Nanomaterials as a game changer in the management and treatment of diabetic foot ulcers
M. V. Vellayappan
a,
S. K. Jaganathan
*bcd and
A. Manikandan
e
aFaculty of Biosciences and Medical Engineering, Universiti Teknologi Malaysia, Johor Bahru 81310, Malaysia
bDepartment for Management of Science and Technology Development, Ton Duc Thang University, 19 Nguyen Huu Tho Street, Tan Phong Ward, District 7, Ho Chi Minh City, Vietnam. E-mail: saravana@tdt.edu.vn
cFaculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, Vietnam
dIJN-UTM Cardiovascular Engineering Centre, Faculty of Biosciences and Medical Engineering, Universiti Teknologi Malaysia, Johor Bahru 81310, Malaysia
eDepartment of Chemistry, Bharath University, Chennai, Tamil Nadu 600 073, India
First published on 1st December 2016
Abstract
Nanoengineered biomaterials have tremendously improved the range of tools utilized for the control of as well as acceleration of healing of diabetic foot ulcers (DFU) over the last few decades. Despite nanoparticles and electrospun nanofibers being used extensively for the treatment of DFU, there has been no review available till now which addresses the utilization of the latest nanodelivery techniques along with modern electrospun nanofiber treatments for DFU. This review thoroughly summarizes the latest mindboggling findings of potential nanomaterials like polymeric and metallic nanoparticles and electrospun nanofibers for DFU treatment. In addition, a succinct insight into the potential of the aforementioned nanomaterials which can be exploited as therapeutic delivery agents for improving the regeneration of damaged dermal and epidermal tissues of DFU is underscored. Ultimately, current challenges and future prospects of nanoparticles and electrospun nanofibers for DFU treatment are highlighted.
1. Introduction
Diabetes mellitus (DM) is a progressive and chronic endocrine problem that occurs most commonly in hyperglycemic (excess of blood glucose level) patients. Diabetes is contemplated to be one of the most dreadful and major health problems in the world, showing an alarming increase. The occurrence of diabetes amongst different age groups across the globe was 2.8% in 2000 (171 million) and it is predicted to increase by 4.4% by 2030 to 366 million. At present it is found that 200 million patients across the globe are affected by diabetes and this count is estimated to rocket by 333 million by 2025. The highest increase in diabetic patients is expected in developing countries (4.2% to 5.6%).1,2 In general, patients with diabetes either completely lack insulin level (type 1 DM) or have very little insulin or cannot use the produced insulin efficiently (type 2 DM). In the case of type 1 DM, the body's immune system kills the cells that release insulin, thereby leading to complete elimination of insulin production in the body. However, in type 2 DM, the patient's body becomes incapable of utilizing the insulin properly. This condition is known as insulin resistance. When the type 2 DM gets worse, the pancreas will produce less insulin and this is known as insulin deficiency. Epidemiological studies have dictated that the worldwide prevalence of type 1 DM has been incremented by 2–5% to almost 1 in 300 at the age of 18 years in the United States.3 The global incidence of type 2 DM has shown tremendous growth over the past few decades.4,5 By the year 2025, it is predicted that there will be 40 million diabetic patients in China and India alone.6 According to the statistics of the International Diabetes Federation, two individuals will develop diabetes and another two will die of diabetes-related complications every 10 seconds across the globe.7 Hence, diabetes has become a critical public health problem that results in a tremendous socioeconomic burden.Nearly 25% of diabetic patients suffer from a higher reported life time risk of developing foot complications,8 and the diabetic foot ulcer (DFU) is the most common complication with an estimated incidence of 25 to 80%.9 Most foot ulceration finally turns into diabetic gangrene if left untreated, resulting in approximately 80% of lower limb amputations.10–13 More than 50% of diabetic wounds can exponentially increase the risk of below-knee amputation,14–16 which appreciably increases mortality apart from resulting in debilitating complications.17–20 Mostly, DFU involves the toes.21 If appropriate therapy is not conferred, the amputation of the affected bone becomes unavoidable.22
Different treatment approaches have been adopted to treat DFU, such as the use of topical wound-care therapies.23 Even though various topical and systemic compounds were used either alone or in combination with different materials to prevent infections, many have been eliminated because of developing bacterial resistance to antiseptics and antibiotics.24,25 These agents have resulted in the emergence and subsequent rapid overgrowth of resistant bacterial strains, drug side effects, and organ-specific toxicity.24–26 Diabetic wound infections due to drug-resistant organisms are now becoming quite common and they have significantly increased resistance to common antibiotics.27
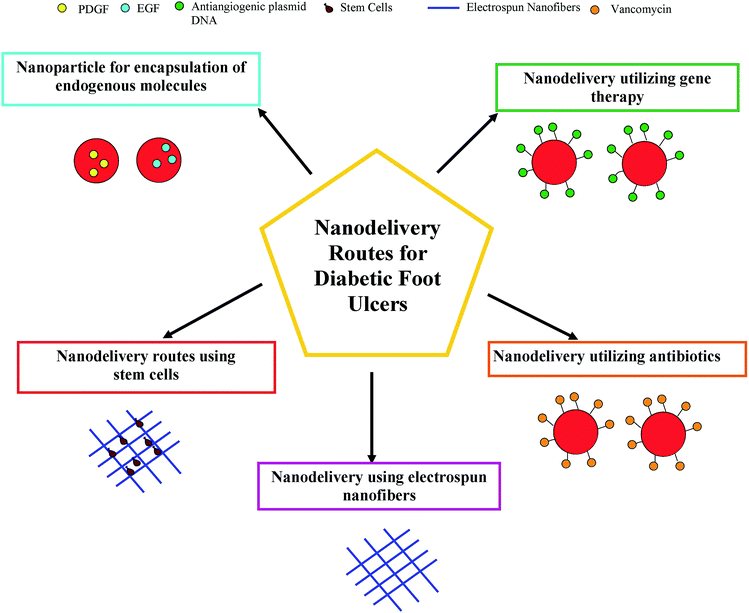
Nanotechnology may play a crucial role in diagnosing, repairing, controlling and even building our human biological systems at the cellular level. This feat is only possible if we can manipulate atoms and molecules at the nanometer scale. The nanomaterials function at a range almost identical to the building blocks of the human cells. Thus, nanotechnology confers fascinating pathways to revolutionize today's medicine. Nanotechnology-based treatment to ameliorate diabetes is one of the research areas most focused on at present. Nanoparticles can be used in different forms, like endogenous bearing molecules, conjugated nanoparticles and pure forms, to treat DFUs. Likewise, electrospun nanofibers may also be exploited for the treatment of DFUs. Hence, in this article the potential application of the above-mentioned two classes of nanomaterials, namely nanoparticles and electrospun nanofibers, in the treatment and management of DFU is presented. In order to understand the pathophysiology of DFUs, the difference between normal wound healing and DFUs is given in the following section.
2. Comparison between normal and DFU wounds
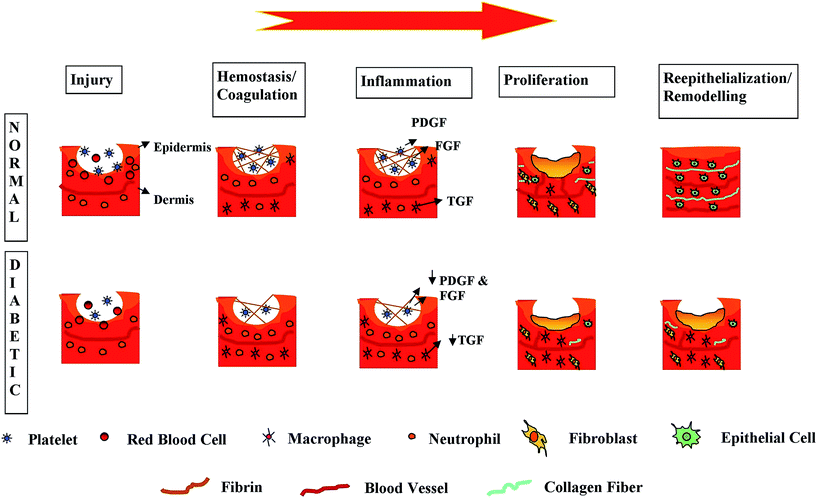
Wound healing is an intricate process involving different types of cells.28,29 The five wound healing stages do not occur in a particular period but may overlap.30–32 The transition between the stages primarily depends on the maturation as well as the differentiation of different skin cells. Fig. 1 shows the different phases involved in wound healing which are impaired for diabetic patients.In general, re-epithelialization commences a few hours after injury. Bioactive growth factors, like the keratinocytes and activated fibroblasts, will arrive at the wound site where they will multiply and form an ECM, accelerating wound closure.28,30 For inducing angiogenesis, protease activity is also necessary.33,34 In the ultimate stage of wound healing, degradation of the granulation tissue and dermis regeneration occur.35 An acute wound generally recovers linearly via the different wound healing phases; however, DFU healing is not the same since it may stop in any one or more of the aforementioned wound healing stages.31 A process similar to the healing of acute wounds is needed in order to accelerate DFU healing processes. This is necessary for long-term healing from contamination/infection, to avert wound dissection and to provide a conducive environment for wound healing.36
Different treatment approaches have been adopted, such as the use of topical wound-care treatment.23 Even though various topical and systemic compounds were used either alone or in combination with different materials to prevent infections, many have been eliminated because of resistance. These agents have resulted in the quick overgrowth of resistant bacterial strains, drug side effects, and organ-specific toxicity.24–26 DFU infections due to drug-resistant microbes are increasing day by day and they have significantly increased resistance to common antibiotics. This has resulted in increased costs, morbidity, and mortality rate due to diabetic wounds.27 Therefore more research has been carried out on wound care with a special interest in new therapeutic treatment modalities and the development of technologies for acute and chronic wound management.37–39 Hence, in the following section, the potential application of nanoparticles for the treatment of DFU is discussed.
3. Nanoparticles for encapsulation of endogenous molecules for DFU
As discussed earlier, the wound healing pattern can be modulated using proteins like cytokines and growth factors.40 Since the soluble proteins exert a control over wound repair, they have gained increasing interest for DFUs. Studies indicate that the aforementioned exogenous recombinant growth factors were tested in the past decade to accelerate DFU healing.41 Despite there being several pilot studies showing good results, the overall result has been not promising enough. To date, the US FDA have approved only PDGF-BB for diabetic foot ulcer treatment.42 The major setback in the utilization of growth factor for topical use is its short half-life.33 Thus, growth factor utilization in its pristine form is stopped by its breakdown instigated by proteolytic enzymes at the wound site.43 A multitude of efforts has been undertaken to confer resistance to enzymatic degradation to growth factor. At present, the advent of nanoscale systems for drug delivery has opened up a wide range of opportunities to improve the efficiency of endogenous molecules to deliver over a prolonged period in a controlled manner.44 There are basically six potential endogenous molecules for diabetic foot ulcer treatment, which will be discussed in what follows.Thrombin has a notable role in fibrin clot formation as well as platelet activation, where it triggers the chemotaxis and aggregation of neutrophils, lymphocytes, and monocyte cells. Moreover, it also stimulates the growth of fibroblasts, epithelial cells and endothelial cells.45,46 Hence, it has a pivotal role in triggering the early events involved in the cellular events of tissue repair. The thrombin receptor activating peptides may provide a useful mimic of the natural effect of thrombin interaction with various cells.47 Recently, nanotechnology has provided a potential method to improve its bioavailability via conjugation with iron oxide nanoparticles (γ-Fe2-O3 conjugation).48,49 In vivo test results depict that the wound response to treatment with γ-Fe2-O3 was remarkable. Analysis of the wound's tensile strength indicates appreciable acceleration of the healing process in the γ-Fe2-O3 treated wound against untreated wounds.
Nitric oxide is a small radical which is made from amino acid L-arginine. The NO aids wound healing in unique ways in animal wound healing models.50 NO possesses good antimicrobial properties.51 The utilization of NO for wound healing therapy has been unresolved until now. Different trials using novel NO therapies, in the form of nitric oxide donors, have been found to have limited ability in the treatment of cutaneous infection.52 Nanomaterials recently made this challenging task possible by providing the ability to store and deliver small gaseous short-lived NO to exhibit their antibacterial activity. The NO remains trapped within a hydrogel where it is stable until it gets exposed to a moist environment.53 The hydrogel provides an ideal matrix to easily store and use NO when it is required. This sustained release provides an advantage to the nanoparticles compared to other vehicles, like injections, that release a higher concentration of the drug rapidly. Easy storage, application and the provision to change the release rate and concentration with a minimal risk of toxicity make this powder formulation a potential candidate for cutaneous delivery. There are other similar researches that demonstrate the effectiveness of NO-releasing nanodelivery systems in the treatment of DFU.54,55 Similarly, NO-releasing silica nanomolecules possess good bactericidal activity against Pseudomonas aeruginosa56 and Acinetobacter baumannii57 which have high levels of resistance to common antibiotics.58 Moreover, NO was also found to possess antimicrobial activity apart from its direct involvement in the wound healing process. Hence, the utilization of NO-releasing nanoparticles may improve DFU healing by promoting angiogenesis and tissue remodeling as well as by killing bacteria.
