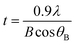Magnetic iron oxide nanoparticles encapsulating horseradish peroxidase (HRP): synthesis, characterization and carrier for the generation of free radicals for potential applications in cancer therapy
Nikesh Guptaa,
Chetna Guptab,
Sandeep Sharmac,
Brijesh Rathibd,
Rakesh Kumar Sharma*c and
H. B. Bohidar*a
aSpecial Centre for Nanosciences (SCNS), Jawaharlal Nehru University, New Delhi – 110067, India. E-mail: bohidarjnu@gmail.com; bohi0700@mail.jnu.ac.in
bDepartment of Chemistry, Hansraj College, University of Delhi, Delhi – 110007, India
cNanotechnology, Drug Delivery and Tissue Culture Research Lab, Department of Chemistry, University of Delhi, Delhi – 110007, India. E-mail: sharmark101@yahoo.com
dDepartment of Chemistry, Massachusetts Institute of Technology (MIT), Cambridge, MA 02139, USA
First published on 28th October 2016
Abstract
This article reports a method of preparation of iron oxide nanoparticles using a reverse micellar (water-in-oil) approach. We have encapsulated horseradish peroxidase (HRP) in iron oxide nanoparticles. HRTEM, XRD, and DLS analyses showed that the average diameter of these particles was around 20 nm, 20.5 nm, and 30 nm, respectively, and the particles were highly monodispersed with spherical morphology. The entrapment efficiency of HRP was found to be as high as 92%. Practically, the entrapped enzyme shows zero leachability for up to 30 days. Enzyme entrapped in iron oxide nanoparticles followed Michaelis–Menten kinetics and showed higher stability towards temperature change as compared to free enzyme. Entrapped enzyme is stable at up to 65 °C; however, the free enzyme starts to lose its activity above 38 °C. The entrapped enzyme, HRP, has been used to convert a benign prodrug, indole-3-acetic acid (IAA), to a toxic oxidized product, and its toxic effect has been tested on cancerous cell lines through thiazolyl blue tetrazolium blue (MTT) assay. MTT assay on two cancer cell lines revealed that indole acetic acid (IAA), the prodrug alone, had no cytotoxic effect, and it became active only after oxidative decarboxylation by HRP. The benign substrate IAA reaches the cells and is oxidized by HRP. IAA, on reacting with HRP, forms free radicals such as indolyl, skatole and peroxyl radicals. This creates severe oxidative stress in the cancer cells, resulting in cell death.
1. Introduction
Nanotechnology, an interdisciplinary research field involving chemistry, engineering, biology, and medicine, has great potential for early detection, accurate diagnosis, and personalized treatment of cancer.1 A number of approaches are available for the synthesis of nanoparticles, for example, reduction in solutions,2–7 microemulsion,8–12 sol–gel method,13 electrochemical,14,15 sonochemical,16 and hydrothermal.17 Cancer nanotechnology is an interdisciplinary area with broad potential applications in fighting cancer, including molecular imaging, molecular diagnosis, targeted therapy, and bioinformatics. The continued development of cancer nanotechnology holds promise for personalized oncology, in which genetic and protein biomarkers can be used to diagnose and treat cancer based on the molecular profile of each individual patient. Nowadays, water-in-oil microemulsion or a reverse micelle approach for the entrapment of biologically active compounds or molecules is receiving tremendous attention. A biologically active compound, such as an enzyme, can be sheltered in the aqueous core and is protected from being denatured by detrimental solvent effects. Water-in-oil microemulsions or reverse micelles provide an interesting route for enzyme catalysis, and they have found wide applications in enzymology, protein chemistry, and biocatalysis.18,19 The activity and stability of biomolecules can be controlled easily through immobilization. With exact knowledge of the phase behaviour and the corresponding activity of enzymes, the application of these media can lead to favorable effects compared to aqueous systems, such as hyperactivity or increased stability of the enzymes.20Most of the volume-confined reactions investigated so far are much simpler and have been localized in either reverse micelles, water-in-oil microemulsions,21,22 or in vesicles. Enzymatic reactions in reverse micelles are an interesting case because the number of water molecules within the core of a reverse micelle is very small. As a result, enzymes in reverse micelles can behave differently than in bulk aqueous solutions, although choosing proper conditions for the correct comparison of the two systems can be difficult.23 Several reports indicate that some enzymes—for example, chymotrypsin24,25 or HRP26,27—appear to act more efficiently when confined in reverse micelles than in bulk solution. However, still there is no clear and general understanding of this enzyme ‘superactivity’. It has been suggested that it may be due to conformational changes in the particular local concentrations of enzyme and substrates, or to the thermodynamic and kinetic properties of the confined water, which may lead to an altered hydration state of the active site of the enzyme. It is likely that different effects play a role, depending on the type of enzyme, the chemical structure of the substrate, and the amphiphiles forming the reverse micelle.28 Enzymes are employed in the body either to replace missing enzymes or enzymes that are not active in the body. The absence of specific enzymes in the body may lead to disease in an individual, and this can be controlled by external injection of the enzymes. However, this process may be contraindicated, as a foreign enzyme protein can cause unwanted reactions inside the body, which may be fatal. Moreover, free enzyme may be unstable inside the body and can be rapidly removed from the body through the natural immune-response system. The introduction of free enzymes into the body, unfortunately, has severe clinical limitations associated with rapid in vivo degradation by proteolysis and their interactions at multiple receptors.29 These limitations can be overcome by encapsulating the enzyme in a biocompatible, non-immunogenic and inert matrix, which prevents the enzyme from degradation, and the enzyme–substrate reaction can take place by the diffusion of substrate through the pores. Many enzymes, such as horseradish peroxidase (HRP),30 glucose oxidase,31 and other oxidase enzymes,32 are used for producing transient free-radical species from specific substrates. Previously, it has been reported33–37 that a combination of indole-3-acetic acid (IAA) and horseradish peroxidase (HRP) is cytotoxic to mammalian cells, and it was suggested that it could be used as a novel cancer therapeutic approach.26–40 IAA, in the presence of peroxidase, forms peroxyl-free radical intermediates, such as indolyl, skatole, and peroxyl radicals (Scheme 1), after fragmentation of oxidation intermediates.41 Free radicals derived from IAA also stimulate the production of reactive oxygen species (ROS), which initiates cellular damage and apoptosis.42 Therefore, when an interaction between HRP and IAA is allowed to take place within the tumor, the IAA is activated and forms free radicals, which kill the cancer cells by inducing oxidative stress in them. Interestingly, neither IAA nor HRP alone showed any cytotoxic effects at therapeutic concentrations, but led to cell death in combination.43 Excessive accumulation of enzyme molecules in the tumor is often achieved through antibody-mediated targeting, and the prodrug, IAA, enters the cancer cells from the systemic circulation. This therapeutic process is known as antibody-directed enzyme prodrug therapy, or ADEPT.44 Free radicals derived from IAA also stimulate the production of reactive oxygen species (ROS), which initiate cellular damage and apoptosis.45 The most well-studied nanoparticles include quantum dots,46,47 carbon nanotubes,48 magnetic nanoparticles,49 liposomes,50 gold nanoparticles,51 and many others.52,53 The use of iron oxide nanoparticles (IONPs) as carriers for enzymes may overcome many limitations, as these are robust, non-toxic and, because of their ultra-small size, can easily be eliminated from the body after their action is completed, through the kidneys. Iron oxide nanoparticles are useful in many applications, such as biosensors, in vivo tracers, dye-doped nanoparticulate biomarkers, and drug and gene delivery,54–56 because of their high biocompatibility and low toxicity. Moreover, these particles can be easily prepared with low polydispersity at ambient temperature.
