 17, 1589–1596 RSC.
- L.-P. Xiao, Z.-J. Shi, F. Xu and R.-C. Sun, BioEnergy Res., 2013, 6, 519–532 CrossRef CAS.
- J. Zhang and N. Yan, Green Chem., 2016, 18, 5050–5058 RSC.
- K. Ninomiya, K. Inoue, Y. Aomori, A. Ohnishi, C. Ogino, N. Shimizu and K. Takahashi, Chem. Eng. J., 2015, 259, 323–329 CrossRef CAS.
- J. Asikkala, T. Tamminen and D. S. Argyropoulos, J. Agric. Food Chem., 2012, 60, 8968–8973 CrossRef CAS PubMed.
- P. Bocchini and G. C. Galletti, Rapid Commun. Mass Spectrom., 1995, 9, 819–826 Search PubMed.
- J. C. Del Rio, J. Rencoret, G. Marques, J. Li, G. r. Gellerstedt, J. s. Jiménez-Barbero, A. n. T. Martínez and A. Gutiérrez, J. Agric. Food Chem., 2009, 57, 10271–10281 CrossRef CAS PubMed.
- W. Sui and H. Chen, Chem. Eng. Sci., 2014, 116, 254–262 CrossRef CAS.
- L.-Z. Qin and H.-Z. Chen, Process Biochem., 2016, 51, 749–758 CrossRef CAS.
- Y. Feng, H. Chen, X. Dong, J. Qu, H. He, B. Xu and Y. Zhang, Polym. Compos., 2014, 37, 915–924 CrossRef.
- E. T. Fabio Araya, R. T. MendonSca and J. Freer, Biotechnol. Bioeng., 2015, 112, 1783–1791 CrossRef PubMed.
- J.-L. Wen, B.-L. Xue, F. Xu and R.-C. Sun, BioEnergy Res., 2012, 5, 886–903 CrossRef CAS.
- J.-L. Wen, S.-L. Sun, B.-L. Xue and R.-C. Sun, Materials, 2013, 6, 359–391 CrossRef CAS.
- M. V. Bule, A. H. Gao, B. Hiscox and S. Chen, J. Agric. Food Chem., 2013, 61, 3916–3925 CrossRef CAS PubMed.
- W. Jin, K. Singh and J. Zondlo, Agriculture, 2013, 3, 12–32 CrossRef CAS.
- C. V. Mihai Brebu, Cellul. Chem. Technol., 2010, 44, 353–363 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24639g |
| This journal is © The Royal Society of Chemistry 2016 |