Growth factors have gained significant attention for wound healing in recent days. They possess the ability to speed up wound healing via cell migration to the wound site (TGF-β), stimulating the proliferation of epithelial cells and fibroblasts (FGF and PDGF), initiating new blood vessel development (FGF and VEGF), and are ultimately involved in scar formation.33 In vivo efficiency of growth factor performance can be improved by preventing its degradation by proteolytic enzymes. This can be achieved by prolonging the duration of growth factors at the delivery site.59,60 Poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) are biodegradable polymers which have good biocompatibility. Long-term in vivo circulation can be achieved using a surface that is generally modified with polyethylene glycol (PEG).61 When biodegradable nanocarriers are used, the release of active constituents like growth factors can be controlled by the polymer degradation rate.62 Recently, hyaluronan-based (HYAFF11) porous nanoparticles were loaded with PDGF for DFU treatment.63 PDGF has been used for the last two decades to accelerate chronic wound healing.64 HYAFF11 is a biopolymer that can be used for biomedical applications like in vitro skin reconstruction.65 HYAFF particles possess the potential to absorb growth factors and release them periodically as well as in a controlled manner. Hence, this technique of specific controlled and localized PDGF release possesses the potential to accelerate DFU wound healing.
Opioids have recently been explored to promote keratinocyte migration.66 This area of research has gained great interest since it accelerates possible wound healing via the topical application of opioids. The common treatment modalities for DFU fail in terms of providing necessary pain control in patients because of the lack of sufficient drug concentrations at the target site.67 As painful wound dressing changes take a long time, pain control must release opioids for an extended duration. Bigliardi et al. demonstrated the ability of nanoparticulate carriers to increase opioid skin penetration and delay the release of the loaded drug.67 Their study carried out on human keratinocytes showed that opioids trigger cell migration and lead to the closure of the experimental wounds. This increased cell migration is concentration dependent. It can be stopped by the opioid receptor antagonist naloxone, dictating a specific opioid-receptor interaction. Similarly, in another study, Kuchler demonstrated that morphine loaded into solid lipid nanoparticles quickened the wound healing process by accelerating the re-epithelialization in a human 3D wound healing model.68 It was observed that the keratinocytes almost covered the wound in just four days, which was in contrast to the case of applying unloaded particles. To conclude, decreased cytotoxicity and irritation, and an extended period of morphine release make this solid lipid nanoparticle a fascinating option for the treatment and management of DFUs.
Matrix metalloproteinases (MMPs) break most of the macromolecules during the maturation phase of the wound.69 This phase requires a perfect balance between collagen production, breakdown, and remodelling. The activity of MMP is controlled by specific tissue inhibitors. Good coordination between the MMP and TI is obligatory to achieve optimum wound maturation. Hence, recent researches have focused on improving the remodeling phase. In recent work, Norling et al. utilized aspirin-triggered D1 and lipoxin A4 analogs and developed a carrier.70,71 Its ability to accelerate wound healing was examined in a mouse model. Polymorphonuclear cell influx displayed a good result in reducing the wound size that ultimately resulted in accelerated healing. Hence, the potential of nanoparticles to bear endogenous molecules for DFU has been discussed in this section. In the upcoming section, the nanoparticles' ability to deliver antibiotic for DFU treatment will now be discussed.
3.1 Nanodelivery utilizing antibiotics for DFU treatment
DFU infection is one of the major challenging issues exacerbating tissue damage.72 Pharmacological treatment faces numerous challenges owing to the increase in antibiotic-resistant strains. For instance, resistance to penicillin by Staphylococcus aureus leads to the failure of treatment against staphylococcal local infections.73 Antibiotic therapy through nanoparticles provides promising solutions for DFU treatment. Exceptional physiochemical traits of nanoparticulate drug delivery systems allow optimal management of size, surface charge, and nature for the delivery of antibiotics in vivo.74The most advanced as well as effective treatment for staphylococcal infection is vancomycin. Vancomycin-modified nanoparticles have been synthesized for increasing the pharmacokinetics of the antibiotic molecule. Hachicha et al. demonstrated the utilization of vancomycin-conjugated nanoparticles for intraocular continuous release injection for endophthalmitis prophylaxis.75 It was observed that the drug concentration was maintained to be more than the minimal inhibitory limit for 24 hours. Likewise, Prasad et al. depicted the use of carboxymethyl chitosan-based nanoparticles loaded with vancomycin as well as proving its effectiveness against drug-resistant staphylococcal strains.76 Similarly, in a recent study by Chakraborty et al., a folic acid tagged nanosized chitosan vehicle was loaded with vancomycin to study its efficacy against drug resistant S. aureus.77 The study results revealed that the tolerance level of the strains to vancomycin and nanoconjugated vancomycin were almost similar. However, in the case of drug-resistant S. aureus strains the tolerance level reduced when nanoconjugated vancomycin was utilized. In addition, it was also observed that the nanoconjugated vancomycin reduced the minimum inhibitory concentration (MIC) and minimum bactericidal concentration values of vancomycin-resistant S. aureus strains. Nevertheless, without folic acid tagging, the chitosan nanodelivery was found to be inefficient against drug-resistant strains. This is because folic acid is a significant nutrient required for nucleotide synthesis by the bacteria; it helps to transport the chitosan nanoparticles loaded with vancomycin drug via endocytosis across the plasma membrane into the cytoplasm.78,79 Hence, this promising solution has tremendous potential in circumventing the problems associated with vancomycin-resistant S. aureus.
The ability of nanobiotechnology to improve the therapeutic potential of antibiotic molecules can be further elucidated by the case of N-methylthiolated β-lactams. These have been recognized to possess efficient antibacterial activity against Staphylococcus bacteria, including MRSA. This β-lactam compound exhibits growth-inhibitory effects on bacteria via a mode of action that is unique compared to other β-lactam antibiotics and it possesses different structure–activity patterns. However, its limitation is its poor water solubility.80 Hence, Turos et al. developed a novel method to obtain an emulsion of polyacrylate nanoparticles where the drug monomer was encapsulated in a polymeric matrix. In vitro studies show that the nanoparticles are nontoxic to human skin cells.81
3.2 Nanodelivery routes for gene therapy in DFU
The polymeric gene delivery system possesses some benefits for plasmid DNA delivery, such as protecting the plasmid DNA. Its potential, which retains the modulation of gene expression while wound healing, has attracted numerous researchers for the development of efficient DFU wound dressing. Transfection capacity has already been tested in vitro where PLGA polymers82 were synthesized to obtain a high plasmid loading efficacy and then it was loaded with antiangiogenic plasmid DNA (pFlt23k).83 The PLGA nanoparticles were produced with the help of a supercritical fluid extraction method of emulsions based on CO2. The results of the study dictate that VEGF secretion by epithelial cells was notably decreased, showing its potential value in the treatment of DFU.Different materials can be considered for the nonviral DNA carriers for gene therapy like polysaccharides and other cationic polymers. Chitosan is a potential material for the long term release of the incorporated drug.84 Masotti and Ortaggi demonstrated a nanofabrication technique to obtain DNA-containing chitosan nanospheres (3.8 ± 4 nm).85 Likewise, Chellat et al. explored the utilization of chitosan to test DNA-loaded nanoparticles.86 The secretion of MMP-9 in cell supernatants was found to increase appreciably after 24 and 48 hours compared to non-treated cells. MMP-2 secretion was found to be greater after 48 hours. Hence, all these results suggest that utilization of nanoparticles in gene therapy may accelerate DFU wound healing.
3.3 Nanodelivery routes using stem cells in DFU
In the last ten years, there have been mindboggling advancements in the field of nanotechnology for stem cell isolation, maintenance, and regulation. The regulation of stem cells involves the utilization of nanoparticles and nano 3D architectures to control stem cell proliferation, differentiation, and maturation.87 In the case of skin regeneration, VEGF high-expressing, transiently modified stem cells have been developed to promote angiogenesis.88,89 To circumvent the problem of lack of expression of angiogenic factors and low cell viability after transplantation, nanotechnology has paved the way to a novel solution through nonviral nanoparticles to deliver hVEGF genes to human mesenchymal stem cells (hMSCs) and human embryonic stem cell-derived cells (hESdCs). This is shown in Fig. 2.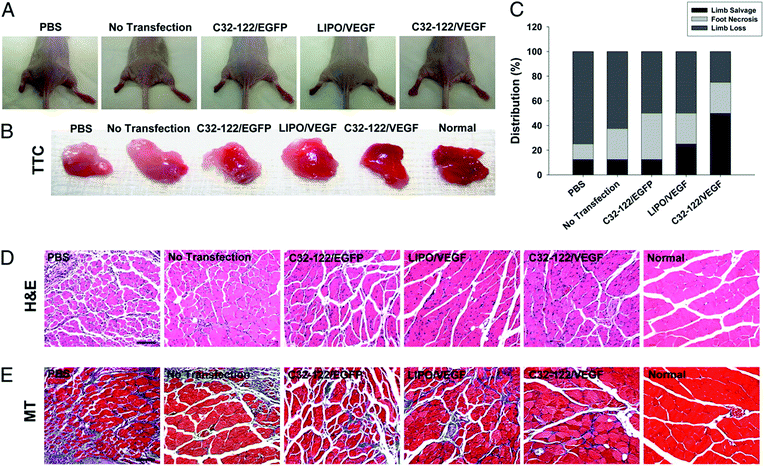 | ||
| Fig. 2 Improved ischemic limb salvage by using biodegradable polymeric nanoparticles. (A) Treated ischemic limbs and controls. (B) TTC staining results of muscles. (C) Status of ischemic limbs which were studied 4 weeks following surgery. This was classified into three levels; limb salvage, foot necrosis, or limb loss. (D) H&E staining showed massive muscle degeneration in the ischemic regions of control limbs (PBS-injection group), and such muscle degeneration was markedly reduced in a group receiving biodegradable polymeric nanoparticles with VEGF. (E) Masson's trichrome staining exhibited notable fibrosis in the control groups.85 | ||
The nanotechnology-modified stem cells showed improved hVEGF production, cell viability, and engraftment into target tissues. The implantation of scaffolds seeded with VEGF-expressing stem cells (hMSCs and hESdCs) was found to form 2 to 4 fold higher vessel densities in just 2 weeks following implantation, in comparison to the control cells or cells transfected with VEGF by using Lipofectamine 2000, which is a leading commercial reagent. Four weeks after intramuscular injection into mouse ischemic hindlimbs, genetically modified hMSCs significantly improved angiogenesis and limb salvage, apart from reducing muscle degeneration and tissue fibrosis.88 These results show that stem cells combined with nanotechnology may play a pivotal role in the treatment and management of DFU.
3.4 Nanoparticles for treatment of DFU
Oxidative stress plays a vital role in the etiology of most diabetic complications.90,91 Among them all, DFU healing has been the main challenge since it is an intricate process.92Here, the impairment in the healing occurs due to late cellular infiltration and granulation tissue formation, decreased angiogenesis, reduced collagen, and its organization.93,94 During the inflammatory phase of wound-healing, neutrophils and macrophages invade the wound. The neutrophils will arrive and are abundant at the wound site within one to three hours after wounding. Later, lymphocytes as well as monocytes will also arrive at the wound tissue and later differentiate into activated macrophages.95 These neutrophils and macrophages will produce large amounts of superoxide radical anions and this phenomenon is commonly described as the “respiratory burst”. In addition, other cells like fibroblasts can be triggered by pro-inflammatory cytokines to produce ROS.96 The production of these reactive molecules is a component of the innate immune system to quickly clean the wound from invading bacteria.97 Apart from the useful role of microbial killing by ROS, it can also have a series of negative effects. For instance, at lower levels, hydrogen peroxide and other ROS restrict the migration and proliferation of different important cells like keratinocytes.98 When the ROS is at a higher level it can lead to severe tissue damage.99 Recently, nanoparticles have been discovered to act as free radical scavengers.100
The cerium oxide (CeO2) nanoparticle is one of the most attractive options to be utilized for DFU healing due to its active potential to serve as a free radical scavenger.101 This metal oxide is a monodispersed particle which possess a single crystal and few twin boundaries with an expanded lattice parameter.102,103 The cerium atom has both +4 and +3 oxidation states. This depicts its dual oxidation state, meaning that these nanoparticles have oxygen vacancies.104 Generally, when the loss of oxygen occurs, this will lead to the reduction of Ce4+ to Ce3+ followed by the creation of an oxygen vacancy. This improves the potential of CeO2 nanoparticles to aid in the wound healing process followed by scavenging properties.
Yttrium oxide (Y2O3) can be considered for DFU treatment owing to its highest free energy for oxide formation from elemental yttrium compared to other metal oxides.105 It is characterized by a minor alteration from stoichiometry at room temperature and pressure.106 These types of nanoparticles are commonly non-toxic to neutrophiles as well as to macrophages. The potential of cerium and yttrium oxide nanoparticles to rescue cells from oxidative stress-induced cell death generally depends on the structure of the nanoparticle. Hence, these nanoparticles may be exploited for the treatment of DFU.
A thorough literature survey depicts three possible mechanisms for the CeO2 and Y2O3 nanoparticles to protect against oxidative stress damage for DFU treatment. (a) They may play a role as direct antioxidants, blocking ROS production, whilst inhibiting the programmed cell death pathway. (b) They may result in a low level of ROS production, which quickly triggers a ROS defense system before the glutamate-induced cell death program is complete. (c) The triggering of the ROS defense system could be caused by the exposure of cells to particulate material known to result in low levels of ROS.107–109
Similarly, nanoparticles made of other metal oxides can also be considered for their potential scavenging to be used for the treatment of DFU. This includes nanoparticles of aluminum oxide (Al2O3), which is commonly called alumina and silver nitrate. Silver nitrate has been widely used in various clinical studies as an antimicrobial agent for treating chronic wounds.110 In addition, it is also effective against a spectrum of bacteria, viruses and fungi.110–112 Novel Ag+-loaded zirconium phosphate nanoparticles were found to play a significant role in the treatment of diabetic wounds.113 Likewise, gold nanoparticles (AuNPs) have been widely explored in recent times for medical applications.114 The potential of AuNPs to inhibit the lipid from peroxidation and to prevent ROS generation has been found to restore the imbalances in the antioxidants.115 Barathmanikanth et al. demonstrated the anti-oxidative and antihyperglycemic potential of AuNPs.116 Hence, these nanoparticles can be utilized for the effective treatment of DFU.
Silver has been used for the treatment of ulcers since the 5th century B.C. Its antimicrobial activity was first reported in the 19th century. However, following the discovery of antibiotics in the 1940s, the utilization of silver salts was reduced. Later on, it was widely used for medical applications, particularly for burn treatment.117
Silver possesses an antibacterial effect and low systemic toxicity.118,119 It also provides an antibacterial effect that could tremendously decrease the possibility of developing resistance. Nanotechnology introduced novel methods for synthesizing pure biostable silver nanoparticles.120 In addition, it can be used along with common antibiotic therapy.120 An extensive in vivo study shows that there are multiple ways through which the silver exhibits its antibiotic activity.121 In addition, it accelerates the wound healing rate by promoting the proliferation and migration of keratinocytes.122 The result of the study is shown in Fig. 3. Thus, silver nanoparticles can be used as a putative candidate for the treatment of DFU.
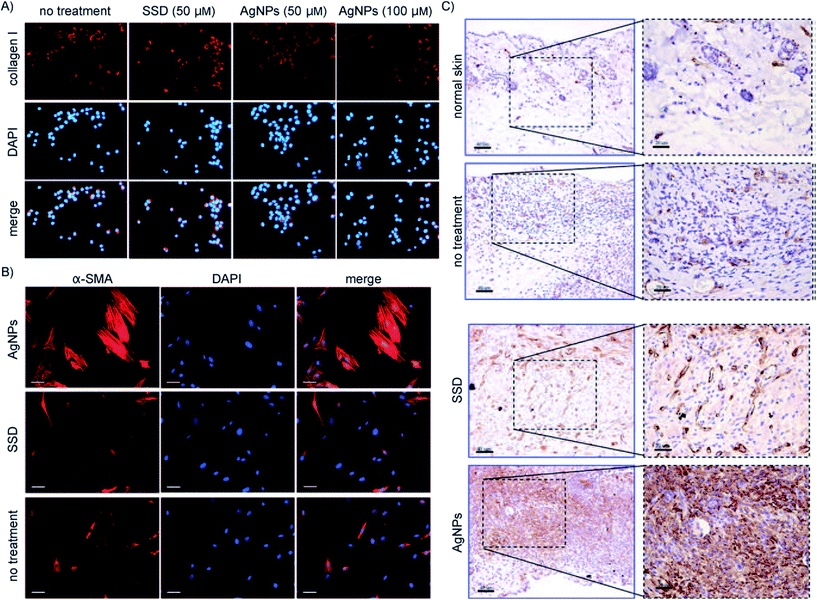 | ||
| Fig. 3 Silver nanoparticles (AgNP) induced differentiation of fibroblasts into myofibroblasts: (A) 3T3 fibroblast cell line with silver sulfadiazine (SSD) and AgNP, and the level of collagen I measured. (B) Myofibroblasts stained using alpha smooth-muscle actins (α-SMA) after culturing 3T3 fibroblasts with AgNPs or SSD for 48 h. (C) α-SMA staining of wounds at day 7 in animals treated with either AgNPs or SSD, and comparison against normal skin.110 | ||
In spite of the advanced development of the therapeutic application of silver nanoparticles, they may pose some side effects which must be addressed. Researchers have been analyzing the biosafety aspect of silver nanoparticles for DFU treatment. They have reported that silver nanoparticles have an acceptable biocompatibility, yet occasionally develop argyria.123 Nanomaterial toxicity needs to be further explored as it is significant to consider in potential health and safety issues. The determinants of particle toxicity have found that they have a large surface area and chemical reactivity when they are at a small size. They also have the ability to penetrate tissues and cells.124 Hence, nanoparticles are likely to be more dangerous compared to the same chemicals in a larger form, and free particles are more toxic than fixed ones.125 In addition, the nanoparticles can reach the blood circulation and be distributed to various parts of our body and may result in toxicity.126 These cases have been researched in vitro and in in vivo animal models. However, extensive studies have to be performed in humans provided that promising results are obtained in animal trials. Thus, the use of nanoparticles in humans demands detailed studies and more caution.
4. Electrospinning for treatment of DFU
Traditional wound dressing generally functions as a transitory barrier for hemostasis and prevents infection.127 Recently, artificial skin grafts have been developed for skin replacement. Yet, the developed products are high in cost, demand extensive care and do not restore complete skin functionality.128 Hydrogel is a fascinating choice for the treatment of wounds, which possesses the advantage of maintaining moisture at the wound site.129 Nevertheless, most hydrogels are non-degradable and too intricate to be used for huge wounds.130 Till now, conventional medical modalities were unsuccessful in attaining satisfactory results in restoring the functionality and the cosmetic appearance of natural skin.131 The combination of electrospinning and growth factors is gaining popularity nowadays as a potential treatment method to accelerate active wound healing.132,133 The platelet-derived growth factor-BB (PDGF-BB) is the only growth factor available on the market which has successfully completed clinical trials.134 The main constraint with growth factors is that the growth factors are easily degraded by proteinases135 or eliminated by exudate prior to reaching the wound bed.136 Recently, efforts have been attempted to deliver growth factors through electrospun nanofibers for healing of DFUs.137–139 In comparison to conventional wound dressings, nanofibers fabricated using electrospinning confer an extracellular matrix (ECM)-like scaffold for supporting skin tissue regeneration.139,140 Conversely, without understanding the fundamentals of biological processes and without a fine-tuned administration technique for releasing the biomolecules at critical phases of healing, optimum diabetic wound healing cannot be achieved.141,142Different techniques are utilized for the fabrication of electrospun meshes with the capacity to aid the diabetic foot healing process whilst eliminating foot ulcer infection. A spectrum of synthetic as well as natural polymers can be integrated for developing materials that can support and supplement healthy tissue deposition. Many techniques have been investigated by researchers for loading the nanofibers with growth factors, vitamins, and other biomolecules for improving the DFU healing processes, as shown in Fig. 4. Moreover, a momentous focus is placed nowadays on the encapsulation of drugs, silver nanoparticles, and plant-derived compounds like essential oils and honey that possess antimicrobial properties. Ultimately, real-time detection of the wound bed environment using pH or temperature, as indicators of the status of the wound also has been explored.143
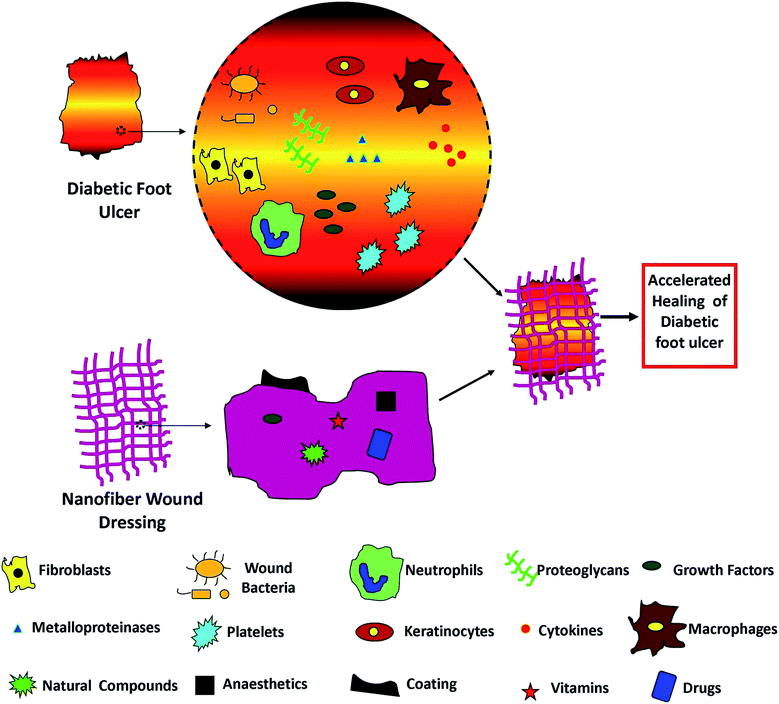 | ||
| Fig. 4 Loading of nanofibers with different natural and synthetic components to accelerate the diabetic foot ulcer healing process. | ||
Similarly, Bertonocelj et al. combined novel nanomaterials with PDGF by electrospinning for treating DFU.144,145 A platelet-rich plasma (PRP) was used in this study as it is a natural source of growth factor. Later, hydrophilic nanofibers encapsulated with PRP were fabricated from chitosan and PEO by an electrospinning technique. Finally, the effects of nanofibers embedded with PRP on cell proliferation, survival, morphology and mobility were studied. In vitro results of the study delineate that PRP triggers keratinocyte and fibroblast cell growth. The optimum concentration of growth medium was found to be 2% (v/v) for both types of skin cells, whilst greater concentrations resulted in modifications in cell morphology, with decreased cell mobility and proliferation. The morphology of the electrospun sheet was observed to be stable even in an aqueous environment for 72 hours. Surprisingly, the electrospinning technique was found not to induce any adverse effects on the biological activity of PRP. The nanofiber sheet was also observed to limit the cell mobility, altered the cell morphology and triggered cell proliferation. Even though only a little amount of blood-derived growth factor was used in cell culture through a PRP-loaded electrospun sheet, such nanofibrillar support drastically triggered cell proliferation, dictating the synergistic effect of nanotopographical morphology and incorporated growth factors. Hence there is a high potential for nanomaterials to deliver PRP to accelerate the diabetic wound healing process.
Biodegradable polymers were electrospun with EGF and/or immobilized on the electrospun sheets for treating DFUs.146,147 Amine-terminated block copolymers consisting of PCL and PEG were electrospun to form a nanofibrous sheet with good biocompatibility properties. It was later given added functional amine groups on the surface via PEG linkers. The EGF was chemically conjugated on the surface of the electrospun sheet. The conjugated quantity of EGF on the electrospun nanofibrous sheet was quantified with XPS. An in vitro cell culture study was performed using human primary keratinocytes on an EGF-conjugated nanofibrous sheet to investigate the impact of EGF nanofibers on the differentiation of keratinocytes. Later, the healing ability of the EGF-conjugated nanofibrous sheet was investigated in diabetic-induced rats with dorsal wounds. It was found that the expression of keratinocyte-specific genes was notably improved by using EGF-conjugated nanofibers. It also demonstrated exceptional in vivo wound healing attributes in comparison to control or EGF solutions. Moreover, immunohistochemical-staining study results showed that the EGF-receptor (EGFR) was expressed the most in the EGF-conjugated nanofibers. Thus, an electrospun EGF-conjugated nanofiber which increases the proliferation and phenotypic expression of keratinocytes is a plausible candidate for DFU wound treatment.
DFU is often regarded as intricate to heal because of the reduced level of cellular and molecular signals necessary for instigating normal wound repair.148 Emulsion electrospinning was implemented in a recent study to imbed bFGF into ultrafine fibers forming a core-sheath structure to induce the wound healing process.138,148 An initial burst release found to be as low as 14.0 ± 2.2% was attained, followed by gradual release up to 4 weeks. In vitro studies on mouse embryo fibroblasts showed that bFGF-loaded core-sheath nanofibers improved cell adhesion, proliferation, and secretion of ECM. The skin regeneration ability of the electrospun nanofiber was tested in vivo by creating skin wounds in the dorsal area of diabetic rats followed by the placing of the wound-healing bFGF-loaded core-sheath nanofibers.
In comparison to fibrous mats infiltrated with free bFGF, bFGF-loaded scaffolds showed an appreciably increased rate of wound healing with complete re-epithelialization and regeneration of skin appendages. A greater density of mature capillary vessels was formed in the second week following treatment with bFGF-loaded fibers, and fiber fragments were absent in the histological sections in the fourth week following the operation. The gradual release of bFGF from nanofibrous mats improved collagen deposition and ECM remodeling. Furthermore, the arrangement and components of collagen fibers were found to be similar to normal tissues. Hence, there is a notable potential for bFGF-loaded electrospun nanofibrous mats to quickly reinstate the structural and functional properties of affected tissues in patients with DFUs.
Lai et al. studied the collagen (Col) and hyaluronic acid (HA) inter-stacking nanofibrous skin equivalent substitute with the programmable release of multiple angiogenic growth factors like vascular endothelial growth factor (VEGF), PDGF, bFGF and EGF. These growth factors were either directly embedded in the nanofibers or encapsulated in the gelatin nanoparticles (GNs) via electrospinning technology.149,150 EGF and bFGF delivery at earlier stages was expected to speed up epithelialization and vasculature sprouting, whilst the release of PDGF and VEGF in the later stage was expected to induce the maturation of blood vessels. Mechanical characterization studies suggest that the Col–HA–GN nanofibrous membrane has mechanical properties almost identical to native human skin. The design of a particle-in-fiber structure was found to permit growth factor release for up to 1 month. Endothelial cells were cultured on a biodegradable Col–HA membrane with four kinds of growth factors (Col–HA w/4GF). It was found that their growth rate increased and formed a better network with a thread-like tubular structure. In vivo studies performed on streptozotocin-induced diabetic rats showed an improved wound closure rate, along with elevated collagen deposition and enhanced maturation of blood vessels. Thus, from the obtained results, it is evident that the electrospun Col–HA–GN composite nanofibrous skin substitute with a stage-wise release pattern of multiple angiogenic factors could be a promising bioengineered construct for chronic DFU treatment.
4.1 Successful cell proliferation and wound healing of coaxial electrospinning nanofibers in the treatment of DFU
The successful application of the coaxial electrospun nanofiber wound dressing is shown in Fig. 5.151–153 Here, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) binary release was achieved from a bFGF/EGF nanofiber patch (Fig. 5a) using a coaxial electrospinning method. The core encapsulated with bFGF was found to be released with an initial burst release profile and almost 30% of the encapsulated bFGF and was released within the first 12 hours. Yet, less than 2% of the EGF was found to be released within 7 days. This may be attributed to the surface immobilization of EGF. There are two methods to immobilize the growth factor with the nanofibers: physical encapsulation and the chemical conjugation method. Even though the surface-immobilized EGF was released by the degradation of the polycaprolactone–polyethylene glycol, a simple diffusion process was used to deliver the bFGF through the shell of the nanofibers. Based on the literature, the thickness of the shell portion of the coaxial electrospun meshes ranges from 100 to 300 nm.154–156 Due to this thin layer, a faster diffusion rate of bFGF was achieved. The high surface-to-volume ratio is one of the major advantages of the nanofibrous matrix since many biological cues can be immobilized on the huge surface area. This results in the efficiency of encapsulated bFGF and surface-immobilized EGF on the surrounding cells and tissues being maximized. Moreover, dual release of bFGF and EGF is very important as the epidermal cells require different growth factors to accelerate the wound healing process. Hence, optimized release of these two growth factors confers accurate control over the differentiation and proliferation of various cells at the time of re-epithelization.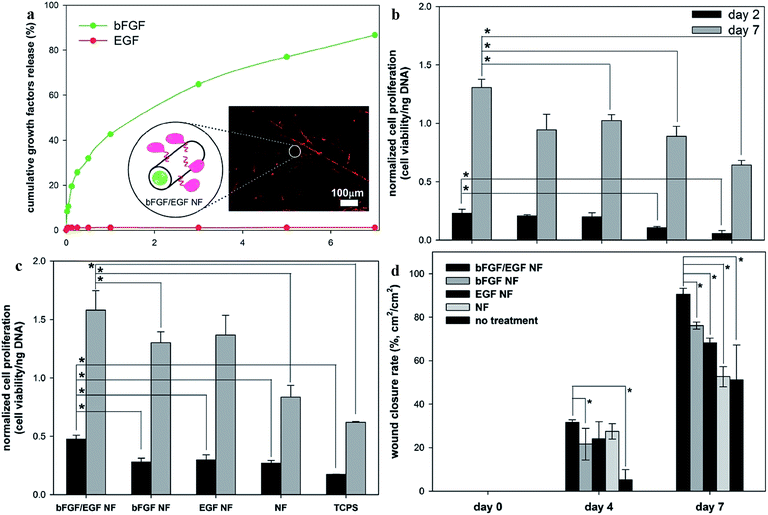 | ||
| Fig. 5 (a) Release profiles of bFGF and EGF from bFGF/EGF NF over 7 days. The inset is a confocal laser scanning microscopic (CLSM) image of fluorescently-labeled proteins that were encapsulated or surface-immobilized in the coaxial nanofibers (shown in green and red) and the illustrated co-axial structures of bFGF/EGF NF. (b) Normalized proliferations of human dermal fibroblasts and (c) human primary keratinocytes. Absorbance values from WST-1 assay results were normalized with respect to the amount of DNA to calculate normalized cell proliferation levels. (d) Wound-closure rates of the diabetic ulcers in C57BL/6 mice. The wound areas were calculated by multiplying length by width (cm2). Wound closure rate (%) = (wound areaday 1 − wound areaday 0)/(wound areaday 0) × 100. Reproduced from ref. 136 with permission from The Royal Society of Chemistry. | ||
In order to examine the combined effect of bFGF and EGF on cell proliferation of epidermal cells, human dermal fibroblasts (HDF) and human primary keratinocytes (HPK) were cultivated separately on the bFGF/EGF NF, the bFGF NF, the EGF surface immobilized nanofibers (EGF NF), and the nanofiber (NF), and degrees of cell proliferation were compared.151 The human dermal fibroblasts (HDF) and human primary keratinocytes (HPK) proliferation are shown in Fig. 5b and c, respectively. On the 7th day, the bFGF/EGF NF exhibited the highest proliferation of HDF compared to the EGF, since the HDF responds to both EGF and bFGF growth factors. However, when the result of the HPK was compared on the 7th day, it was found that the EGF NF induced a better proliferation rate compared to the bFGF/EGF NF. This is because, even though both EGF and bFGF improve the HPK proliferation, the EGF seems to be a more specialized growth factor in promoting the proliferation of keratinocytes. Thus, HPK and HDF demonstrate different preference of growth factors to improve their proliferation.
In vivo testing was performed in the dorsal area of diabetic-induced mice.151 After four days, it was found that the wound healing rate of mice subjected to a nanofiber patch demonstrated higher wound closure rate than the untreated groups, but no difference was observed among the different groups of nanofibers with different growth factors. This substantiated that neither of the growth factors induces proliferation of epidermal cells at the initial stage of wound healing. However, the nanofibers with the dual growth factors greatly outperformed the other nanofibers for improving the proliferation rate of the epidermal cells, improving the wound closure rate by day 7. It was observed that bFGF/EGF NF has 14.5% better wound healing compared to the bFGF NF and a 22.5% improved wound healing rate compared to the EGF NF. The in vivo wound closure rates of the diabetic ulcers of the nanofibers fabricated using electrospinning are represented in Fig. 5d. Hence, this clearly shows the potential of electrospinning in the treatment of DFUs.
4.2 Nanoparticle-in-fiber system for fast and slow biphasic release through electrospinning for the treatment of DFUs
Utilization of gradually released growth factors as a “cocktail” treatment is an effective approach for curing intricate DFUs. Layer by layer, core–shell electrospinning, etc. have been explored, yet the diffusion between layers remains a crucial issue to be addressed.157 The nanoparticle-in-fiber system is a potential strategy to achieve fast and slow biphasic release of two varying growth factors.158 Vascular endothelial growth factor (VEGF) and platelet-derived growth factor-BB (PDGF-BB) from nanoparticles embedded in the nanofiber release profile are shown in Fig. 6a. An initial burst release of 63% VEGF was observed from nanofibers within one hour. Moreover, the VEGF loaded in the nanofibers of a chitosan (CS)/poly(ethylene oxide) (PEO) scaffold achieved 100% release in one day. However, PDGF loaded in poly-lactic-co-glycolic acid (PLGA) nanoparticles demonstrated only an initial burst release of just 28% within 2 hours. Moreover, the PDGF-BB release was observed to have a sustained and controlled profile even at day 7. Hence, a distinct difference can be observed between the release of growth factor which was loaded into the nanofibers and growth factor loaded in a nanoparticle within the nanofibers. In the wound healing process, VEGF can accelerate angiogenesis at the wound site in the early stage of the wound, so a rapid delivery of VEGF is required. In contrast, the PDGF-BB encapsulated in the PLGA nanoparticles enables a slower delivery at the wound site to induce fibroblast growth in a sustained manner. This fascinating approach of combining nanofibers, growth factors and nanoparticles by electrospinning enables us to control the release of different growth factors to achieve better wound healing of DFUs.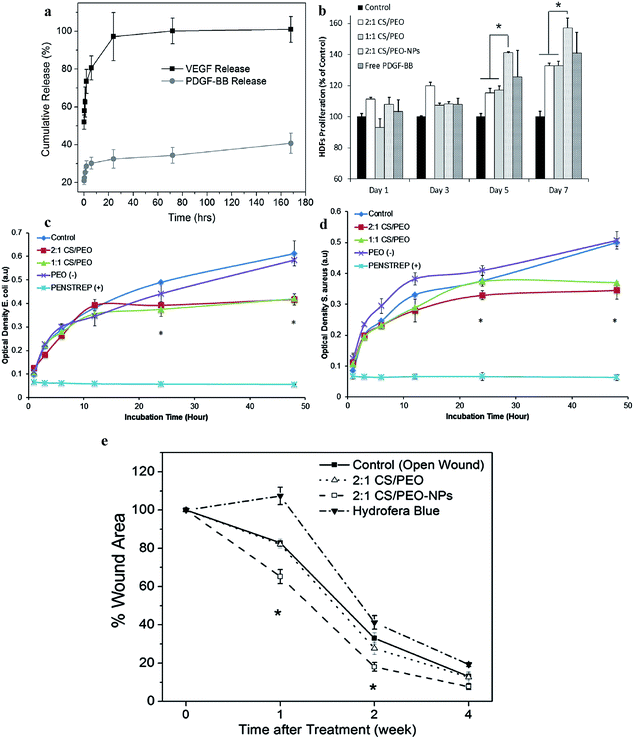 | ||
Fig. 6 (a) Growth factor release kinetics from nanofibers and nanoparticles within fibers as determined by ELISA. VEGF was released from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs nanofibers in PBS at 37 °C. PDGF-BB was released from PLGA nanoparticles encapsulated in 1 1 CS/PEO-NPs nanofibers in PBS at 37 °C. PDGF-BB was released from PLGA nanoparticles encapsulated in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs nanofibers under the same conditions. (b) Cell proliferation on nanofiber scaffolds: 1 1 CS/PEO-NPs nanofibers under the same conditions. (b) Cell proliferation on nanofiber scaffolds: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO, 2 1 CS/PEO, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO without growth factors nanoparticles, 2 1 CS/PEO without growth factors nanoparticles, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO with PLGA nanoparticles loaded with PDGF-BB (2 1 CS/PEO with PLGA nanoparticles loaded with PDGF-BB (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs). Tissue culture plate and free PDGF-BB were served as negative and positive controls, respectively. Nanofiber meshes and controls were seeded with adult human dermal fibroblast (HDFs) and MTS assay was used to quantify the cell viability. Antibacterial assessment of chitosan/PEO-NP scaffolds compared to (c) negative controls (cell suspension and PEO scaffold) and (d) a positive control (Penstrep solution). 1 1 CS/PEO-NPs). Tissue culture plate and free PDGF-BB were served as negative and positive controls, respectively. Nanofiber meshes and controls were seeded with adult human dermal fibroblast (HDFs) and MTS assay was used to quantify the cell viability. Antibacterial assessment of chitosan/PEO-NP scaffolds compared to (c) negative controls (cell suspension and PEO scaffold) and (d) a positive control (Penstrep solution). 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs and 2 1 CS/PEO-NPs and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs scaffolds showed antibacterial activities against both E. coli and S. aureus compared to negative controls. (e) Quantitative measurement of wound size reduction. Reprinted with permission from ref. 141. Copyright 2016, Elsevier. 1 CS/PEO-NPs scaffolds showed antibacterial activities against both E. coli and S. aureus compared to negative controls. (e) Quantitative measurement of wound size reduction. Reprinted with permission from ref. 141. Copyright 2016, Elsevier. | ||
HDF was placed on the electrospun nanofibers and MTS assays were performed to determine the efficiency of the nanoparticle-in-fiber system to accelerate the growth of the HDF.158 Cell proliferation increased significantly for all the samples compared to the control. It was observed that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs sample with PDGF-BB loaded nanoparticles demonstrated quicker cell growth compared to the control and scaffolds without growth factors, as shown in Fig. 6b. Even in comparison to PDGF-BB, this sample was found to improve cell growth specifically on days 5 and 7 due to the high surface area conferred. These results show that nanofibrous meshes are cytocompatible and capable of conferring a sustained release profile.
1 CS/PEO-NPs sample with PDGF-BB loaded nanoparticles demonstrated quicker cell growth compared to the control and scaffolds without growth factors, as shown in Fig. 6b. Even in comparison to PDGF-BB, this sample was found to improve cell growth specifically on days 5 and 7 due to the high surface area conferred. These results show that nanofibrous meshes are cytocompatible and capable of conferring a sustained release profile.
The chitosan possesses good antibacterial activity.158 As shown in Fig. 6c and d, the incorporation of chitosan into the system resulted in improved antibacterial activity after 10 hours of culture. A similar trend was observed for both Gram positive and Gram negative bacteria, namely E. coli and S. aureus. Fig. 6e shows the in vivo wound healing efficacy of the nanoparticle-in-fiber system.158 It was found that the wound area for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs samples with growth factors was significantly smaller than other samples at weeks 1 and 2, whilst 2
1 CS/PEO-NPs samples with growth factors was significantly smaller than other samples at weeks 1 and 2, whilst 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO without growth factors was found to be almost the same as the open wound control. Observation of the wound 1, 2 and 4 weeks after implantation is shown in Fig. 7a.158 A scab was seen on Hydrofera Blue samples only, while nanofibers fabricated by electrospinning obtained almost complete remodeling and hair coverage. Traditionally, when the growth factor activity is increased, it could lead to scar formation.159,160
1 CS/PEO without growth factors was found to be almost the same as the open wound control. Observation of the wound 1, 2 and 4 weeks after implantation is shown in Fig. 7a.158 A scab was seen on Hydrofera Blue samples only, while nanofibers fabricated by electrospinning obtained almost complete remodeling and hair coverage. Traditionally, when the growth factor activity is increased, it could lead to scar formation.159,160
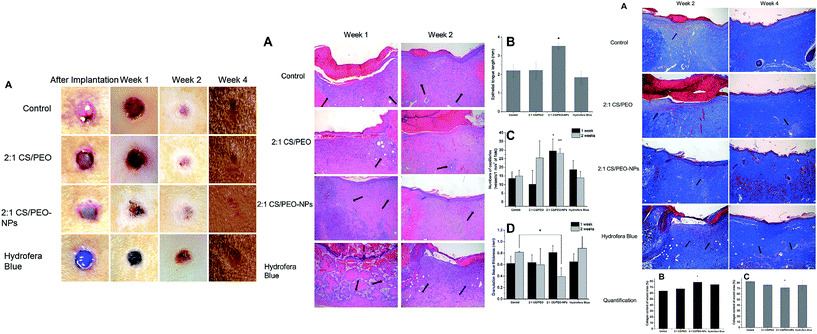 | ||
Fig. 7 (a) Representative macroscopic appearance of wound closure at 0, 1, 2, and 4 weeks after treatment of skin wound of control, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO, 2 1 CS/PEO, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs, and Hydrofera Blue after 0, 1, 2, and 4 weeks; histological evaluation of wounds treated by CS/PEO-NP meshes. (b) H&E staining for skin wound samples of control (open wound), 2 1 CS/PEO-NPs, and Hydrofera Blue after 0, 1, 2, and 4 weeks; histological evaluation of wounds treated by CS/PEO-NP meshes. (b) H&E staining for skin wound samples of control (open wound), 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO, 2 1 CS/PEO, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs, Hydrofera Blue after 1 and 2 weeks of treatment; inflammatory cells are indicated by arrows; (c) epithelial length after 1 week of treatment; (d) capillary density at wound site after 1 and 2 weeks of treatment; and (e) granulation tissue thickness after 1 and 2 weeks of treatment. Collagen staining images and quantification of wounds treated by CS/PEO-NP meshes. (f) Masson's trichrome staining of each wound sample: control, 2 1 CS/PEO-NPs, Hydrofera Blue after 1 and 2 weeks of treatment; inflammatory cells are indicated by arrows; (c) epithelial length after 1 week of treatment; (d) capillary density at wound site after 1 and 2 weeks of treatment; and (e) granulation tissue thickness after 1 and 2 weeks of treatment. Collagen staining images and quantification of wounds treated by CS/PEO-NP meshes. (f) Masson's trichrome staining of each wound sample: control, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO, 2 1 CS/PEO, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs, and Hydrofera Blue after 2 and 4 weeks of treatment; inflammatory cells are indicated by arrows; collagen quantification of each wound area after 2 (g) and 4 (h) weeks of treatment. Reprinted with permission from ref. 141. Copyright 2016, Elsevier. 1 CS/PEO-NPs, and Hydrofera Blue after 2 and 4 weeks of treatment; inflammatory cells are indicated by arrows; collagen quantification of each wound area after 2 (g) and 4 (h) weeks of treatment. Reprinted with permission from ref. 141. Copyright 2016, Elsevier. | ||
MT and H&E staining were performed to ascertain the efficacy of the nanoparticle-in-fiber system for DFU healing.158 Fig. 7b shows H&E staining of the wound sites after 1 and 2 weeks of treatment whilst Fig. 7c shows that longer epithelial tongues formed on 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs samples, elucidating that GF releasing meshes facilitated epithelium regeneration in the short term. At 1 week, more newly formed capillaries for 2
1 CS/PEO-NPs samples, elucidating that GF releasing meshes facilitated epithelium regeneration in the short term. At 1 week, more newly formed capillaries for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs within the wound site were observed compared to an open wound, and a scaffold without GFs (Fig. 7d). Following 2 weeks of treatment, complete coverage of new epithelium was observed for all samples except the Hydrofera Blue. Fast creeping of the epithelium is a supporting factor for faster skin healing post-wounding.161 Finally, the epithelium of the wound closes completely and the granulation tissue thickness for 2
1 CS/PEO-NPs within the wound site were observed compared to an open wound, and a scaffold without GFs (Fig. 7d). Following 2 weeks of treatment, complete coverage of new epithelium was observed for all samples except the Hydrofera Blue. Fast creeping of the epithelium is a supporting factor for faster skin healing post-wounding.161 Finally, the epithelium of the wound closes completely and the granulation tissue thickness for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs at week 2 was reduced to a great extent compared with the control, substantiating that it changed from the inflammation phase to the proliferation phase (Fig. 7e).
1 CS/PEO-NPs at week 2 was reduced to a great extent compared with the control, substantiating that it changed from the inflammation phase to the proliferation phase (Fig. 7e).
Fig. 7f shows the MT staining results which are used to quantify the amount of collagen deposited at the wound site.158 The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO demonstrated significantly higher collagen deposited compared to Hydrofera Blue after 2 weeks of treatment (Fig. 7g). A high number of myofibroblasts, more mature collagen fibers, and fewer inflammatory cells were observed for the 2
1 CS/PEO demonstrated significantly higher collagen deposited compared to Hydrofera Blue after 2 weeks of treatment (Fig. 7g). A high number of myofibroblasts, more mature collagen fibers, and fewer inflammatory cells were observed for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/PEO-NPs (in corroboration with H&E results). A greater collagen content at the wound site may aid in the reconstruction of the ECM and support skin growth (Table 1).162
1 CS/PEO-NPs (in corroboration with H&E results). A greater collagen content at the wound site may aid in the reconstruction of the ECM and support skin growth (Table 1).162
| S. No. | Technique | Base material | Endogenous molecules | Key findings | Ref. |
|---|---|---|---|---|---|
| 1 | Conjugation | Iron oxide nanoparticles (γ-Fe2-O3 conjugation) | Thrombin | • Wound response to the treatment of γ-Fe2-O3 was remarkable | 48 and 49 |
| • Analysis of the wound's tensile strength indicates appreciable acceleration of the healing process | |||||
| 2 | NO-releasing nanodelivery | Hydrogel | Nitric oxide | • Sustained release provides an advantage to the nanoparticles compared to other vehicles | 50, 54 and 55 |
| • Easy storage, application and the provision to change the release rate and concentration | |||||
| • Minimal risk of toxicity | |||||
| • Possesses good bactericidal activity | |||||
| 3 | Biodegradable nanocarriers | Hyaluronan-based (HYAFF11) porous nanoparticles | PDGF | • Release of PDGF periodically as well as in a controlled manner | 63–65 |
| • Accelerate chronic wound healing via a stimulation of chemotaxis, proliferation and ECM deposition | |||||
| 4 | Nanoparticulate carriers | Lipid nanoparticles | Opioids, morphine | • Opioids trigger cell migration and lead to the closure of the experimental wounds | 66–68 |
| • Accelerate the re-epithelialization in a human 3D wound healing modal | |||||
| • Keratinocytes almost covered the wound in just four days | |||||
| • Decreased cytotoxicity and irritation, extended period of morphine release | |||||
| 5 | Managing the enzymatic activity of matrix metalloproteinases (MMPs) | MMPs | Protease inhibitors, aspirin-triggered D1 and lipoxin A4 | • Polymorphonuclear cell influx displayed a good result in reducing the wound size | 69–71 |
| 6 | Nanoparticulate drug delivery systems | Carboxymethyl chitosan-based nanoparticles and folic acid tagged chitosan | Vancomycin | • Drug concentration was maintained to be more than the minimal inhibitory limit for 24 hours | 75–79 |
| • Proved its effectiveness against drug-resistant staphylococcal strains | |||||
| • The tolerance level of drug-resistant S. aureus strains reduced when nanoconjugated vancomycin was used | |||||
| 7 | Drug encapsulation within nanoparticles | Emulsion of polyacrylate nanoparticle | N-Methylthiolated β-lactams | • It possesses different structure–activity patterns | 80 and 81 |
| • Putative application is limited due to its poor water solubility | |||||
| • In vitro studies show that the nanoparticles are nontoxic to human skin cells | |||||
| 8 | Polymeric gene delivery system | PLGA nanoparticles | Plasmid DNA | • Retains the modulation of gene expression while wound healing | 82 and 83 |
| • Transfection capacity has already been tested in vitro | |||||
| • VEGF secretion by epithelial cells was notably decreased | |||||
| 9 | Polymeric gene delivery system | Chitosan nanospheres | Plasmid DNA | • The secretion of MMP-9 in cell supernatants was found to increase appreciably after 24 and 48 hours | 84–86 |
| • MMP-2 secretion was found to be greater after 48 hours | |||||
| 10 | Nanotechnology modified stem cells | Human mesenchymal stem cells (hMSCs) and human embryonic stem cell-derived cells (hESdCs) | hVEGF gene | • Implantation of scaffolds seeded with VEGF-expressing stem cells (hMSCs and hESdCs) was found to form 2 to 4 fold higher vessel densities | 87–89 |
| • Genetically modified hMSCs significantly improved angiogenesis and limb salvage | |||||
| • Reduced muscle degeneration and tissue fibrosis | |||||
| 11 | Free radical scavenging utilizing nanotechnology | Cerium oxide | — | • Reduction of Ce4+ to Ce3+ followed by creation of an oxygen vacancy | 101–103 |
| • Improved wound healing | |||||
| 12 | Protection against oxidative stress damage | Cerium oxide and yttrium oxide nanoparticles | — | • Block ROS production | 105–109 |
| • Inhibiting programmed cell death pathway | |||||
| • Triggers ROS defense system | |||||
| 13 | Metal oxides for scavenging | Aluminum oxide and gold nanoparticles (AuNP) | — | • Possess biocompatibility | 110–116 |
| • High surface reactivity | |||||
| • Resistance to oxidation and plasmon resonance | |||||
| • AuNP inhibit the lipid from peroxidation and prevent the ROS generation | |||||
| • Restore the imbalances in the antioxidants | |||||
| 14 | Biostable nanoparticles synthesis using nanotechnology | Silver nanoparticles (AgNP) | — | • Antibacterial and antifungal activity | 118–122 |
| • Used along with common antibiotic therapy | |||||
| • Promotion of proliferation and migration of keratinocytes | |||||
| • AgNP have an acceptable biocompatibility yet occasionally develop argyria |
Yet, in Fig. 7h, it can be seen that the collagen content was the least for the growth factor releasing nanofibers and this result may be due to the mature collagen formation and because more hair follicles have regenerated.158 It can be concluded that the nanofiber/nanoparticles patch fabricated using electrospinning confers not only a synergistic delivery of growth factors, but also plays a vital role of ECM mimicry and anti-infection fibrous structure to accelerate the DFU healing process. Table 2 presents electrospinning in the treatment of DFU and its key findings.
| S. No. | Technique | Base material | Growth factor used | Form of electrospun material | Key findings | Ref. |
|---|---|---|---|---|---|---|
| 1 | Co-axial electrospinning | Amine-functionalized block copolymers comprised of poly(ε-caprolactone) (PCL) and poly(ethyleneglycol) (PEG) | Basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) | Fibers | • X-ray photoelectron spectroscopy revealed distinctive peaks of nitrogen atoms, which confirm the presence of a surface-immobilized EGF | 146 and 147 |
| • bFGF demonstrated a high initial burst in 24 h, whilst the EGF displayed no negligible release in 7 days | ||||||
| • Human primary keratinocyte and fibroblast cells cultivated on the nanofiber meshes demonstrated the highest cellular proliferation on mesh comprised of the bFGF and the EGF | ||||||
| • Wound closure rates of diabetic ulcers were notably increased in 7 days | ||||||
| • Keratin 14, 5, 1 possessed higher expression levels against the control groups | ||||||
| • Biphasic release of bFGF and EGF significantly supported tissue recovery with similar phenotypes to the original keratinized tissues | ||||||
| • Resultant nanofibrous mesh improves the accumulation of both collagen and a cemented matrix of keratin | ||||||
| 2 | Electrospinning | Chitosan, poly(ethylene oxide) (PEO) | Vascular endothelial growth factor (VEGF) and platelet derived growth factor-BB (PDGF-BB) encapsulated poly(lactic-co-glycolic acid) nanoparticles | Combination of fibers and particles | • Obtained nanofibrous composites delivered VEGF rapidly and PDGF-BB in a relayed manner | 157 and 158 |
| • It was found to support the fibroblast growth and meanwhile exhibited anti-bacterial activities | ||||||
| • Nanofiber/nanoparticle scaffolds appreciably accelerated the wound healing process by inducing angiogenesis, improving re-epithelialization and controlling granulation tissue formation in rats | ||||||
| • Quicker collagen deposition and earlier remodeling at the injured site to achieve a faster full regeneration of skin compared to commercial Hydrofera Blue wound dressing material | ||||||
| 3 | Electrospinning | Chitosan and PEO | Platelet-rich plasma | Fibers | • The platelet-rich plasma stimulates keratinocyte and fibroblast cell growth in vitro | 144 and 145 |
| • Optimum concentration in growth medium was found to be 2% (v/v) for both types of skin cells | ||||||
| • Higher concentrations resulted in modification in cell morphology, with decreased cell mobility and proliferation | ||||||
| • The morphology of nanofibers observed to be stable even in aqueous conditions for 72 h | ||||||
| • Electrospinning does not induce adverse effects on the biological activity of platelet-rich plasma | ||||||
| • Nanofibrillar support drastically triggered cell proliferation, indicating the synergistic effect of nanotopography and incorporated growth factors | ||||||
| 4 | Electrospinning | Amine-terminated block copolymers consisting of PCL and PEG | EGF | Fibers | • Conjugated quantity of EGF on the nanofibers was quantified with X-ray photoelectron scattering | 146 and 147 |
| • Expression of keratinocyte-specific genes notably increased with the utilization of EGF-conjugated nanofibers | ||||||
| • Demonstrated exceptional in vivo wound healing activities in comparison to control groups | ||||||
| • Immunohistochemical-staining results show that the EGF-receptor (EGFR) was highly expressed in the EGF nanofiber group | ||||||
| 5 | Emulsion electrospinning | Poly(ethylene glycol)–poly(DL-lactide) (PELA) | bFGF | Fibers | • Initial burst release as low as 14.0 ± 2.2% was attained | 138 and 148 |
| • This is followed by gradual release for around 4 weeks | ||||||
| • In vitro studies on mouse embryo fibroblasts showed that bFGF-loaded fibrous mats improved cell adhesion, proliferation, and secretion of extracellular matrix (ECM) | ||||||
| • bFGF-loaded scaffolds showed appreciably increased wound recovery rate with complete re-epithelialization and regeneration of skin appendages | ||||||
| • Greater density of more mature capillary vessels were generated during the second week | ||||||
| • There were no fiber fragments observed in the histological sections during the fourth week after the operation | ||||||
| • Gradual release of bFGF from nanofibrous mats improved collagen deposition and ECM remodeling | ||||||
| • Arrangement and component of collagen fibers were found to be similar to normal tissues | ||||||
| 6 | Electrospinning | Collagen (Col) and hyaluronic acid (HA) | VEGF, PDGF, bFGF and EGF either directly embedded or encapsulated in the gelatin nanoparticles (GNs) | Combination of fibers and nanoparticles | • Physiochemical characterizations suggest that the Col–HA–GN nanofibrous membrane has mechanical properties almost identical to human native skin | 149 and 150 |
| • Fiber structure was found to permit slow controlled release of growth factors for up to 1 month | ||||||
| • Endothelial cells were not only found to be increased in growth rate but also observed to form a better network with a thread-like tubular structure | ||||||
| • In vivo results show an improved wound closure rate, along with elevated collagen deposition and enhanced maturation of vessels |
5. Current challenges and future prospects
Recently, nanotechnology has revolutionized the medical field. Advanced nanodelivery methods using nanoparticles and nanofibers fabricated through electrospinning are being explored extensively by researchers. This is represented in Fig. 8. There are various nanodelivery routes like polymeric gene delivery systems, nanoparticulate drug delivery systems, free radical scavenging utilizing nanotechnology, etc. These methods are found to be very effective for the treatment of DFUs. This is because nanomaterials provide different ways to overcome the dimensional barrier of conventional DFU therapies. Nanocarriers or nanodelivery have a notable potential to amplify the therapeutic potential of biological and synthetic molecules. But, the impact of nanotechnology treatment on the human system has to be assessed meticulously prior to its implementation in clinical trials.It has been suggested that the wound dressing of the future may incorporate a color map of the wound, a pH readout, infection feedback, and an indicator showing if a DFU wound dressing change is necessary. To develop such an intricate wound management system, many fundamental milestones need to be reached. A potential strategy to overcome biofilm formation in the DFU wound bed could be to develop a nanofiber mesh using electrospinning that is able to attract bacteria and trap them within its fibrous structure. By integrating such a system with sensors for triggered drug release, a smart dressing would be achieved, that would be capable of reducing the bacterial load in the diabetic wound bed, treat infection only if it occurs, and stimulate the growth of healthy tissue.
Nanofibers seem to possess potential advantages compared to nanoparticles. Based upon our knowledge, this advantage lies mostly in the morphology of the fibers in the applications to DFU treatment. As we know, skin regeneration requires proper scaffolding and this is naturally provided by the extracellular matrix (ECM). This ECM contains several proteins like collagen, fibronectin, laminin, etc. and the morphology of these proteins is closer to that of the nanofiber and hence bio-mimicking is expected.163,164 However, the larger surface area of the nanoparticles makes them highly functional and therefore we surmise that combining both provides synergism in the DFU treatments.
Synergetic functioning of nanofibers with active properties using electrospinning can lay a solid foundation for DFU wound dressings of the future. Biosensors which can be integrated within the dressing must be capable of detecting low levels of bacterial contamination and emit a recognizable output indicative of infection risk. The sensor will trigger a material response that releases a pre-loaded drug with a required release profile as per the conditions of the DFU. The triggered release could be attained by combining switchable surfaces or stimuli-responsive materials into the DFU wound dressing material.165 At the same time, the sensor must provider the doctor/patient with values of different parameters indicative of the status of the DFU wound, specifically pH, temperature, moisture, and exudate production.
Acknowledgements
This work was supported partly by the Ministry of Higher Education Malaysia with the FRGS Grant Vot number: Q.J130000.2545.12H80.References
- M. Bahrami, A. Ataie-Jafari, S. Hosseini, M. H. Foruzanfar, M. Rahmani and M. Pajouhi, Int. J. Food Sci. Nutr., 2009, 60, 618–626 CrossRef CAS PubMed.
- S. Wild, G. Roglic, A. Green, R. Sicree and H. King, Diabetes Care, 2004, 27, 1047–1053 CrossRef PubMed.
- D. M. Maahs, N. A. West, J. M. Lawrence and E. J. Mayer-Davis, Endocrinol. Metab. Clin. North Am., 2010, 39, 481–497 Search PubMed.
- C. C. Cowie, K. F. Rust, E. S. Ford, M. S. Eberhardt, D. D. Byrd-Holt, C. Li, D. E. Williams, E. W. Gregg, K. E. Bainbridge, S. H. Saydah and L. S. Geiss, Diabetes Care, 2009, 32, 287–294 CrossRef PubMed.
- E. W. Gregg, B. L. Cadwell, Y. J. Cheng, C. C. Cowie, D. E. Williams, L. Geiss, M. M. Engelgau and F. Vinicor, Diabetes Care, 2004, 27, 2806–2812 CrossRef PubMed.
- K. H. Yoon, J. H. Lee, J. W. Kim, J. H. Cho, Y. H. Choi, S. H. Ko, P. Zimmet and H. Y. Son, Lancet, 2006, 368, 1681–1688 CrossRef.
- Y. Wenying, Indian J. Med. Res., 2010, 132, 475–477 Search PubMed.
- N. Singh, D. G. Armstrong and B. A. Lipsky, J. Am. Med. Assoc., 2005, 293, 217–228 CrossRef CAS PubMed.
- L. A. Lavery, K. R. Higgins, D. R. Lanctot, G. P. Constantinides, R. G. Zamorano, K. A. Athanasiou, D. G. Armstrong and C. M. Agrawal, Diabetes Care, 2007, 30, 14–20 CrossRef PubMed.
- R. E. Pecoraro, G. E. Reiber and E. M. Burgess, Diabetes Care, 1990, 13, 513–521 CrossRef CAS PubMed.
- J. Larsson, C. D. Agardh, J. Apelqvist and A. Stenstrom, Clin. Orthop. Relat. Res., 1998, 149–158 CAS.
- J. A. Mayfield, G. E. Reiber, L. J. Sanders, D. Janisse and L. M. Pogach, Diabetes Care, 1998, 21, 2161–2177 CrossRef CAS PubMed.
- American Diabetes Association, Diabetes Care, 1999, 22, 1354–1360 Search PubMed.
- D. G. Armstrong and B. A. Lipsky, Int. Wound J., 2004, 1, 123–132 CrossRef PubMed.
- L. A. Lavery, D. G. Armstrong, R. P. Wunderlich, M. J. Mohler, C. S. Wendel and B. A. Lipsky, Diabetes Care, 2006, 29, 1288–1293 CrossRef PubMed.
- B. A. Lipsky, A. R. Berendt, H. G. Deery, J. M. Embil, W. S. Joseph, A. W. Karchmer, J. L. LeFrock, D. P. Lew, J. T. Mader, C. Norden and J. S. Tan, Clin. Infect. Dis., 2004, 39, 885–910 CrossRef PubMed.
- T. Zgonis, J. J. Stapleton, V. A. Girard-Powell and R. T. Hagino, AORN J., 2008, 87, 935–946 CrossRef.
- N. Tentolouris, S. Al-Sabbagh, M. G. Walker, A. J. Boulton and E. B. Jude, Diabetes Care, 2004, 27, 1598–1604 CrossRef PubMed.
- C. J. Schofield, G. Libby, G. M. Brennan, R. R. MacAlpine, A. D. Morris and G. P. Leese, Diabetes Care, 2006, 29, 2252–2256 CrossRef PubMed.
- D. Boutoille, A. Feraille, D. Maulaz and M. Krempf, Foot Ankle Int., 2008, 29, 1074–1078 CrossRef PubMed.
- G. E. Reiber, B. A. Lipsky and G. W. Gibbons, Am. J. Surg., 1998, 176, 5s–10s CrossRef CAS PubMed.
- J. M. Robbins, G. Strauss, D. Aron, J. Long, J. Kuba and Y. Kaplan, J. Am. Podiatr. Med. Assoc., 2008, 98, 489–493 CrossRef PubMed.
- J. Majtan, J. Evidence-Based Complementary Altern. Med., 2011, 2011, 5 Search PubMed.
- S. M. Tambe, L. Sampath and S. M. Modak, J. Antimicrob. Chemother., 2001, 47, 589–598 CrossRef CAS PubMed.
- P. Appelgren, V. Bjornhagen, K. Bragderyd, C. E. Jonsson and U. Ransjo, Burns, 2002, 28, 39–46 CrossRef PubMed.
- A. M. Abd-El Aal, M. R. El-Hadidy, N. B. El-Mashad and A. H. El-Sebaie, Ann. Burns Fire Disasters, 2007, 20, 83–88 CAS.
- D. J. Payne, M. N. Gwynn, D. J. Holmes and D. L. Pompliano, Nat. Rev. Drug Discovery, 2007, 6, 29–40 CrossRef CAS PubMed.
- S. Enoch and D. J. Leaper, Surgery, 2008, 26, 31–37 Search PubMed.
- J. Li, J. Chen and R. Kirsner, Clin. Dermatol., 2007, 25, 9–18 CrossRef PubMed.
- L. da Silva, E. Carvalho and M. T. Cruz, Expert Opin. Biol. Ther., 2010, 10, 1427–1439 CrossRef CAS PubMed.
- V. Falanga, Lancet, 2005, 366, 1736–1743 CrossRef.
- B. M. Delavary, W. M. van der Veer, M. van Egmond, F. B. Niessen and R. H. J. Beelen, Immunobiology, 2011, 216, 753–762 CrossRef CAS PubMed.
- A. J. Singer and R. A. F. Clark, N. Engl. J. Med., 1999, 341, 738–746 CrossRef CAS PubMed.
- S. Schreml, R. M. Szeimies, L. Prantl, M. Landthaler and P. Babilas, J. Am. Acad. Dermatol., 2010, 63, 866–881 CrossRef PubMed.
- M. P. Rodero and K. Khosrotehrani, Int. J. Clin. Exp. Pathol., 2010, 3, 643–653 CAS.
- M. Jannesari, J. Varshosaz, M. Morshed and M. Zamani, Int. J. Nanomed., 2011, 6, 993–1003 CAS.
- T. Velnar, T. Bailey and V. Smrkolj, J. Int. Med. Res., 2009, 37, 1528–1542 CrossRef CAS PubMed.
- S. Guo and L. A. DiPietro, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed.
- H. Brem and M. Tomic-Canic, J. Clin. Invest., 2007, 117, 1219–1222 CrossRef CAS PubMed.
- S. Werner and R. Grose, Physiol. Rev., 2003, 83, 835–870 CAS.
- G. L. a. P. Mansson, Pharm. Technol., 1991, 15, 34–38 Search PubMed.
- P. Senet, E. Vicaut, N. Beneton, C. Debure, C. Lok and O. Chosidow, Arch. Dermatol., 2011, 147, 926–930 CrossRef CAS PubMed.
- G. Murphy and H. Nagase, Mol. Aspects Med., 2008, 29, 290–308 CrossRef CAS PubMed.
- A. MaHam, Z. Tang, H. Wu, J. Wang and Y. Lin, Small, 2009, 5, 1706–1721 CrossRef CAS PubMed.
- D. H. Carney, R. Mann, W. R. Redin, S. D. Pernia, D. Berry, J. P. Heggers, P. G. Hayward, M. C. Robson, J. Christie, C. Annable, J. W. Fenton Ii and K. C. Glenn, J. Clin. Invest., 1992, 89, 1469–1477 CrossRef CAS PubMed.
- U. Amara, D. Rittirsch, M. Flierl, U. Bruckner, A. Klos, F. Gebhard, J. D. Lambris and M. Huber-Lang, in Advances in Experimental Medicine and Biology, 2008, vol. 632, pp. 71–79 Search PubMed.
- K. C. Glenn, G. H. Frost, J. S. Bergmann and D. H. Carney, Pept. Res., 1988, 1, 65–73 CAS.
- O. Ziv-Polat, M. Topaz, T. Brosh and S. Margel, Biomaterials, 2010, 31, 741–747 CrossRef CAS PubMed.
- S. Das and A. B. Baker, Front. Bioeng. Biotechnol., 2016, 4, 82 Search PubMed.
- M. R. Schaffer, P. A. Efron, F. J. Thornton, K. Klingel, S. S. Gross and A. Barbul, J. Immunol., 1997, 158, 2375–2381 CAS.
- R. Weller, R. J. Price, A. D. Ormerod, N. Benjamin and C. Leifert, J. Appl. Microbiol., 2001, 90, 648–652 CrossRef CAS PubMed.
- L. Englande and A. Friedman, J. Clin. Aesthet. Dermatol., 2010, 3, 45–50 Search PubMed.
- A. J. Friedman, G. Han, M. S. Navati, M. Chacko, L. Gunther, A. Alfieri and J. M. Friedman, Nitric Oxide, 2008, 19, 12–20 CrossRef CAS PubMed.
- C. Miller, B. McMullin, A. Ghaffari, A. Stenzler, N. Pick, D. Roscoe, A. Ghahary, J. Road and Y. Av-Gay, Nitric Oxide, 2009, 20, 16–23 CrossRef CAS PubMed.
- L. R. Martinez, G. Han, M. Chacko, M. R. Mihu, M. Jacobson, P. Gialanella, A. J. Friedman, J. D. Nosanchuk and J. M. Friedman, J. Invest. Dermatol., 2009, 129, 2463–2469 CrossRef CAS PubMed.
- N. Barraud, D. Schleheck, J. Klebensberger, J. S. Webb, D. J. Hassett, S. A. Rice and S. Kjelleberg, J. Bacteriol., 2009, 191, 7333–7342 CrossRef CAS PubMed.
- M. R. Mihu, U. Sandkovsky, G. Han, J. M. Friedman, J. D. Nosanchuk and L. R. Martinez, Virulence, 2010, 1, 62–67 CrossRef PubMed.
- A. Y. Peleg, H. Seifert and D. L. Paterson, Clin. Microbiol. Rev., 2008, 21, 538–582 CrossRef CAS PubMed.
- P. A. Tran, L. Zhang and T. J. Webster, Adv. Drug Delivery Rev., 2009, 61, 1097–1114 CrossRef CAS PubMed.
- Z. Deǧim, J. Drug Targeting, 2008, 16, 437–448 CrossRef PubMed.
- G. Molineux, Cancer Treat. Rev., 2002, 28, 13–16 CrossRef CAS PubMed.
- O. I. Corrigan and X. Li, Eur. J. Pharm. Sci., 2009, 37, 477–485 CrossRef CAS PubMed.
- B. Zavan, V. Vindigni, K. Vezzù, G. Zorzato, C. Luni, G. Abatangelo, N. Elvassore and R. Cortivo, J. Mater. Sci.: Mater. Med., 2009, 20, 235–247 CrossRef CAS PubMed.
- D. R. Knighton, K. F. Ciresi, V. D. Fiegel, L. L. Austin and E. L. Butler, Ann. Surg., 1986, 204, 322–330 CrossRef CAS PubMed.
- S. Lepidi, G. Abatangelo, V. Vindigni, G. P. Deriu, B. Zavan, C. Tonello and R. Cortivo, FASEB J., 2006, 20, 103–105 CAS.
- N. B. Wolf, S. Kuchler, M. R. Radowski, T. Blaschke, K. D. Kramer, G. Weindl, B. Kleuser, R. Haag and M. Schafer-Korting, Eur. J. Pharm. Biopharm., 2009, 73, 34–42 CrossRef CAS PubMed.
- P. L. Bigliardi, D. J. Tobin, C. Gaveriaux-Ruff and M. Bigliardi-Qi, Exp. Dermatol., 2009, 18, 424–430 CrossRef CAS PubMed.
- S. Kuchler, N. B. Wolf, S. Heilmann, G. Weindl, J. Helfmann, M. M. Yahya, C. Stein and M. Schafer-Korting, J. Biotechnol., 2010, 148, 24–30 CrossRef PubMed.
- C. L. Baum and C. J. Arpey, Dermatol. Surg., 2005, 31, 674–686 CrossRef CAS PubMed.
- L. V. Norling, M. Spite, R. Yang, R. J. Flower, M. Perretti and C. N. Serhan, J. Immunol., 2011, 186, 5543–5547 CrossRef CAS PubMed.
- M. P. Caley, V. L. C. Martins and E. A. O'Toole, Adv. Wound Care, 2015, 4, 225–234 CrossRef PubMed.
- J. Hurlow and P. G. Bowler, Ostomy Wound Manage., 2009, 55, 38–49 Search PubMed.
- M. Qadan and W. G. Cheadle, Surg. Clin. North Am., 2009, 89, 295–310 CrossRef PubMed.
- S. M. Moghimi, A. C. Hunter and J. C. Murray, Pharmacol. Rev., 2001, 53, 283–318 CAS.
- W. Hachicha, L. Kodjikian and H. Fessi, Int. J. Pharm., 2006, 324, 176–184 CrossRef CAS PubMed.
- C. Subhankari Prasad, S. Sumanta Kumar, M. Santanu Kar, S. Susmita, B. Manjusri, R. Somenath and P. Panchanan, Nanotechnology, 2010, 21, 105103 CrossRef PubMed.
- S. P. Chakraborty, S. K. Sahu, S. K. Mahapatra, S. Santra, M. Bal, S. Roy and P. Pramanik, Nanotechnology, 2010, 21, 105103 CrossRef PubMed.
- G. J. Russell-Jones, Adv. Drug Delivery Rev., 1996, 20, 83–97 CrossRef CAS.
- I. Tamai and A. Tsuji, Adv. Drug Delivery Rev., 1996, 20, 5–32 CrossRef CAS.
- K. Greenhalgh and E. Turos, Nanomedicine, 2009, 5, 46–54 CAS.
- E. Turos, J. Y. Shim, Y. Wang, K. Greenhalgh, G. S. K. Reddy, S. Dickey and D. V. Lim, Bioorg. Med. Chem. Lett., 2007, 17, 53–56 CrossRef CAS PubMed.
- I.-S. Kim, S.-K. Lee, Y.-M. Park, Y.-B. Lee, S.-C. Shin, K. C. Lee and I.-J. Oh, Int. J. Pharm., 2005, 298, 255–262 CrossRef CAS PubMed.
- A. S. Mayo, B. K. Ambati and U. B. Kompella, Int. J. Pharm., 2010, 387, 278–285 CrossRef CAS PubMed.
- A. Masotti, F. Bordi, G. Ortaggi, F. Marino and C. Palocci, Nanotechnology, 2008, 19, 5 CrossRef PubMed.
- A. Masotti and G. Ortaggi, Mini-Rev. Med. Chem., 2009, 9, 463–469 CrossRef CAS PubMed.
- F. Chellat, A. Grandjean-Laquerriere, R. Le Naour, J. Fernandes, L. H. Yahia, M. Guenounou and D. Laurent-Maquin, Biomaterials, 2005, 26, 961–970 CrossRef CAS PubMed.
- X. Yang, J. D. Shah and H. Wang, Bioorg. Med. Chem. Lett., 2009, 15, 945–956 CAS.
- F. Yang, S. M. Son, S. R. Bogatyrev, D. Singh, J. J. Green, Y. Mei, S. Park, S. H. Bhang, B. S. Kim, R. Langer and D. G. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 3317–3322 CrossRef CAS PubMed.
- E. A. Wahl, T. L. Schenck, H.-G. Machens and E. R. Balmayor, Sci. Rep., 2016, 6, 36879 CrossRef CAS PubMed.
- D. Giugliano, A. Ceriello and G. Paolisso, Diabetes Care, 1996, 19, 257–267 CrossRef CAS PubMed.
- E. L. Feldman, M. J. Stevens and D. A. Greene, Clinical Neuroscience, 1997, 4, 365–370 CAS.
- R. A. Clark, J. Am. Acad. Dermatol., 1985, 13, 701–725 CrossRef CAS PubMed.
- D. L. Coleman, Diabetes, 1982, 31, 1–6 CrossRef CAS PubMed.
- D. K. Yue, S. McLennan, M. Marsh, Y. W. Mai, J. Spaliviero, L. Delbridge, T. Reeve and J. R. Turtle, Diabetes, 1987, 36, 295–299 CrossRef CAS PubMed.
- A. J. Singer and R. A. Clark, N. Engl. J. Med., 1999, 341, 738–746 CrossRef CAS PubMed.
- B. Meier, H. H. Radeke, S. Selle, M. Younes, H. Sies, K. Resch and G. G. Habermehl, Biochem. J., 1989, 263, 539–545 CrossRef CAS PubMed.
- R. A. F. Clark, in The Molecular and Cellular Biology of Wound Repair, ed. R. A. F. Clark and P. M. Henson, Springer US, Boston, MA, 1988, pp. 3–33 Search PubMed.
- E. A. O'Toole, M. Goel and D. T. Woodley, Dermatol. Surg., 1996, 22, 525–529 CrossRef.
- P. A. Cerutti and B. F. Trump, Cancer Cells, 1991, 3, 1–7 CAS.
- D. B. Warheit, Mater. Today, 2004, 7, 32–35 CrossRef CAS.
- F. Zhang, S.-W. Chan, J. E. Spanier, E. Apak, Q. Jin, R. D. Robinson and I. P. Herman, Appl. Phys. Lett., 2002, 80, 127–129 CrossRef CAS.
- V. Perebeinos, S.-W. Chan and F. Zhang, Solid State Commun., 2002, 123, 295–297 CrossRef CAS.
- Y. Y. Tsai, J. Oca-Cossio, K. Agering, N. E. Simpson, M. A. Atkinson, C. H. Wasserfall, I. Constantinidis and W. Sigmund, Nanomedicine, 2007, 2, 325–332 CrossRef CAS PubMed.
- R. D. Robinson, J. E. Spanier, F. Zhang, S.-W. Chan and I. P. Herman, J. Appl. Phys., 2002, 92, 1936–1941 CrossRef CAS.
- T. S. Sudarshan, Mater. Manuf. Processes, 1996, 11, 1059 CrossRef CAS.
- C. G. Enke, Mater. Corros., 1974, 25, 801–802 Search PubMed.
- S. Becker, J. M. Soukup and J. E. Gallagher, Toxicol. In Vitro, 2002, 16, 209–218 CrossRef CAS PubMed.
- G. Zhou, Y. Li, Y. Ma, Z. Liu, L. Cao, D. Wang, S. Liu, W. Xu and W. Wang, J. Nanopart. Res., 2016, 18, 135 CrossRef.
- A. Hosseini, A. M. Sharifi, M. Abdollahi, R. Najafi, M. Baeeri, S. Rayegan, J. Cheshmehnour, S. Hassani, Z. Bayrami and M. Safa, Biol. Trace Elem. Res., 2015, 164, 80–89 CrossRef CAS PubMed.
- J. B. Wright, K. Lam, D. Hansen and R. E. Burrell, Am. J. Infect. Control, 1999, 27, 344–350 CrossRef CAS PubMed.
- W. W. Monafo and C. A. Moyer, Ann. N. Y. Acad. Sci., 1968, 150, 937–945 CrossRef CAS PubMed.
- H. Q. Yin, R. Langford and R. E. Burrell, J. Burn Care Rehabil., 1999, 20, 195–200 CrossRef CAS PubMed.
- D. Ruggiero, M. Lecomte, E. Michoud, M. Lagarde and N. Wiernsperger, Diabetes Metab., 1997, 23, 30–42 CAS.
- R. Guo, Y. Song, G. Wang and R. W. Murray, J. Am. Chem. Soc., 2005, 127, 2752–2757 CrossRef CAS PubMed.
- N. O. Yakimovich, A. A. Ezhevskii, D. V. Guseinov, L. A. Smirnova, T. A. Gracheva and K. S. Klychkov, Russ. Chem. Bull., 2008, 57, 520–523 CrossRef CAS.
- S. Barathmanikanth, K. Kalishwaralal, M. Sriram, S. R. Pandian, H. S. Youn, S. Eom and S. Gurunathan, J. Nanobiotechnol., 2010, 8, 16 CrossRef PubMed.
- H. J. Klasen, Burns, 2000, 26, 131–138 CrossRef CAS PubMed.
- A. Melaiye and W. J. Youngs, Expert Opin. Ther. Pat., 2005, 15, 125–130 CrossRef CAS.
- J. Fong and F. Wood, Int. J. Nanomed., 2006, 1, 441–449 CrossRef CAS PubMed.
- J. Jain, S. Arora, J. M. Rajwade, P. Omray, S. Khandelwal and K. M. Paknikar, Mol. Pharm., 2009, 6, 1388–1401 CrossRef CAS PubMed.
- A. D. Widgerow, Burns, 2010, 36, 965–974 CrossRef PubMed.
- X. Liu, P. Y. Lee, C. M. Ho, V. C. H. Lui, Y. Chen, C. M. Che, P. K. H. Tam and K. K. Y. Wong, ChemMedChem, 2010, 5, 468–475 CrossRef CAS PubMed.
- J. Ai, E. Biazar, M. Jafarpour, M. Montazeri, A. Majdi, S. Aminifard, M. Zafari, H. R. Akbari and H. G. Rad, Int. J. Nanomed., 2011, 6, 1117–1127 CAS.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- S. Cavaliere-Jaricot, M. Darbandi, E. Kuçur and T. Nann, Microchim. Acta, 2008, 160, 375–383 CrossRef CAS.
- Z. Chen, H. Meng, G. Xing, C. Chen, Y. Zhao, G. Jia, T. Wang, H. Yuan, C. Ye, F. Zhao, Z. Chai, C. Zhu, X. Fang, B. Ma and L. Wan, Toxicol. Lett., 2006, 163, 109–120 CrossRef CAS PubMed.
- J. S. Boateng, K. H. Matthews, H. N. E. Stevens and G. M. Eccleston, J. Pharm. Sci., 2008, 97, 2892–2923 CrossRef CAS PubMed.
- S. P. Zhong, Y. Z. Zhang and C. T. Lim, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 510–525 CrossRef CAS PubMed.
- B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef CAS PubMed.
- L. Yildirimer, N. T. K. Thanh and A. M. Seifalian, Trends Biotechnol., 2012, 30, 638–648 CrossRef CAS PubMed.
- G. F. Pierce and T. A. Mustoe, Annu. Rev. Med., 1995, 46, 467–481 CrossRef CAS PubMed.
- K. Obara, M. Ishihara, T. Ishizuka, M. Fujita, Y. Ozeki, T. Maehara, Y. Saito, H. Yura, T. Matsui, H. Hattori, M. Kikuchi and A. Kurita, Biomaterials, 2003, 24, 3437–3444 CrossRef CAS PubMed.
- M. C. Robson, L. G. Phillips, L. E. Robson, A. Thomason and G. F. Pierce, Lancet, 1992, 339, 23–25 CrossRef CAS.
- G. S. Schultz, R. G. Sibbald, V. Falanga, E. A. Ayello, C. Dowsett, K. Harding, M. Romanelli, M. C. Stacey, L. Teot and W. Vanscheidt, Wound Repair Regen., 2003, 11, S1–S28 CrossRef PubMed.
- R. Jain, A. Agarwal, P. R. Kierski, M. J. Schurr, C. J. Murphy, J. F. McAnulty and N. L. Abbott, Biomaterials, 2013, 34, 340–352 CrossRef CAS PubMed.
- Y. Yang, T. Xia, W. Zhi, L. Wei, J. Weng, C. Zhang and X. Li, Biomaterials, 2011, 32, 4243–4254 CrossRef CAS PubMed.
- Y. Yang, T. Xia, F. Chen, W. Wei, C. Liu, S. He and X. Li, Mol. Pharm., 2012, 9, 48–58 CrossRef CAS PubMed.
- J. S. Choi, K. W. Leong and H. S. Yoo, Biomaterials, 2008, 29, 587–596 CrossRef CAS PubMed.
- C. Arun Richard, J. Venugopal, S. Sundarrajan and S. Ramakrishna, Biomed. Mater., 2011, 6, 015001 CrossRef PubMed.
- M. Ackermann, T. Wolloscheck, A. Wellmann, V. W. Li, W. W. Li and M. A. Konerding, Eur. Surg. Res., 2011, 47, 81–89 CrossRef CAS PubMed.
- M. Ackermann, T. Wolloscheck, A. Wellmann, V. W. Li, W. W. Li and M. A. Konerding, Int. J. Mol. Med., 2011, 27, 647–653 CAS.
- N. Mehmood, A. Hariz, R. Fitridge and N. H. Voelcker, J. Biomed. Mater. Res., Part B, 2014, 102, 885–895 CrossRef PubMed.
- V. Bertonocelj, J. Pelipenko, J. Kristl, M. Jeras, M. Cukjati and P. Kocbek, Eur. J. Pharm. Biopharm., 2014, 88, 64–74 CrossRef PubMed.
- K. M. Lacci and A. Dardik, Yale J. Biol. Med., 2010, 83, 1–9 CAS.
- J. S. Choi, K. W. Leong and H. S. Yoo, Biomaterials, 2008, 29, 587–596 CrossRef CAS PubMed.
- Y. J. Son, J. Kang, H. S. Kim and H. S. Yoo, Biomacromolecules, 2016, 17, 1067–1074 CrossRef CAS PubMed.
- Y. Yang, T. Xia, W. Zhi, L. Wei, J. Weng, C. Zhang and X. Li, Biomaterials, 2011, 32, 4243–4254 CrossRef CAS PubMed.
- H. J. Lai, C. H. Kuan, H. C. Wu, J. C. Tsai, T. M. Chen, D. J. Hsieh and T. W. Wang, Acta Biomater., 2014, 10, 4156–4166 CrossRef CAS PubMed.
- K. Lee, E. A. Silva and D. J. Mooney, J. R. Soc., Interface, 2011, 8, 153–170 CrossRef CAS PubMed.
- J. S. Choi, S. H. Choi and H. S. Yoo, J. Mater. Chem., 2011, 21, 5258–5267 RSC.
- S. Akita, K. Akino and A. Hirano, Adv. Wound Care, 2013, 2, 44–49 CrossRef PubMed.
- H.-X. Shi, C. Lin, B.-B. Lin, Z.-G. Wang, H.-Y. Zhang, F.-Z. Wu, Y. Cheng, L.-J. Xiang, D.-J. Guo, X. Luo, G.-Y. Zhang, X.-B. Fu, S. Bellusci, X.-K. Li and J. Xiao, PLoS One, 2013, 8, e59966 CAS.
- H. Jiang, Y. Hu, Y. Li, P. Zhao, K. Zhu and W. Chen, J. Controlled Release, 2005, 108, 237–243 CrossRef CAS PubMed.
- Y. Zhang, Z.-M. Huang, X. Xu, C. T. Lim and S. Ramakrishna, Chem. Mater., 2004, 16, 3406–3409 CrossRef CAS.
- I. C. Liao, S. Y. Chew and K. W. Leong, Nanomedicine, 2006, 1, 465–471 CrossRef CAS PubMed.
- K. C. Wood, H. F. Chuang, R. D. Batten, D. M. Lynn and P. T. Hammond, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10207–10212 CrossRef CAS PubMed.
- Z. Xie, C. B. Paras, H. Weng, P. Punnakitikashem, L. C. Su, K. Vu, L. Tang, J. Yang and K. T. Nguyen, Acta Biomater., 2013, 9, 9351–9359 CrossRef CAS PubMed.
- P. M. Krein, Y. Huang and B. W. Winston, Expert Opin. Ther. Pat., 2001, 11, 1065–1079 CrossRef CAS.
- T. A. Wilgus, A. M. Ferreira, T. M. Oberyszyn, V. K. Bergdall and L. A. DiPietro, Lab. Invest., 2008, 88, 579–590 CrossRef CAS PubMed.
- P. Li, P. Liu, R.-P. Xiong, X.-Y. Chen, Y. Zhao, W.-P. Lu, X. Liu, Y.-L. Ning, N. Yang and Y.-G. Zhou, J. Pathol., 2011, 223, 659–671 CrossRef CAS PubMed.
- J. H. Sung, M.-R. Hwang, J. O. Kim, J. H. Lee, Y. I. Kim, J. H. Kim, S. W. Chang, S. G. Jin, J. A. Kim, W. S. Lyoo, S. S. Han, S. K. Ku, C. S. Yong and H.-G. Choi, Int. J. Pharm., 2010, 392, 232–240 CrossRef CAS PubMed.
- R. Vasita and D. S. Katti, Int. J. Nanomed., 2006, 1, 15–30 CrossRef CAS PubMed.
- P. Karuppuswamy, J. R. Venugopal, B. Navaneethan, A. L. Laiva, S. Sridhar and S. Ramakrishna, Appl. Surf. Sci., 2014, 322, 162–168 CrossRef CAS.
- R. Ravichandran, S. Sundarrajan, J. R. Venugopal, S. Mukherjee and S. Ramakrishna, Macromol. Biosci., 2012, 12, 286–311 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |