Novel polymer with ionic liquid moieties for biodiesel synthesis from waste oils
Junqiao Li,
Siyi Zhang,
Weiyong Tian and
Xuezheng Liang*
School of Chemistry and Chemical Engineering, Shaoxing University, Shaoxing, 312000, China. E-mail: xzliang@usx.edu.cn
First published on 7th November 2016
Abstract
A novel polymer with ionic liquid moieties was synthesized via a one-pot copolymerization and quaternization of 4-vinylpyridine, divinylbenzene and 1,2-dibromoethane. Then, acidic sites were introduced by ion-exchange. The acidic copolymer was applied to catalyze biodiesel synthesis from waste oils. The results show that the copolymer is very efficient for the reaction with a total yield of over 99% under mild conditions. The polymerization and quaternization were carried out simultaneously to form evenly dispersed ionic liquid moieties in the polymer matrix. The inlaid structure effectively prevents the bulky ionic liquid molecules blocking pores, which provides easily accessible active sites for reactants and results in high activity. The polymer combines the advantages of both ionic liquid and solid catalyst, and shows great potential in green chemistry.
1. Introduction
Ionic liquids (ILs) have a wide range of application in areas such as electrolytes, dye-sensitized solar cells, solvents and catalysis.1 The ionic liquids display high solubility. Some ILs can act as both solvent and catalyst for reactions and achieve high activities.2,3 However, the recovery of ILs from a homogeneous system is quite difficult. In addition, ILs are preferably solidified to develop certain devices. The solidification of ILs enhances their safety and long-term stability, and has become an important research field.4 The supports used are very important for the solidification process. Commercial silica gel with high surface area is widely used due to its low cost and ease of availability. The acidic IL modified silica gel is very efficient for the hydrolysis of cellulose.5 Mesoporous molecular sieves such as SBA-15 (ref. 6) and MCM-41 (ref. 7) have been used as supports. Besides silica, polymers have also been used. Nanoporous polydivinylbenzene (PDVB) with superhydrophobicity and superoleophilicity has been widely applied as a support for ILs.8 However, bulky IL molecules greatly decrease the surface area and cause pore blocking, which results in low IL loading amounts. It is essential to fabricate materials with both high IL amounts and BET surface areas.Biodiesel is a well-known renewable diesel fuel,9 which is generally synthesized via the transesterification of vegetable oils with methanol.10 The high cost and limited availability of vegetable oils are the main problems in the biodiesel industry. Vegetable oils account for 75% of the cost of total biodiesel synthesis, which causes the cost of biodiesel to be much higher than diesel derived from fossil fuels.11 Therefore, a decrease in the cost of biodiesel was highly desired and various cheap oils were used. Among these, waste oils such as frying cooking oil and trap grease will be good feedstocks for biodiesel synthesis that are 2–3 times cheaper than fresh oils and widely available in countries with large populations such as China.12 When compared to fresh oils, waste oils often contain a high free fatty acids (FFAs) content. Efficient alkaline-catalyzed processes are unsuitable for waste oils due to the high FFAs content. Acid-catalyzed pre-esterification treatment of the waste oils is often carried out to transform the FFAs. Liquid acids such as sulfuric acid and p-toluenesulfonic acid suffer from the drawbacks of equipment corrosion and tedious post-treatment.13 Recyclable catalysts are more suitable. Acidic ILs show high activities for the esterification of fatty acids and short chain alcohols with conversions of up to 96%.14,15 Solid superacid catalysts are also used for the reaction.16 However, the transformation of FFAs in waste oils is more difficult than that of pure fatty acids. The transesterification becomes a competitive reaction. Acidic ILs17,18 and carbon-based solid acids19 have been used during the pretreatment step. However, high reaction temperatures are needed to achieve satisfactory conversions. A two-step process adds to the operational complexity and production cost. Acid catalysts can catalyze both esterification and transesterification reactions.20 Various solid acids such as silica sulfuric acid,21 mono-vacant silicotungstate anchored to MCM-41,22 sulfated tin oxide,23 carbon-based solid acid,24 Zr-SBA-15 (ref. 25) and clay-based catalysts26 have been applied during the one-step biodiesel synthesis from waste oils. Harsh reaction conditions (220 °C) are needed to improve both FFAs and triglycerides conversion.27 Heteropolyacid showed yields below 88.6% at 80 °C.28 A zeolite-based catalyst gave a maximum yield of 46% at 70 °C.29 Efficient one-pot processes under mild conditions were presented using acidic ILs immobilized on hydrophobic polydivinylbenzene (DVB) in our previous report.30–33 However, the bulky IL molecules greatly decreased the BET surface area and IL loading amount. Herein, a novel polymer with IL moieties was synthesized via the copolymerization and quaternization of 4-vinylpyridine (VPy), divinylbenzene (DVB) and 1,2-dibromoethane. Dibromoethane was quaternized with vinylpyridine to form a cross-linked structure in the polymer matrix. The IL moieties were embedded in the framework with three chemical bonds, which avoided pore blocking. Then, ion exchange was carried out to introduce the acidic sites (Scheme 1). The novel copolymer was applied to catalyze biodiesel synthesis from waste oils. The copolymer showed high activity for the reaction with an optimal yield of over 99% at 70 °C.
