Shear induced self-thickening of chitosan/β-cyclodextrin compound solution†
Abstract
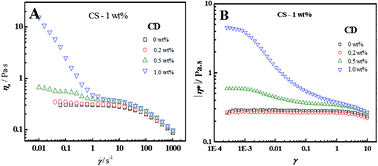
The molecular association and rheological behavior of the compound solutions of chitosan (CS) and β-cyclodextrin (CD) were investigated. The results show that an appropriate amount of CD could significantly affect the viscoelastic behavior of CS aqueous solution. The CS/CD compound solution presents an obvious multiple shear-thinning and a subsequent shear induced thickening (SIT) behavior which is similar to that of chitosan-grafted polyacrylamide aqueous solution, depending on the mass ratio of CD and CS. Too high or too low CD concentration is unfavorable for shear induced self-thickening of the CS/CD compound solution when CS concentration is fixed. TEM observations of the CS/CD compound solution show that larger CS–CD aggregates induced by hydrogen bonding appear with CD concentration increasing, and result in the increases of viscosity and moduli, which are in agreement with the results of steady and oscillation flows. The increased viscosity of the CS/CD compound solution after strong shearing is attributed mainly to the larger and irregular hydrogen-bonded aggregates inclined to connect into large pieces instead of isolated spheres. This superior and unique rheological property of the compound solution can be promisingly applied for various industrial fields in which accurate control of the solution rheology is required.

 Please wait while we load your content...
Please wait while we load your content...