 | ||
| Scheme 1 Diagrammatic representation of oxidation of indole-3-acetic acid by horseradish peroxidase to form free radicals. | ||
In our study, we have protected the HRP by encapsulating it inside the cavities of iron oxide nanoparticles. The non-biodegradable nature of these nanoparticles keeps the enzyme protected within the cavity of the nanoparticles as long as the particles are in the system. We have synthesized HRP-encapsulating iron oxide nanoparticles (HRP@IONPs). With the help of experiments, we have shown that the enzyme is not leachable through the pores of the nanoparticles; it remains in the cores, and the substrate diffuses through the pores of the nanoparticles and reacts with the enzyme to give the oxidized product. In earlier work from our group,18 it was shown that when we conjugate o-DA with dextran, then this small molecule of size ∼10 kD is not able to pass through the pores of nanoparticles; thereby, no reaction occurs between the substrate (o-DA–dextran) and the enzyme, and we observed no peaks in the UV-Vis spectrum. Now, when such a small molecule of size ∼10 kD is not able to penetrate through the pores of nanoparticles, then the enzyme, HRP (size ∼ 44 kD), which is sitting inside the cavities of the nanoparticles, will definitely not be able to leach out through its pores. The entrapped enzyme was allowed to interact with IAA within the cavity of IONPs to produce free radicals, which are responsible for killing the cancer cells. In the present study, we have demonstrated the enzyme–prodrug interaction, using IONPs as host, and the consequent impact of the resultant free radicals on the death of cancer cells, such as SiHa, a squamous cell carcinoma, and MCF-7 breast cancer cells, through oxidative stress.
2. Experimental
2.1 Materials
Aerosol-OT (AOT), i.e. sodium bis-(2-ethylhexyl) sulphosuccinate (99%) was purchased from ACROS Organics. Iron salts, ferric chloride, and ferrous chloride/sulphate were purchased from Alfa Aesar. Horseradish peroxidase (Rz = 3), indole acetic acid (IAA), o-dianisidine, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), and 2,7-dichlorofluorescein diacetate (DCFH-DA) were procured from Sigma Aldrich. Hexane (AR grade), hydrogen peroxide (30%) and ammonia were obtained from Rankem. Absolute ethanol was obtained from Merck. All the reagents were used without further purification. Aqueous solutions were prepared using double distilled water. Nitrogen gas was procured from Sigma Gases (India).2.2 Method of preparation of iron oxide nanoparticles encapsulating HRP
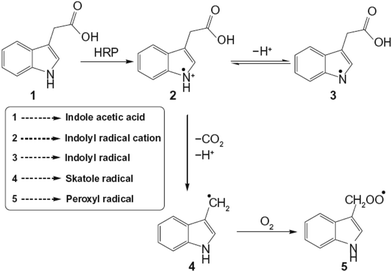
The overall procedure for the preparation of iron oxide nanoparticles is based on a reverse micellar technique under nitrogen (N2) atmosphere. Three micellar solutions A, B, and C were prepared and mixed to form a resultant micellar solution D (Scheme 2).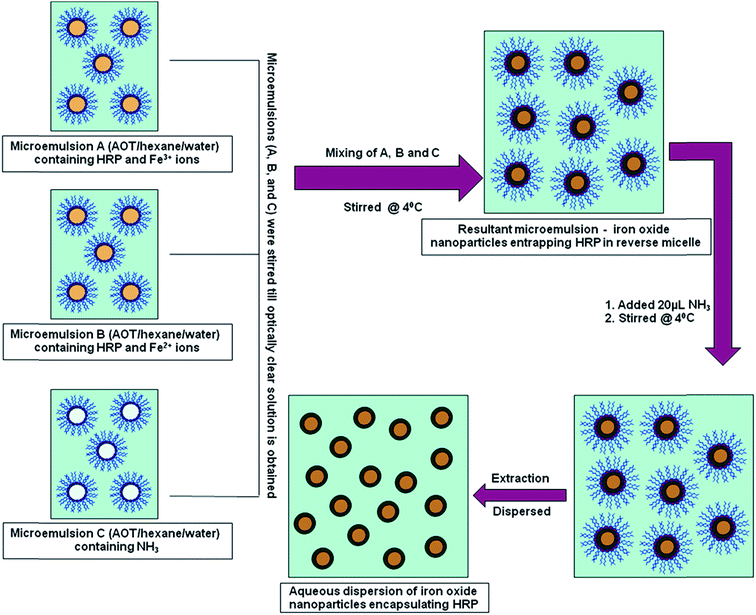 | ||
| Scheme 2 Schematic representation for the synthesis of iron oxide nanoparticles encapsulating horseradish peroxidase (HRP@IONPs) using water-in-oil microemulsion. | ||
Solution A consists of: 20 mL of 0.1 M AOT, 150 μL water, 150 μL HRP (5 mg mL−1), and 150 μL ferric salt (Fe3+) solution (4%) in a 100 mL round bottom flask.
Solution B consists of: 20 mL of 0.1 M AOT, 150 μL water, 150 μL HRP (5 mg mL−1), and 150 μL ferrous salt (Fe2+) solution (2%) in a 100 mL round bottom flask.
Solution C consists of: 20 mL of 0.1 M AOT, 150 μL water, and 20 μL ammonia solution in a 100 mL round bottom flask.
All the three solutions, A, B, and C, were stirred overnight under cold conditions at 4 °C until an optically clear solution was obtained. Solution B was then added dropwise to A, and after complete addition, the mixture was stirred for 6 hours. Solution C was then added dropwise to this mixture, and 20 μL excess ammonia solution was added to this solution. This resultant solution was further stirred for 12 hours. All the reactions were carried out under nitrogen atmosphere. Then, 10 mL of absolute ethanol was added and stirred vigorously for 10 min. The system was kept undisturbed for 30 min under cold conditions to allow the formation of an interfacial layer.
2.3 Extraction and coating of iron oxide nanoparticles
The interfacial layer was collected and washed twice with hexane and three times with ethanol, using a sonicator and microcentrifuge. Finally, the particles were dispersed in 6 mL of double distilled water by sonication under cold conditions. The dispersed NPs were dialyzed for 24 hours against water using a 12 kDa dialysis membrane bag to completely remove the surfactant and other unreacted and unwanted species. The synthesized nanoparticles were dispersed in 50 mL of water and stirred for 40 min at ∼60 °C. Then, the solution was cooled to room temperature and centrifuged. The pellets thus obtained were dispersed in 5 mL of water and to this, 10 mg tannic acid solution was added and stirred for 24 h under cold conditions at 4 °C. Then, the particles were centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm under cold conditions and the pellet thus formed was dispersed in 5 mL of water, and the system was kept undisturbed to determine its aqueous stability.
000 rpm under cold conditions and the pellet thus formed was dispersed in 5 mL of water, and the system was kept undisturbed to determine its aqueous stability.
Void nanoparticles were prepared in the same manner, i.e. instead of HRP, an equal amount of water was added to maintain the same Wo.
2.4 Size determination of nanoparticles
where t is the particle diameter, λ is the wavelength of the incoming X-rays (CuKα radiation), B is the breadth of the peak (or full width at half maximum, FWHM) in radian units, and θ is the half angle of diffraction.
2.5 Activity of the entrapped enzyme
Synthesized IONPs encapsulating HRP (HRP@IONPs) were dispersed in 3 mL of phosphate buffer (PB; 0.1 M, pH = 7.2). The enzyme kinetics of the HRP-catalyzed o-dianisidine oxidation reaction with H2O2 were studied in a 3.5 mL quartz cuvette, by adding 50 μL of o-dianisidine solution (1% w/v), followed by 20–100 μL (20×) of H2O2 (1 M) and 100 μL of the IONPs dispersed in water/buffer, to 3 mL of 0.1 M PB. The activity of the HRP enzyme is determined from the initial slope between the curve of the increase in absorbance of the colored product measured at 445 nm vs. time. The kinetic parameters, Km and kcat, were calculated from the Lineweaver–Burk plot of the Michaelis–Menten equation.
Enzyme activity studies were performed as follows:
Iron oxide nanoparticles entrapping HRP were dispersed in 5 mL of buffer (0.1 M, pH = 7.2), while 100 μL of dispersed nanoparticles, 50 μL of o-dianisidine (o-DA) (1% w/v), 50 μL H2O2 (1 M), and 2.7 mL buffer were placed in a 3.5 mL quartz cuvette. The gradual increase in concentration of the oxidized product of o-dianisidine was measured spectrophotometrically. In the case of the free enzyme, HRP entrapped in iron oxide nanoparticles was replaced by free HRP. The temperature of the thermostat was varied from 20 °C to 70 °C using a circulator water bath, and the gradual change in the concentration of the oxidized product of o-DA was assayed. The activity of the HRP at a desired temperature was calculated from its corresponding absorbance versus time graph.
2.6 Free radical determination
Free radicals formed by the IAA–HRP combination oxidize DCFH-DA to dichlorofluorescein (DCF). DCFH-DA was activated by taking 350 μL of 1 mM DCFH-DA in ethanol, followed by the addition of 1750 μL of 0.01 N NaOH. This mixture was allowed to stand in the dark for 30 minutes, followed by the addition of 17.9 mL of 25 mM sodium PB (pH = 7.2). The reaction mixture contains 300 μL of the activated form of DCFH-DA, 40 μL of IAA (500 μM), 40 μL of free or entrapped HRP (1.2 μg mL−1), and 3 mL of double-distilled water in a 3.5 mL cuvette. The reaction was studied at room temperature every 10 minutes at λext. = 490 nm and λem. = 522 nm, using a Cary Eclipse fluorescence spectrophotometer (Varian, Palo Alto, CA).2.7 Entrapment efficiency
The entrapment efficiency (E%) of HRP in iron oxide nanoparticles was determined from the difference in the total amount of enzyme added to the reverse micelles during preparation and the amount of enzyme remaining unentrapped. Here, we have neglected the loss of enzyme, if any, during extraction and washing. The IONPs entrapping enzyme was well dispersed in phosphate buffer solution (0.1 M, pH = 7.2) by sonication for 5 min. The buffer solution was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, so that the free enzyme was dissolved in the supernatant liquid and the particles settled. This procedure was repeated three times, so that the entire free enzyme, if present, enters the supernatant solution. Then, the concentration of free enzyme was determined by treating the supernatant solution with o-dianisidine and H2O2, and measuring the absorbance (activity) at 445 nm using a UV-Vis spectrophotometer. The calculated activity of the enzyme was then plotted on a standard curve of HRP, and the entrapment efficiency was determined by using the formula:
000 rpm for 10 min, so that the free enzyme was dissolved in the supernatant liquid and the particles settled. This procedure was repeated three times, so that the entire free enzyme, if present, enters the supernatant solution. Then, the concentration of free enzyme was determined by treating the supernatant solution with o-dianisidine and H2O2, and measuring the absorbance (activity) at 445 nm using a UV-Vis spectrophotometer. The calculated activity of the enzyme was then plotted on a standard curve of HRP, and the entrapment efficiency was determined by using the formula:| Entrapment efficiency (E%) = ({[enzyme]total − [enzyme]free}/[enzyme]total) × 100 |
2.8 Leachability of the enzyme from the nanoparticles
The particles obtained were dispersed in buffer and stirred under cold conditions for 30 days. The leachability of the enzyme from the particles was checked after regular intervals of time (after every 24 h). For this, a small volume of the nanoparticles dispersed in buffer was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C so that the particles settled. The enzyme activity in the supernatant buffer solution was then measured spectrophotometrically at 445 nm by treating it with o-dianisidine and H2O2.
000 rpm for 15 min at 4 °C so that the particles settled. The enzyme activity in the supernatant buffer solution was then measured spectrophotometrically at 445 nm by treating it with o-dianisidine and H2O2.
2.9 MTT (3-(4,5-dimethylthiazole-2yl)-2,5-diphenyltetrazolium bromide) assay
The cytotoxicity of void iron oxide nanoparticles, as well as that of iron oxide nanoparticles containing HRP and free enzyme, was measured by MTT assay. Two cancer cell samples (SiHa and MCF-7) were purchased from the National Cell Repository, NCCS, Pune (India) and were maintained according to the instructions provided. Briefly, these were seeded at a density of 1 × 104 cells per well in a 96-well plate containing 100 μL of RPMI complete media per well. After two hours, a cell monolayer was incubated in the medium with increasing concentrations of IAA mixed with (i) free HRP, (ii) void iron oxide nanoparticles, and (iii) iron oxide nanoparticles encapsulating HRP, and the final volume was adjusted to 200 μL with complete RPMI. Cells were incubated at 37 °C in a humidified environment of 5% CO2 for 48 h. Two hours prior to termination of the incubation, 20 μL of MTT (5 mg mL−1) was added to each well. Two hours post incubation with MTT, the precipitate formed was dissolved in 100 μL isopropanol, and quantified by measuring the absorbance at 570 nm (reference wavelength 620 nm), using a microplate reader (Biotek, USA). Cells cultured without IAA and nanoparticles were taken as a negative control.3. Results and discussions
Iron oxide nanoparticles encapsulating HRP were prepared in the aqueous core of a microemulsion of AOT in hexane. The size of the HRP@IONPs was measured by using HRTEM and DLS techniques. HRTEM and DLS indicated that the average diameter of the particles was about 20 nm (Fig. 1) and 30 nm (Fig. 2), respectively. The particles were spherical in shape with a narrow size distribution. The crystallinity of the particles was confirmed from their SAED (Fig. 3) and XRD patterns (Fig. 4). The average particle size calculated from XRD was around 20.5 nm, which matches well with the average diameter of nanoparticles determined from HRTEM. The aqueous dispersion of HRP@IONPs was stable for 10 days under cold conditions. To examine whether the entrapped enzyme leached out from the nanoparticles, and to demonstrate its enzymatic activity while entrapped within the nanoparticles, we studied the enzyme kinetics of entrapped HRP using o-dianisidine as a substrate. The HRP@IONPs dispersed in water were centrifuged for 30 min at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and the supernatant aqueous solution was tested for 60 min to determine the presence of enzyme by adding o-dianisidine and H2O2. The pellet was again dispersed in water and the dispersion was stirred for 24 h before centrifuging. The enzyme concentration of the supernatant solution was again measured using the above procedure. The process was repeated for 30 days. Each time, no detectable concentration of HRP was found in the supernatant solution, indicating that HRP entrapped inside the nanoparticles was not leachable for up to 30 days. The E% of these nanoparticles, as measured by the difference between the enzyme added and the enzyme remaining outside, was calculated to be around 92%. The IAA–HRP combination leads to the formation of indolyl, skatole, and peroxyl radicals; hence, we measured the production of these radicals using DCFH-DA. From Fig. 5, it can be concluded that DCFH-DA is oxidized to DCF, and the formation of DCF from DCFH-DA depends purely upon free radicals, such as indolyl, skatole, and peroxyl. Therefore, it can be stated that the IAA–HRP combination leads to the formation of free radicals, which are responsible for killing cancer cells. The temperature-dependent activity of HRP entrapped in iron oxide nanoparticles, and that of free HRP in aqueous buffer (all at pH 7.2), is shown in Fig. 6. The activity of free HRP in aqueous buffer increases with increase in temperature up to a maximum activity at 38 °C. Thereafter, the activity starts to decrease with a further rise in temperature. Similar behavior was obtained in the case of entrapped HRP, except that the maximum activity was found to be at 65 °C (activity measurements beyond 70 °C are not reliable due to the formation of bubbles in the cuvette). This shows that, although the entrapped enzyme is less active than the free enzyme, it is also less sensitive to temperature changes than the free enzyme. The temperature profile for the activity of entrapped enzymes is quite broad. This is perhaps because the IONPs shell protects the entrapped enzyme from temperature variation and restricts its mobility, hindering temperature-induced denaturation, thereby increasing its stability over a wider range of temperature. Analysis of the Michaelis–Menten parameters of free HRP and HRP-entrapped in iron oxide nanoparticles (HRP@IONPs) was performed using the Lineweaver–Burk (L–B) plots, which revealed that the kinetics was diffusion-dependent. The encapsulated enzyme follows Michaelis Menten kinetics, which is evident from the straight line obtained in the graph of 1/v vs. 1/[S]. The Lineweaver Burk plot (LB-plot) is shown in Fig. 7. The relatively high affinity of the free enzyme for the substrate was noted from the Michaelis constant, Km, of 1.78 mmol mL−1, while the corresponding value for enzyme entrapped in the cavities of the nanoparticles was 15.81 mmol mL−1. The kcat values calculated from the L–B plots were 106 min−1 for free enzyme and 104 min−1 for entrapped enzyme (Table 1). The reduced affinity for the substrate, as revealed from a comparison of the Km values, may be accounted for by factors such as diffusional constraint of the substrate and conformational distortion of the entrapped enzyme in the restricted volume of the cavity.
000 rpm, and the supernatant aqueous solution was tested for 60 min to determine the presence of enzyme by adding o-dianisidine and H2O2. The pellet was again dispersed in water and the dispersion was stirred for 24 h before centrifuging. The enzyme concentration of the supernatant solution was again measured using the above procedure. The process was repeated for 30 days. Each time, no detectable concentration of HRP was found in the supernatant solution, indicating that HRP entrapped inside the nanoparticles was not leachable for up to 30 days. The E% of these nanoparticles, as measured by the difference between the enzyme added and the enzyme remaining outside, was calculated to be around 92%. The IAA–HRP combination leads to the formation of indolyl, skatole, and peroxyl radicals; hence, we measured the production of these radicals using DCFH-DA. From Fig. 5, it can be concluded that DCFH-DA is oxidized to DCF, and the formation of DCF from DCFH-DA depends purely upon free radicals, such as indolyl, skatole, and peroxyl. Therefore, it can be stated that the IAA–HRP combination leads to the formation of free radicals, which are responsible for killing cancer cells. The temperature-dependent activity of HRP entrapped in iron oxide nanoparticles, and that of free HRP in aqueous buffer (all at pH 7.2), is shown in Fig. 6. The activity of free HRP in aqueous buffer increases with increase in temperature up to a maximum activity at 38 °C. Thereafter, the activity starts to decrease with a further rise in temperature. Similar behavior was obtained in the case of entrapped HRP, except that the maximum activity was found to be at 65 °C (activity measurements beyond 70 °C are not reliable due to the formation of bubbles in the cuvette). This shows that, although the entrapped enzyme is less active than the free enzyme, it is also less sensitive to temperature changes than the free enzyme. The temperature profile for the activity of entrapped enzymes is quite broad. This is perhaps because the IONPs shell protects the entrapped enzyme from temperature variation and restricts its mobility, hindering temperature-induced denaturation, thereby increasing its stability over a wider range of temperature. Analysis of the Michaelis–Menten parameters of free HRP and HRP-entrapped in iron oxide nanoparticles (HRP@IONPs) was performed using the Lineweaver–Burk (L–B) plots, which revealed that the kinetics was diffusion-dependent. The encapsulated enzyme follows Michaelis Menten kinetics, which is evident from the straight line obtained in the graph of 1/v vs. 1/[S]. The Lineweaver Burk plot (LB-plot) is shown in Fig. 7. The relatively high affinity of the free enzyme for the substrate was noted from the Michaelis constant, Km, of 1.78 mmol mL−1, while the corresponding value for enzyme entrapped in the cavities of the nanoparticles was 15.81 mmol mL−1. The kcat values calculated from the L–B plots were 106 min−1 for free enzyme and 104 min−1 for entrapped enzyme (Table 1). The reduced affinity for the substrate, as revealed from a comparison of the Km values, may be accounted for by factors such as diffusional constraint of the substrate and conformational distortion of the entrapped enzyme in the restricted volume of the cavity.
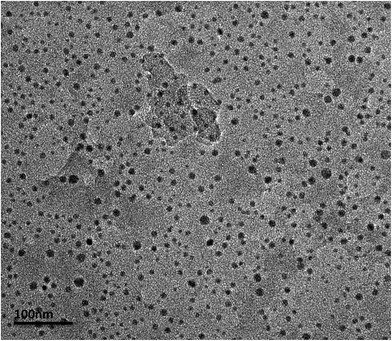 | ||
| Fig. 1 High resolution transmission electron microscopy (HRTEM) image of iron oxide nanoparticles encapsulating HRP, showing an average size of 20 nm. | ||
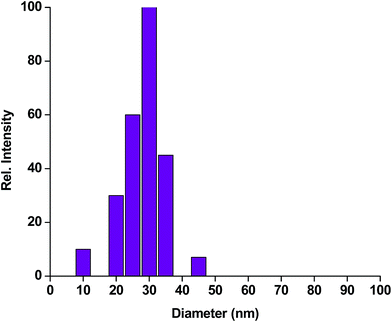 | ||
| Fig. 2 Dynamic light scattering (DLS) pattern of iron oxide nanoparticles encapsulating HRP, showing maximum distribution at 30 nm. | ||
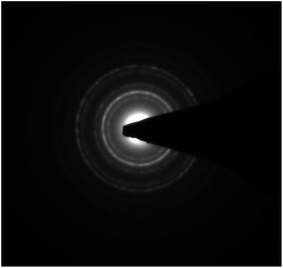 | ||
| Fig. 3 Selected area electron diffraction (SAED) pattern of iron oxide nanoparticles encapsulating HRP. | ||
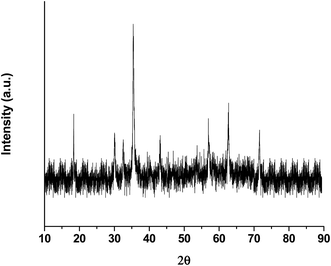 | ||
| Fig. 4 X-ray diffraction pattern of iron oxide nanoparticles encapsulating HRP, showing a particle size of 20.5 nm. | ||
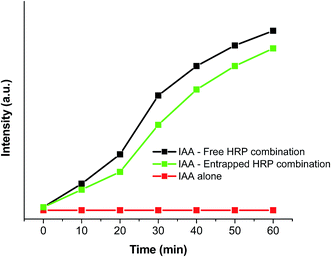 | ||
| Fig. 5 Kinetic study of the formation of dichlorofluorescein (DCF) from 2,7-dichlorofluorescein diacetate (DCFH-DA) due to IAA/HRP combination. | ||
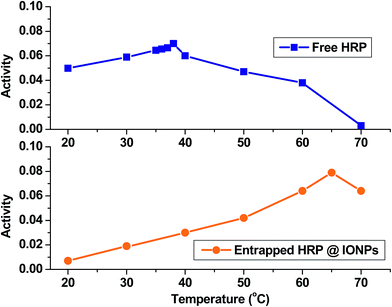 | ||
| Fig. 6 Temperature-dependent enzymatic activity of free HRP and entrapped HRP for the oxidation of o-dianisidine by H2O2 in phosphate buffer (pH = 7.2), measured in the range 20 °C to 70 °C. | ||
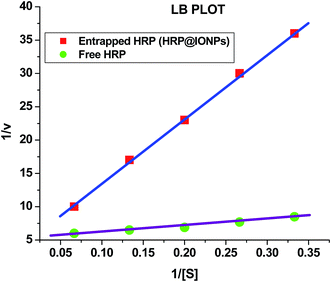 | ||
| Fig. 7 Lineweaver–Burk plot for comparison of Michaelis–Menten parameters of free HRP and iron oxide nanoparticles encapsulating HRP (HRP@IONPs). | ||
| Kinetic parameters | Free HRP | HRP@IONPs |
|---|---|---|
| Km (mmol mL−1) | 1.78 | 15.81 |
| kcat (min−1) | 106 | 104 |
In order to determine the possibility of using these HRP-entrapped IONPs for converting a benign prodrug, IAA, into a cytotoxin, we performed an in vitro cytotoxicity assay. For this assay, we used human cancer cell lines, such as SiHa, a squamous cell carcinoma, and MCF-7, a breast cancer cell line. These cells were incubated with increasing concentrations of IAA (0 to 2 μM) in either medium alone or in combination with free HRP, void IONPs, or HRP@IONPs. Cell viability was measured using the MTT assay after 48 hours of treatment. From the results (shown in Fig. 8), we observed significant cell death in cancer cells incubated with the IAA–HRP combination. Furthermore, when cells were treated with the HRP@IONPs-IAA combination, MCF-7 cells were found to be more sensitive compared to SiHa cells. Within 48 hours, about 50% of MCF-7 cells died at 1 μM IAA with the entrapped HRP combination treatment, whereas relatively fewer cell deaths (30–35%) were induced in SiHa cells with the same treatment. These differences between cell lines are not surprising. The observed difference between MCF-7 and SiHa cells could be, among others, caused by different uptake rates. The rates of proliferation of the cell lines were different. Neither IAA alone nor its combination with void IONPs resulted in cell death. The results thus show that the IAA–HRP combination can kill tumor cells. Entrapment did not result in any significant reduction in the activity of the enzyme. Also, entrapped HRP can be active for a relatively longer duration and over a wider temperature range. Additionally, in order to rule out any influence of HRP in formazan bioreduction in the MTT assay, we incubated the free HRP or HRP@IONPs without cells for 48 hours and added MTT to this solution in a similar manner as to the treated cells. We did not observe any significant difference in OD compared to the blank wells where the MTT was added to the medium alone without cells, suggesting that the formazan bioreduction observed in cells with and without IAA–HRP treatment was due to the presence of live cells. In our in vitro study, the MTT assay showed that the viability of cancer cells remained unaltered with an increasing concentration of IAA, when administered alone. On the other hand, IAA combined with entrapped HRP in the nanoparticles led to a significant decrease in cell viability with increasing concentration of IAA, similar to that observed with the free HRP/IAA combination.
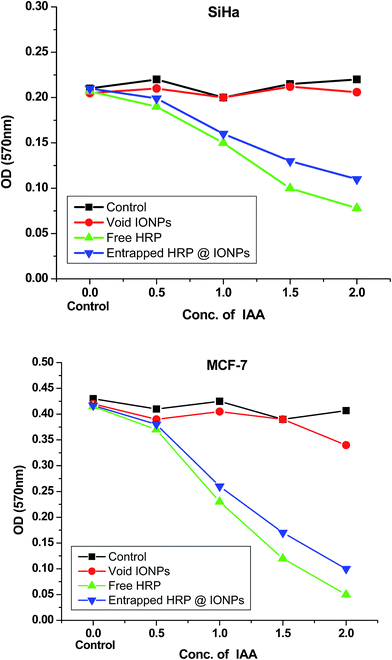 | ||
| Fig. 8 Cytotoxicity studies (MTT assay) of void iron oxide nanoparticles (void IONPs), free HRP, and iron oxide nanoparticles encapsulating HRP (HRP@IONPs) on SiHa and MCF-7 human cancer cell lines. | ||
The HRP/IAA combination produces reactive oxygen species (ROS). Normally, cells defend themselves against ROS damage with various enzymes, antioxidants such as ascorbic acids, and uric acids. Oxidative stress is an imbalance between the systemic manifestation of ROS and the ability of a biological system to readily detoxify the reactive intermediates or to repair the resulting damage. HRP/IAA toxicity is due to high oxidative stress, and tumor cells typically have higher levels of ROS scavengers. Since tumor cells usually possess higher levels of scavengers for ROS, they may be more vulnerable to the combination of iron oxide NP-entrapped HRP and IAA (see Scheme 3). The cancer cells have higher levels of ROS as compared to normal cells, and if we further increase the ROS level, by whatever strategy, cancer cell death occurs.57
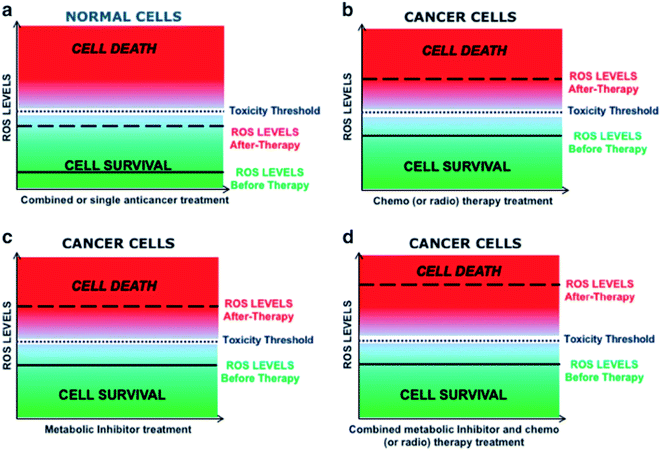 | ||
| Scheme 3 Schematic representation of ROS levels in normal and cancer cells. Strategies to manipulate ROS levels as anticancer therapy. Effect of different therapeutic manipulations on the intracellular ROS levels and relative toxicity in both normal and cancer cells.57 | ||
4. Conclusion
Horseradish peroxidase can be effectively and efficiently encapsulated in the core of iron oxide nanoparticles by utilizing the aqueous core of a water-in-oil microemulsion as a host nanoreactor. The particles are around 20 nm in diameter with spherical morphology, and are reasonably monodispersed. The enzyme entrapment efficiency of the nanoparticles was more than 92% with zero leachability of the enzyme over up to 30 days. The kinetic parameters of the entrapped enzyme were determined and compared with those of the free enzymes. The catalytic activity of the entrapped enzyme is less than that of the free enzymes, which may be due to the diffusional constraint of the substrate, since the molecule (o-DA) has to enter into nanoparticles through their pores and react with HRP, and then the oxidized product escapes through its pores. Although the activity of the entrapped enzyme is less, it is still sufficient to be used for enzyme therapeutic purposes. An MTT assay with these particles shows that neither IAA alone nor its combination with void nanoparticles resulted in tumor cell death, whereas the IAA–HRP combination can kill the cells. Entrapment did not result in a significant reduction in the activity of the enzyme. Also, entrapped HRP can be active for a relatively longer duration.Acknowledgements
NG is highly thankful to the Dr D. S. Kothari Post Doctoral Fellowship Cell, University Grants Commission (UGC), India, for financial assistance. CG is highly thankful to the University of Delhi, India for providing financial assistance in the form of an Innovation Project. SS is thankful to the University Grants Commission (UGC), India, for financial assistance in the form of a Senior Research Fellowship. BR is thankful to the University Grants Commission (UGC), India for the award of Raman postdoctoral fellowship. RKS is highly thankful to the University of Delhi for financial assistance in the form of an R&D program. HBB is highly thankful to the Department of Science and Technology, Delhi, India and Jawaharlal Nehru University, Delhi, India for providing financial assistance for research.References
- W. Cai and X. Chen, Small, 2007, 3, 1840 CrossRef CAS PubMed.
- M. R. Johan, L. C. Chong and N. A. Hamizi, Int. J. Electrochem. Sci., 2012, 7, 4567 CAS.
- D. V. Goia and E. Matijevic, New J. Chem., 1998, 22, 1203 RSC.
- N. Gupta, C. Gupta, S. Sharma, R. K. Sharma and H. B. Bohidar, Chemical Biology Letters, 2015, 2, 41–44 Search PubMed.
- N. Gupta, H. P. Singh and R. K. Sharma, J. Mol. Catal. A: Chem., 2011, 335, 248–252 CrossRef CAS.
- H. P. Singh, N. Gupta and R. K. Sharma, Journal of Biomedical and Therapeutic Sciences, 2014, 1(1), 34–40 Search PubMed.
- N. Gupta, H. P. Singh and R. K. Sharma, Colloids Surf., A, 2010, 367, 102–107 CrossRef CAS.
- A. K. Ganguli, T. Ahmad, S. Vaidya and J. Ahmed, Pure Appl. Chem., 2008, 80, 2451 CrossRef CAS.
- H. K. Wang, C. Y. Yi, L. Tian, W. J. Wang, J. Fang, J. H. Zhao and W. G. Shen, J. Nanomater., 2012, 453915 Search PubMed.
- S. López-Cuenca, L. A. Pérez Carrillo, M. R. Velasco, R. Díaz de León, H. Saade, R. G. López, E. Mendizábal and J. E. Puig, J. Nanomater., 2011, 431382 Search PubMed.
- R. K. Sharma, S. Das and A. Maitra, J. Colloid Interface Sci., 2005, 284, 358 CrossRef CAS PubMed.
- N. Gupta, A. Shrivastava and R. K. Sharma, Int. J. Nanomed., 2012, 7, 5491 Search PubMed.
- M. Epifani, C. Giannini, L. Tapfer and L. Vasanelli, J. Am. Ceram. Soc., 2000, 83, 2385 CrossRef CAS.
- R. A. Khaydarov, R. R. Khaydarov, O. Gapurova, Y. Estrin and T. Scheper, J. Nanopart. Res., 2009, 11, 1193 CrossRef CAS.
- H. Ma, B. Yin, S. Wang, Y. Jiao, W. Pan, S. Huang, S. Chen and F. Meng, ChemPhysChem, 2004, 5, 68 CrossRef CAS PubMed.
- J. J. Zhu, S. W. Liu, O. Palchik, Y. Koltypin and A. Gedanken, Langmuir, 2000, 16, 6396 CrossRef CAS.
- W. Yan, B. Chen, S. M. Mahurin, V. Schwartz, D. R. Mullins, A. R. Lupini, S. J. Pennycook, S. Dai and S. H. Overbury, J. Phys. Chem. B, 2005, 109, 10676 CrossRef CAS PubMed.
- R. Kumar, A. Maitra, P. K. Patanjali and P. Sharma, Biomaterials, 2005, 26, 6743 CrossRef CAS PubMed.
- T. K. Jain, I. Roy, T. K. De and A. Maitra, J. Am. Chem. Soc., 1998, 120, 11092 CrossRef CAS.
- B. Orlich and R. Schomäcker, Adv. Biochem. Eng./Biotechnol., 2002, 75, 185 CrossRef CAS PubMed.
- S. Kumar, V. K. Meena, P. P. Hazari and R. K. Sharma, Int. J. Pharm., 2016, 506, 242 CrossRef CAS PubMed.
- F. P. Chang, Y. Hung, J. H. Chang, C. H. Lin and C. Y. Mou, ACS Appl. Mater. Interfaces, 2014, 6, 6883 CAS.
- P. L. Luisi, M. Giomini, M. P. Pileni and B. H. Robinson, Biochim. Biophys. Acta, 1988, 947, 209 CrossRef CAS.
- T. E. Sintra, S. P. M. Ventura and J. A. P. Coutinho, J. Mol. Catal. B: Enzym., 2014, 107, 140 CrossRef CAS.
- F. Moyano, R. D. Falcone, J. C. Mejuto, J. J. Silber and N. M. Correa, Chem.–Eur. J., 2010, 16, 8887 CrossRef CAS PubMed.
- R. Biswas, et al., J. Phys. Chem. B, 2008, 112, 6620 CrossRef CAS PubMed.
- M. Moniruzzaman, N. Kamiya and M. Goto, Langmuir, 2009, 25, 977 CrossRef CAS PubMed.
- A. Küchler, M. Yoshimoto, S. Luginbühl, F. Mavelli and P. Walde, Nat. Nanotechnol., 2016, 11, 409 CrossRef PubMed.
- M. Pernot, R. Vanderesse, C. Frochot, F. Guillemin and M. Barberi-Heyob, Expert Opin. Drug Metab. Toxicol., 2011, 7, 793 CrossRef CAS PubMed.
- D. S. Kim, S. E. Jeon, Y. M. Jeong, S. Y. Kim, S. B. Kwon and K. C. Park, FEBS Lett., 2006, 580, 1439 CrossRef CAS PubMed.
- S. Trivic, V. Leskovac, J. Zeremski, M. Vivic and G. W. Winston, Bioorg. Chem., 2002, 30, 95 CrossRef CAS PubMed.
- K. H. Cheeseman and T. F. Slater, Br. Med. Bull., 1993, 49, 481 CAS.
- G. Bonifert, L. Folkes, C. Gmeiner, G. Dachs and O. Spadiut, Cancer Med., 2016, 5, 1194 CrossRef CAS PubMed.
- M. Kimura, Y. Umemoto and T. Kawano, Front. Plant Sci., 2014, 5, 1 Search PubMed.
- M. Kimura and T. Kawano, Plant Signaling Behav., 2015, 10(11), 1 Search PubMed.
- F. W. Krainer and A. Glieder, Appl. Microbiol. Biotechnol., 2015, 99, 1611 CrossRef CAS PubMed.
- L. K. Folkes, L. P. Candeias and P. Wardman, Int. J. Radiat. Oncol., Biol., Phys., 1998, 42, 917 CrossRef CAS.
- G. Manda, M. T. Nechifor and T. M. Neagu, Curr. Chem. Biol., 2009, 3, 342 CrossRef CAS.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579 CrossRef CAS PubMed.
- S. Y. Huh, J. I. Na, C. H. Huh and K. C. Park, Curr. Pharm. Des., 2002, 8, 1363 CrossRef.
- L. P. Candeias, L. K. Folkes, M. Porssa, J. Parrick and P. Wardman, Free Radical Res., 1995, 23, 403 CrossRef CAS PubMed.
- D. S. Kim, S. E. Jeon and K. C. Park, Cell. Signalling, 2004, 16, 81 CrossRef CAS PubMed.
- S. Pugine, T. Piza, E. Costa and M. De Melo, WEBMED CENTRAL Microbiology, 2010, 1, WMCOO695 Search PubMed.
- C. Andrady, S. K. Sharma and K. A. Chester, Immunotherapy, 2011, 3, 193 CrossRef CAS PubMed.
- D. S. Kim, S. E. Jeon and K. C. Park, Cell. Signalling, 2004, 16, 81 CrossRef CAS PubMed.
- W. Cai, D. W. Shin, K. Chen, O. Gheysens, Q. Cao, S. X. Wang, S. S. Gambhir and X. Chen, Nano Lett., 2006, 6, 669 CrossRef CAS PubMed.
- W. Cai, A. R. Hsu, Z. B. Li and X. Chen, Nanoscale Res. Lett., 2007, 2, 265 CrossRef CAS PubMed.
- Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47 CrossRef CAS PubMed.
- D. L. Thorek, A. K. Chen, J. Czupryna and A. Tsourkas, Ann. Biomed. Eng., 2006, 34, 23 CrossRef PubMed.
- J. W. Park, C. C. Benz and F. J. Martin, Semin. Oncol., 2004, 31, 196 CrossRef CAS PubMed.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nanomedicine, 2007, 2, 681 CrossRef CAS PubMed.
- M. Ferrari, Nat. Rev. Cancer, 2005, 5, 161 CrossRef CAS PubMed.
- P. Grodzinski, M. Silver and L. K. Molnar, Expert Rev. Mol. Diagn., 2006, 6, 307 CrossRef CAS PubMed.
- G. Bhakta, R. K. Sharma, N. Gupta, S. Cool, V. Nurcombe and A. Maitra, Nanomedicine, 2011, 7, 472 CAS.
- D. J. Bharali, I. Klejbor and E. K. Stachowiak, et al., Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11539 CrossRef CAS PubMed.
- S. Santra, H. Yang, D. Dutta, J. T. Stanley, P. H. Holloway, W. Tan, B. M. Moudgil and R. A. Mericle, Chem. Commun., 2004, 24, 2810 RSC.
- E. Panieri and M. M. Santoro, Cell Death Dis., 2016, 7, e2253, DOI:10.1038/cddis.2016.105.
| This journal is © The Royal Society of Chemistry 2016 |