2. Experimental
All organic reagents were commercial products of the highest purity available and used in the reactions without further purification.2.1 Synthesis of the polymer with IL moieties
A mixture of AIBN (0.05 g), DVB (2.0 g), VPy, 1,2-dibromoethane (half molar to VPy) and n-butanol (20 mL) was stirred at room temperature for 2 h to form a homogeneous solution. Then, the solution was transferred to a 50 mL Teflon-lined stainless steel autoclave and heated in an oven at 140 °C for 48 h. After cooling, a light yellow hard gel was obtained. The resulting gel was ground into a powder and washed three times with ethyl acetate, acetone and ethanol, sequentially to remove the unreacted materials and oligomer. Then, the solid was dried in an oven at 80 °C overnight to form a fluffy polymer with IL moieties. Then, the Br− ions were exchanged with HSO4−. The polymer was dispersed in 5 wt% sulfuric acid ethanol solution at a ratio of 1 g solid to 20 mL liquid. The mixture was heated to 85 °C overnight. The mixture was filtered and washed with ethanol to completely remove the Br− ions and excess sulfuric acid. After drying at 80 °C overnight, the acidic polymer was obtained.2.2 The procedure for biodiesel synthesis
Waste frying cooking oil obtained from a restaurant was used. Dehydration and decolorization with active carbon were carried out to remove the water and solid impurities. The waste oil with 22 wt% FFAs was used as raw material. A mixture of waste oil, methanol and catalyst was stirred at 70 °C for certain time. A condenser was used to avoid the loss of methanol via vaporization. The reaction process was monitored by GC analysis. After the reaction, the catalyst was recycled by filtration. The polymer was washed three times with ethyl acetate and dried at 80 °C for 4 h. Then, the catalyst was applied in subsequent cycles. The reaction mixture was distilled to recycle the excess methanol. Then, the reaction mixture was divided into two layers. The upper biodiesel layer was collected for quantitative analysis using a Shimadzu (GC-14C) gas chromatograph according to the method by Alcantara et al.34 Three moles of FFAs were regarded as one mole triglyceride. The esterification conversion was calculated from acid titration.3. Results and discussion
3.1 Characterization of the polymer with IL moieties
The acid sites in the polymer were introduced by ion exchange. The ion capacity could be controlled by the IL content. The bulky cations of the IL were inlaid in the polymeric framework, which effectively avoided the pore blocking. As a result, the active IL content was increased. Also, the hydrophobic and hydrophilic properties of the surface could be adjusted via the molar ratio of hydrophilic IL moieties and hydrophobic PDVB. Generally, more polar IL moieties benefited the hydrophilic surface. For biodiesel synthesis from waste oils, a hydrophobic surface is very important,28 which can efficiently separate the by-product water from the active sites. Herein, DVB was used to form the rigid hypercross-linked structure, which provides a high hydrophobic surface area. The BET surface area greatly decreased when the bulky IL was immobilized onto the PDVB. In order to obtain the appropriate acidity and hydrophobic surface, the ratio of DVB and VPy (IL moieties) was investigated (Table 1). The ratio of DVB and IL moieties greatly affected the polymers properties. Pure PDVB displays a high hydrophobic BET surface area of 946 m2 g−1 and acts as the support. The BET surface decreased with an increase in the VPy (IL moieties) amount, which was attributed to the pore blocking by bulky IL. For the pure polymer obtained using the IL, a high acidity of 4.9 mmol g−1 could be obtained, however, the BET surface was very low (only 2 m2 g−1). The polymer was unsuitable to catalyze the desired reaction due to the low BET surface. The polymer displayed both high acidity and surface area when 2.0 g of DVB and 0.7 g of VPy were used during the catalyst's synthesis. After ion exchange, the Br− was totally exchanged with HSO4− and an acidity of 1.8 mmol g−1 was obtained. Besides the IL content, the acidity could also be adjusted via controlling the extent of ion exchange. Br− can be partially exchanged with a certain amount of HSO4− to obtain a certain acidity. As a result, the polymer with a DVB and VPy mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 was chosen.
0.7 was chosen.
| Ratio/m/m (DVB/VPy) | Acidity/mmol g−1 | BET surface area/m2 g−1 |
|---|---|---|
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
4.9 | 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
2.5 | 135 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
1.8 | 538 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
0.8 | 746 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.2 | 946 |
The IR spectra of the polymer with Br− and HSO4− are shown in Fig. 1, respectively. The polymer showed almost the same absorption peaks, which indicated the same chemical bonding structure in both of the polymers. The mild ion exchange process did not destroy the original polymer structure, which accounted for the similar IR spectra. The strong absorption at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cm−1 was the C–N stretching vibration, which indicated that the VPy was successfully quaternized to form the pyridinium ionic liquid moieties in the polymer. The peaks at 1347 cm−1 are the C–H bending vibrations, which are derived from the alkyl groups in the polymer. The FT-IR spectra also showed the weak C
650 cm−1 was the C–N stretching vibration, which indicated that the VPy was successfully quaternized to form the pyridinium ionic liquid moieties in the polymer. The peaks at 1347 cm−1 are the C–H bending vibrations, which are derived from the alkyl groups in the polymer. The FT-IR spectra also showed the weak C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1601 cm−1) absorption peak, which originate from the residual double bonds in the polymer. There are also other functionality peaks including Ar–H (2925 cm−1) and OH (3400 cm−1). The FT-IR spectra also displayed multi-substituted aromatic ring peaks below 1000 cm−1, which were derived from DVB and VPy. On the other hand, the polymer with HSO4− anions gave additional absorption peaks at 1037 and 963 cm−1, which were assigned to S
C (1601 cm−1) absorption peak, which originate from the residual double bonds in the polymer. There are also other functionality peaks including Ar–H (2925 cm−1) and OH (3400 cm−1). The FT-IR spectra also displayed multi-substituted aromatic ring peaks below 1000 cm−1, which were derived from DVB and VPy. On the other hand, the polymer with HSO4− anions gave additional absorption peaks at 1037 and 963 cm−1, which were assigned to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from HSO4−. These peaks further indicated that the acidic sites were successfully introduced into the polymer.
O from HSO4−. These peaks further indicated that the acidic sites were successfully introduced into the polymer.
Elemental analysis of the polymer with IL moieties gave the following results: C 73.4%; H 6.5%; S 6.1% and N 2.7%. According to the S content, the acidity was a little lower. There may be some SO42− instead of HSO4− in the polymer, which lowers the acidity. On the other hand, SO42− shows a stronger interaction with H+ ion, which can effectively prevent the acidic sites from releasing H+. The N content agreed well with VPy amount in the raw material, which indicated that the copolymerization and quaternization occurred smoothly. Furthermore, the XPS of N showed a single peak, which confirmed that all the pyridine rings were quaternized by dibromoethane to form the IL structure. Also, the ICP analysis of the exchanged polymer showed no existence of the Br element, which indicates that both Br atoms of dibromoethane were involved in quaternization reaction to form the inlaid IL structure.
The BET surface area of the polymer with different anions was investigated. The copolymer with Br− gave the highest surface area of 623 m2 g−1, whereas the polymer with HSO4− has a BET surface area of 538 m2 g−1. The polymer displays a much higher BET surface area than the supported ILs catalysts comprised of even lower IL loading amounts.30–33 When compared to the traditional grafted structure, here the pyridinium IL moieties were introduced via a quaternization reaction and the bulky pyridinium cations were inlaid in the polymer framework, which effectively avoids pore blocking. Therefore, the polymer has a high BET surface area with more IL moieties. During the ion exchange process, the surface area decreased a little due to the larger volume of HSO4− when compared to Br−. The pore volume of the polymer decreased from 1.13 to 0.98 cm3 g−1 after ion exchange with the same average pore size of 17.3 nm. The high BET surface also benefited the ion exchange step, in which the ions could move freely. The high BET surface is very important for a heterogeneous catalyst and can greatly reduce the mass transfer hindrance.
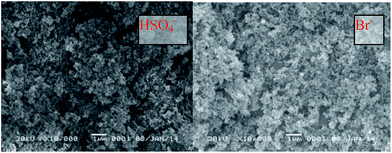
The scanning electron microscopy (SEM) images of the polymers with different anions are shown in Fig. 2. The resulting particles have microsphere structures with particle sizes of about 10–20 nm. The microspheres gathered together to form the packed structures, which was quite similar to the hypercross-linked PDVB.8 The IL moieties were introduced via the quaternization reaction with dibromoethane to form the cross-linked structure, which effectively avoids the formation of bulky particles. Furthermore, the hydrophobic surface of PDVB increased the affinity of the particles. Although the particles were inclined to accumulate, most of the particles still kept their clear boundary. Herein, the pyridinium cations inlaid in the polymer framework also formed some electrostatic repulsion between particles, which results in the well-dispersed microsphere structure. The gathered structures also formed some packed pores in the polymer, which provide additional surface area. The SEM image changed a little after ion exchange due to the mild reaction process. The particles appeared to gather more closely with the HSO4− anions, which may be caused by the stronger ion interactions between the HSO4− anions. The high BET surface benefits the mass transfer efficiency during the catalytic process.
3.2 Catalytic activity for biodiesel synthesis
First, the polymer was applied to catalyze the esterification (Fig. 3). Soapstocks are the main waste generated during the oil refining process in the oil industry. FFAs can be obtained via the simple acidification of these soapstocks. Herein, oleic acid was used as a representative for FFAs. The polymer showed high activity for the esterification reaction with a conversion over 99% after 3.5 h, which was superior to an IL immobilized on DVB.30–33 The high acidity and BET surface area accounted for the high activity. When compared to traditional acidic ILs (<95%),15 the polymer had a high hydrophobic BET surface, which made the acidic sites easily accessible to the reactants. The esterification was a typical reversible reaction. The amount of by-product water increased with the reaction time and the acidic ions interacted with water more easily to promote the reverse hydrolysis reaction, which reduced the reaction conversion. In regard to the polymer, the acidic sites are embedded in the hydrophobic surface. The by-product water was separated from the acidic sites by the hydrophobic surface, whereas the hydrophobic reactants could easily enter into the acidic sites,29 which was quite beneficial for the forward reaction. As a result, the reaction was carried out at relatively high speed of 2.07 mol (L h)−1 with a reaction conversion of over 45% after only 0.5 h. Moreover, the by-product water could hardly reach the acidic sites, which slowed the reverse reaction during the last stage of the reaction. As a result, the polymer showed high conversion. When compared to the ILs immobilized on DVB, the polymer provides more accessible active sites with its higher BET surface area and IL amount.30–33 The high activity for the esterification indicates that the polymer has great potential for biodiesel synthesis from waste soapstocks.Besides the FFAs, waste frying cooking oil was also used as a raw material (Fig. 4). The polymer also showed high activity for the reactions with a total yield of 99% at 70 °C only after 8 h. The reactions of the waste frying cooking oil were complex. The transesterification of triglycerides and esterification of FFAs were carried out simultaneously. Both reactions are reversible, which adds to the difficulty to achieve high yields. Furthermore, both reactions formed the same biodiesel and caused a high biodiesel concentration, which was unfavorable for the reversible reactions. Therefore, harsh reaction conditions (220 °C) are often applied to achieve satisfactory yields.19,23,24 Also, the reaction occurs at 220 °C to give a high yield of 99% after 2.5 h, which further confirmed the high activity of the polymer. The polymer displays excellent performance for the reactions with high yields obtained at low temperature (70 °C). The hydrophobic BET surface attached a lot of importance to the high activity. The reactants with long carbon chains showed a high affinity with the hydrophobic polymer and the catalyst was well dispersed in the reaction mixture. Moreover, the high BET surface added to the mass transfer efficiency, which enriched the reactants in the active sites and confirmed the high activity. On the other hand, the polar by-products water and glycerol were repulsed by the hydrophobic surface, which benefited the reaction equilibrium forward. The esterification of the FFAs occurred faster and the FFAs conversion was higher than the total yield during the first stage of the reactions. When compared to the pure FFA (oleic acid, Fig. 3), the esterification has a much lower speed. The waste oil contained a high triglycerides content, which caused the transesterification to be more competitive than esterification. Although triglycerides have a higher steric hindrance, several acidic sites were still occupied by triglycerides due to their high concentration in the reaction system. As a result, the FFAs conversion was greatly slowed and only 50% of the FFAs were converted after 1 h. The biodiesel concentration increased with the reaction time, which was a lot higher than that of the pure FFAs or triglycerides raw material. A high biodiesel concentration accelerates the reverse reactions. Therefore, the esterification reached its peak value after 8 h. For the triglycerides, the transesterification reaction was generally completed via three steps. The triglycerides transformed to diglycerides, monoglycerides and glycerol. Triglycerides cannot mix well with methanol and formed a heterogeneous reaction system. The reaction occurred at low speed in the beginning. The intermediate products such as diglycerides and monoglycerides with both hydroxyl groups and hydrophobic fatty chain showed good solubility in methanol. The reaction system gradually became homogeneous and the transesterification accelerated after 3 h. The total yield gradually exceeded the FFAs conversion due to the higher transesterification speed. The total yield reached its peak value of 99.1% after 8 h. Although the reaction time seemed to be much longer than the traditional catalyst,19,23,24 the reaction temperature was quite low. According to the relationship of the reaction rate and temperature, the reaction was relatively quick. Furthermore, the polymer showed high activity at 220 °C with a very short reaction time. The well-dispersed acidic IL moieties in the hydrophobic surface made the active sites easily accessible to reactants, which resulted in the high activity.
3.3 The recycled activity of the catalyst
The recovery of the polymer was simpler than that of the traditional acidic ILs. The filtration was easier than the time-consuming delamination process. After the reaction, the polymer was recovered by filtration. The recycled activities were investigated carefully (Fig. 5). For the acidic ILs immobilized on silica, the recycled activities dropped quickly.35 The polymer showed a high stability during the recycling process. The recycled polymer still showed a total yield of 98.7% for the sixth run. The polymer has highly cross-linked structures with bulky pyridinium cations inlaid in the framework, which prevent the active sites releasing. Moreover, the high hydrophobic surface provides enough space for the anions, which confirmed the active sites are easily accessible to the reactants. The elemental analysis of the recycled polymer showed no evident change. In addition, the element analysis of the filtrate also showed no nitrogen or sulfur residues. These results confirmed the high stability of the catalyst. The acidity of the polymer decreased slightly after six runs, which indicated that the HSO4− interacted well with the cations. Furthermore, the acidity could be regained through a simple ion exchange step.3.4 A comparative study of the different catalysts
The activities of different acid catalysts were compared and the results are shown in Table 2. The reaction conditions of all the catalysts were optimized to obtain optimal yields. Table 2 indicates that the novel polymer showed the highest activity for the reactions. For the Lewis acidic IL,36 the catalytic activity was the lowest for waste oils. The IL was quite sensitive to water. A large amount of by-product water was produced from the esterification of FFAs in the waste oils. The Lewis acidic IL was decomposed by water, which greatly decreased the catalytic activity. More by-product water destroyed more active sites with the reaction time. The transesterification can hardly be activated with the small amount of active sites available. Therefore, the total yield was even lower than the FFAs conversion. In regard to the water-proof acidic IL [SO3H–Bpy][HSO4], the catalyst showed much higher activity. The IL can totally dissolve in the reaction mixture and the active sites are well dispersed in the reaction system, which benefits the mass transfer efficiency and confirms the high activity. However, the free H+ ions dispersed in the reaction mixture interact with the polar water and triglycerides, which promotes the reverse reactions particularly during the last stage of the reaction. As a result, the relatively low yield of 94% was obtained after a long reaction time (16 h). Moreover, the IL was difficult to separate from the reaction mixture with high water content, which added to the operation cost. The widely-used acid catalyst H2SO4 showed an even lower catalytic activity than the acidic IL. H2SO4 has strong acidity, which was also very effective for the hydrolysis reaction. Therefore, the hydrolysis reaction was greatly promoted using the H2SO4 catalyst, which decreased the yield of biodiesel. H2SO4 also suffered from serious side reactions such as polymerization and carbonization, which reduced the product quality. The unrecyclability of the homogeneous catalytic process is another drawback. Amberlyst-15 showed a low activity for the reaction due to its low acidity 0.8 mmol g−1. When compared to the IL immobilized on PDVB,30 the polymer shows a higher activity with a lower catalyst amount and shorter reaction time. The polymer has a highly cross-linked structure, which prevents the bulky cations blocking the pores in the polymer. On the other hand, the cations embedded in the polymeric framework are involved in the pore formation process, which benefits the BET surface area. When compared to the IL immobilized on PDVB, the grafted bulky IL moieties would greatly block the pores in PDVB, which added the mass transfer hindrance. The high hydrophobic surface of PDVB is very important for the reaction, which promotes the reaction forwards.37 Furthermore, the use of propane sulfone added to the catalyst's cost. Besides high activity, the polymer demonstrates the advantages of low cost due to the cheap synthetic materials used in its synthesis. Therefore, the novel polymer is an optimal choice for biodiesel synthesis from waste oils.| Catalyst | Catalyst amount/mg | Reaction time/h | FFA conversion/% | Yield/% |
|---|---|---|---|---|
| a Reaction conditions: waste oil (5 g) and methanol (2.91 g) at 70 °C.b The yield was calculated using GC with an internal standard. | ||||
| Polymer | 40 | 8 | 98.9 | 99.1 |
| IL on PDVB24 | 50 | 9 | 98.3 | 99.1 |
| [Et3NH]Cl–AlCl3 | 60 | 24 | 86.5 | 78.8 |
| [SO3H–Bpy][HSO4] | 60 | 16 | 90.3 | 94.5 |
| H2SO4 | 70 | 18 | 88.5 | 89.7 |
| Amberlyst-15 | 500 | 18 | 79.8 | 81.4 |
4. Conclusions
A novel polymer with IL moieties has been synthesized under solvothermal conditions. Bulky pyridinium cations are inlaid in the polymeric framework, which effectively avoids pore blocking. The polymer has high activity for biodiesel synthesis from waste oils such as soapstocks and frying cooking oil under mild reaction conditions. The low catalyst cost, high activity, high stability, simple recovery and good reusability are key features of this novel polymer.Notes and references
- T. Yasuda, S. Nakamura, Y. Honda, K. Kinugawa, S.-Y. Lee and M. Watanabe, ACS Appl. Mater. Interfaces, 2012, 4, 1783 CAS.
- D. Tian, Y. Han, C. Lu, X. Zhang and G. Yuan, Carbohydr. Polym., 2014, 113, 83 CrossRef CAS PubMed.
- X. Zhang, W. Zhang, D. Tian and C. Lu, RSC Adv., 2013, 3, 7722 RSC.
- J. Yuan, S. Soll, M. Drechsler, A. H. E. Muller and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 17556 CrossRef CAS PubMed.
- A. S. Amarasekara and O. S. Owereh, Catal. Commun., 2010, 11, 1072 CrossRef CAS.
- S. Rostamnia, A. Hassankhani, H. G. Hossieni, B. Gholipour and H. Xin, J. Mol. Catal. A: Chem., 2014, 395, 463 CrossRef CAS.
- B. Wang, J. Zhang, X. Zou, H. Dong and P. Ya, Chem. Eng. J., 2015, 260, 172 CrossRef CAS.
- Y. Zhang, S. Wei, F. Liu, Y. Dua, S. Liu, Y. Ji, T. Yokoi, T. Tatsumi and F.-S. Xiao, Nano Today, 2009, 4, 135 CrossRef CAS.
- D. E. López, J. G. Goodwin, D. A. Bruce and S. Furuta, Appl. Catal., A, 2008, 339, 76 CrossRef.
- H. Yun, M. Wang, W. Feng and T. Tan, Energy, 2013, 54, 84 CrossRef CAS.
- S. Morais, T. M. Mata, A. A. Martins, G. A. Pinto and C. A. V. Costa, J. Cleaner Prod., 2010, 18, 1251 CrossRef CAS.
- P. Felizardo, M. J. N. Correia and I. Raposo, Waste Manag., 2006, 26, 487 CrossRef CAS PubMed.
- J. Y. Park, D. K. Kim and J. S. Lee, Bioresour. Technol., 2009, 101, S62 CrossRef PubMed.
- L. Zhang, M. Xian, Y. He, L. Li, J. Yang and S. Yu, et al., Bioresour. Technol., 2009, 100, 4368 CrossRef CAS PubMed.
- D. Fang, J. Yang and C. Jiao, ACS Catal., 2011, 1, 42 CrossRef CAS.
- A. Patel, V. Brahmkhatri and N. Singh, Renewable Energy, 2013, 51, 227 CrossRef CAS.
- Z. Mana, Y. A. Elsheikhb, M. A. Bustama, S. Yusupa, M. I. A. Mutaliba and N. Muhammada, Ind. Crops Prod., 2013, 41, 144 CrossRef.
- Y. A. Elsheikh, Z. Man, M. A. Bustam, S. Yusup and C. D. Wilfred, Energy Convers. Manage., 2011, 52, 804 CrossRef CAS.
- Q. Shu, Z. Nawaz, J. Gao, Y. Liao, Q. Zhang and D. Wang, et al., Bioresour. Technol., 2010, 101, 5374 CrossRef CAS PubMed.
- B. X. Peng, Q. Shu, J. F. Wang, G. R. Wang, D. Z. Wang and M. H. Han, Process Saf. Environ. Prot., 2008, 86, 441 CrossRef CAS.
- K. A. Shah, J. K. Parikh and K. C. Maheria, BioEnergy Res., 2014, 7, 206 CrossRef CAS.
- A. Patel and N. Narkhede, Catal. Sci. Technol., 2013, 3, 3317 CAS.
- M. K. Lam, K. T. Lee and A. R. Mohamed, Appl. Catal., B, 2009, 93, 134 CrossRef CAS.
- Q. Shu, J. Gao, Z. Nawaz, Y. Liao, D. Wang and J. Wang, Appl. Energy, 2010, 87, 2589 CrossRef CAS.
- J. A. Melero, L. F. Bautista, J. Iglesias, G. Morales and R. Sánchez-Vázquez, Catal. Today, 2012, 195, 44 CrossRef CAS.
- M. A. Olutoye and B. H. Hameed, Appl. Catal., A, 2013, 450, 57 CrossRef CAS.
- P. Kumaran, N. Mazlini, I. Hussein, M. Nazrain and M. Khairul, Energy, 2011, 36, 1386 CrossRef CAS.
- A. Talebian-Kiakalaieh, N. Aishah Saidina Amin, A. Zarei and I. Noshadi, Appl. Energy, 2013, 102, 283 CrossRef CAS.
- M. Hassani, G. D. Najafpour, M. Mohammadi and M. Rabiee, J. Sci. Ind. Res., 2014, 73, 129 CAS.
- X. Z. Liang, Energy, 2013, 63, 103 CrossRef CAS.
- X. Z. Liang, H. Q. Xiao and C. Z. Qi, Fuel Process. Technol., 2013, 110, 109 CrossRef CAS.
- X. Z. Liang, Ind. Eng. Chem. Res., 2013, 52, 6894 CrossRef CAS.
- X. Z. Liang, Appl. Catal., A, 2013, 455, 206 CrossRef CAS.
- R. Alcantara, J. Amores, L. Canoira, E. Fidalgo, M. J. Franco and A. Navarro, Biomass Bioenergy, 2000, 18, 515 CrossRef CAS.
- Z. Xu, H. Wan, J. Miao, M. Han, C. Yang and G. Guan, J. Mol. Catal. A: Chem., 2010, 332, 152 CrossRef CAS.
- X. Liang, G. Gong, H. Wu and J. Yang, Fuel, 2009, 88, 613 CrossRef CAS.
- F. Liu, L. Wang, Q. Sun, L. Zhu, X. Meng and F.-S. Xiao, J. Am. Chem. Soc., 2012, 134, 16948 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |