Curcumin inhibits the Al(III) and Zn(II) induced amyloid fibrillation of β-lactoglobulin in vitro†
Sampa Pala,
Sanhita Maitya,
Subrata Sardara,
Hasan Parveja,
Niloy Dasb,
Jishnu Chakrabortya and
Umesh Chandra Halder*a
aDepartment of Chemistry, Jadavpur University, Kolkata-700032, India. E-mail: uhalder2002@yahoo.com
bDepartment of Chemistry, Durgapur Govt. College, Durgapur, West Bengal 713214, India
First published on 16th November 2016
Abstract
Accumulation of ordered protein aggregates (or amyloids) is responsible for several neurodegenerative diseases. The behaviour of amyloidal fibril formation of β-lactoglobulin (β-lg) during heat treatment depends on the environmental conditions. In this study the Al(III) and Zn(II) induced amyloid fibrillations of β-lg, in the absence and presence of curcumin, were evaluated using fluorescence, Thioflavin T, Congo red, Rayleigh scattering, dynamic light scattering analysis, FT-IR, CD spectroscopy and transmission electron microscopy. Curcumin, a natural phenolic antioxidant, is capable of binding with Al3+, Zn2+ and β-lg. Our experimental findings demonstrate that the metal–curcumin mixture can inhibit the transition from less structured oligomers to β-sheet rich protofibrils which act as seeding factors for further fibrillization. The Al(III)–curcumin mixture has greater inhibition capability than the Zn(II)–curcumin mixture of heat treated metal induced aggregation of β-lg.
1 Introduction
Amyloid plaques containing amyloid β (Aβ) peptides are conceived as pathological hallmarks of Alzheimer's disease (AD). The progressive accumulation of Aβ aggregates via a fibrillation process is widely believed to be fundamental to initialize the neurodegenerative pathology and trigger a cascade of events which include neurotoxicity, oxidative stress and inflammation during the progression of AD.1–3 Other peptides and proteins generate morphologically similar or different amyloid fibrils under carefully chosen conditions through dimer and oligomer formation. Their growth into the protofibrils and fibrils is really a complicated nucleation process.4 Recently, the leading role of the soluble aggregates in the neurodegenerative disorders has been widely accepted.5Several attempts have been made in searching the therapeutic agents which can inhibit the formation of such toxic oligomers or disintegrate the preformed fibrils. A number of experimental parameters (protein concentration, pH, temperature, ionic strength, presence of co-solvents, additives, etc.) can be varied to modulate the protein aggregation process and diversify the morphology, such as worm-like or rigid fibrils and amorphous aggregates.6–8 Interactions with metal ions can deeply affect protein aggregation, causing rapid precipitation, increased fibrillogenesis, and morphology alterations.9–11 It has been shown that the senile plaques, typical of the Alzheimer's disease, contain a great amount of transition metals ions such as Cu(III), Fe(III) and Zn(II). So far, their role is not very well clarified. The formation of amyloid fibrils from the β-amyloid peptide, main constituent of amyloid plaques in the brain of Alzheimer's disease/patients, became faster in the presence of copper and zinc.12,13 A similar behaviour has been noticed in α-synuclein, a protein involved in Parkinson's disease. The generally accepted argument on the function of divalent metals in protein aggregation is based on their ability to act as bridges, as well as to provide an electrostatic screening between the negatively charged groups of the neighbouring protein molecules.14 In fact, aggregation of protein is generally promoted by the electrostatic screening due to the action of monovalent and/or divalent metal ions.15
In our experiment we have selected the β-lg because of its two important interest – (i) it is a model β-protein in the aggregation process and (ii) used as a thermal marker in the industrial processes involved in preparation of milk.16 In general, β-lg is the major protein in whey of ruminant milk. The globular molecule has molecular weight of approximately 18.3 kDa and is made of 162 amino acid residues. β-lg is composed of nine β-strands and one α-helix, in which the hydrophobic sequences are mostly buried.17,18 In addition, β-lg possesses two intramolecular disulfide bonds (Cys66–Cys160, Cys106–Cys119) and one free thiol group (Cys121) which is buried between the β-barrel and the major α-helix.19 Furthermore, it consists of 162 amino acids and capable of binding and transporting small hydrophobic molecules. This carrier property makes it an attractive candidate to serve as a transporter for delivering important hydrophobic nutrients to improve their bioavailability. β-lg exists in the form of a dimmer at room temperature and neutral pH. However, it dissociates into monomers at acidic pH (<pH 3) due to electrostatic repulsions between the subunits.
Although the mechanism of β-lg heat-aggregation has been extensively studied, it is not still completely understood and controlled.20–23 As a consequence of β-lg aggregation, either amorphous aggregates or amyloid fibrils can be formed, depending on the experimental conditions.21,24 β-lg can form fibrils when heated above 75 °C under acidic conditions. However, longer treatment at 80 °C initiates the oxidation process promoting the exchange between thiol groups and disulphide bridges.25 Cu(II) and Zn(II) ions have a different physiological role and earlier reports show that Cu(II) or Zn(II) ions changes in the β-lg structure on thermal treatment and accelerate the formation of aggregates of β-lg having different morphologies.10,11 On the other hand Fe3+ ions almost completely inhibited fibrillation of β-lg at high temperature.26
Recently, a series of potential small molecule drugs, concerned with neurodegenerative disorders, were reported. Among them, curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), a natural poly phenol compound having potent antioxidant and anti-inflammatory activities, is under development as a potential chemotherapeutic agent of cancer and neurodegenerative disorders.27,28 Earlier studies have also shown that curcumin can interact with Aβ, thus inhibiting the Aβ aggregation and deposition.29 Previous reports demonstrated that curcumin inhibits the aggregation of α-synuclein and lysozyme27,30 and this prompted us to further explore its influence on the fibrillation of other proteins. In the present work we investigate, the role of curcumin as a potential inhibitor of Al(III) and Zn(II) catalysed aggregation and fibrillation of bovine β-lg is examined. The results evidence that β-lg, under particular thermal conditions, forms amyloid fibrils. Curcumin–Al(III) mixture, compared to curcumin–Zn(II) mixture, stabilize the native conformation of the protein and retards the aggregation process.
2 Materials and methods
2.1. Reagents and chemicals
Bovine beta lactoglobulin (β-lg) was isolated and purified from cow milk as described earlier.31 The final product was lyophilized and stored at 4 °C. Since the extinction coefficient of β-lg (0.96 mg−1 mL−1 cm−1 at 280 nm) is known, different concentrations of protein samples were prepared by dissolving β-lg samples in milli-Q-water and then measuring the O.D. at 280 nm. Sodium dihydrogen phosphate, ZnCl2 and AlCl3 (AR grade) was purchased from Merck (Mumbai, India). Curcumin and different fluorescent probes, viz., 8-anilinonaphthalene-1-sulfonic acid ammonium salt (ANS), Congo Red (CR) as well as Thioflavin T (ThT) were obtained from Sigma Chemical Co. (St. Louis, USA) and used as received without further purification. The other chemicals used were of highest purity available.2.2. Protein sample preparation
All the samples used in each experiment were aqueous protein solution prepared in 10 mM sodium phosphate buffer of pH 7 and having protein concentration 3 mM. For the experiments in the presence of aluminum and zinc, the ions were dissolved in the in mili-Q-water and the final concentration of metal solution were 1 mM and protein was added to the aqueous-metal solutions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The chloride salts of Al3+ and Zn2+ were chosen, since they do not display any IR or Raman bands that can interfere with the protein spectrum. A 1 mM curcumin stock solution was prepared dissolving 99.9% pure ethanol. The final concentration of protein–metal and protein–metal–curcumin (metal–curcumin mixture was in 1
1 molar ratio. The chloride salts of Al3+ and Zn2+ were chosen, since they do not display any IR or Raman bands that can interfere with the protein spectrum. A 1 mM curcumin stock solution was prepared dissolving 99.9% pure ethanol. The final concentration of protein–metal and protein–metal–curcumin (metal–curcumin mixture was in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) mixture were 3
1 molar ratio) mixture were 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.32,33 Each sample was freshly prepared and filtered through Sartorius filter paper (pore size 0.20 μm). All solutions were heated at 70 °C for 2 h. All measurements maintained the same concentration ratio described above.
1.32,33 Each sample was freshly prepared and filtered through Sartorius filter paper (pore size 0.20 μm). All solutions were heated at 70 °C for 2 h. All measurements maintained the same concentration ratio described above.
2.3. Intrinsic fluorescence study
Intrinsic fluorescence measurements were performed with all above described protein solutions in Na-phosphate buffer, pH-7.0 on a Shimadzu spectrofluorimeter (Shimadzu 5301 PC). The temperature was maintained at 25 °C. The fluorescence spectra were measured using 1 cm path-length rectangular quartz cell keeping the protein (β-lg) concentration 13.6 μM. The excitation and emission both slits were set at 5 nm and each spectrum was an average of three scans. Intrinsic fluorescence spectra were recorded in the wavelength region 310 to 400 nm after exciting the protein sample at wavelength of 295 nm.2.4. Dynamic light scattering (DLS) measurement
The diffusion of particulates in solution induces fluctuations in the intensity of the scattered light. DLS detects these fluctuations using an auto correlate on a microsecond time scale and is used to analyze the distribution of the molecules and supramolecular aggregates as it is very sensitive to particle size.34 Different sizes of molecules in the solution can be observed in different peaks provided their sizes vary sufficiently. In our experiment, DLS measurements were performed with the samples of 1 mg mL−1 β-lg concentration and all ratios were maintained in same Na-phosphate buffer, pH-7.0. All samples were heated at 70 °C for 2 h and DLS measurements were performed with β-lg solutions in absence and presence metals and metal–curcumin mixture employing Zeta-sizer Nanos (Malvern Instrument, U.K.) equipped with 633 nm laser and using 2 mL rectangular cuvette (path length 10 mm) at 20 °C. The time-dependent auto correlation function was acquired with twelve acquisitions for each run. Each data is an average of five such acquisitions.2.5. Monitoring the secondary structural change by FT-IR spectroscopy
For FT-IR measurements, 50 μL thermally incubated β-lg solutions with Al(III) and Zn(II) (in the molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), thermally incubated β-lg with Al(III) + curcumin mixture (1
1), thermally incubated β-lg with Al(III) + curcumin mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and with Zn(II) + curcumin mixture (1
1) and with Zn(II) + curcumin mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) having β-lg concentrations 20 mg mL−1 were taken in a Microcon filter device and diluted with 200 μL of D2O. It was then quickly centrifuged at 4000 × g for 8 min until the volume reached ∼50 μL. After that 200 μL of D2O was added again and centrifuged for another 8–10 min. This process of D2O exchange was repeated 3–4 times.35 Finally, the D2O exchanged β-lg samples were placed between two CaF2 windows separated by a 50 μm thick Teflon spacer. FT-IR scans were collected in the range of 1550–1750 cm−1 at a resolution of 2 cm−1 in N2 environment using a Spectrum 100 FT-IR spectrometer (Perkin Elmer). Spectrum of D2O at pD 7.0 was collected and subtracted from sample spectrum.
1) having β-lg concentrations 20 mg mL−1 were taken in a Microcon filter device and diluted with 200 μL of D2O. It was then quickly centrifuged at 4000 × g for 8 min until the volume reached ∼50 μL. After that 200 μL of D2O was added again and centrifuged for another 8–10 min. This process of D2O exchange was repeated 3–4 times.35 Finally, the D2O exchanged β-lg samples were placed between two CaF2 windows separated by a 50 μm thick Teflon spacer. FT-IR scans were collected in the range of 1550–1750 cm−1 at a resolution of 2 cm−1 in N2 environment using a Spectrum 100 FT-IR spectrometer (Perkin Elmer). Spectrum of D2O at pD 7.0 was collected and subtracted from sample spectrum.
2.6. Rayleigh light scattering measurement
The effect of addition of the antioxidant curcumin on the thermal aggregation β-lg in presence of Al(III) and Zn(II) ions were monitored by Rayleigh Light Scattering (RLS) measurements. The emission spectra were measured at 350 nm after exciting the solution at 350 nm using 10 mM phosphate buffer at pH 7.0 in a Shimadzu spectrofluorimeter (Shimadzu 5301 PC). The fluorescence spectra were collected at 25 °C using 1 cm path-length cell. The excitation and emission slits were set at 5 nm.2.7. Thoiflavin T (ThT) assay
ThT is a dye which shows enhanced fluorescence at 480 nm when bound to amyloid fibrils.36 Thus to investigate and compare the aggregates formed by heat exposed β-lg in absence and presence of Al(III) and Zn(II) and Al(III) + curcumin mixture or Zn(II) + curcumin mixture, the following ThT assay was employed. Briefly 250 μL of β-lg samples having concentration 54.3 μM was taken. It was then added to 40 μL ThT solution (stock 3.13 mM ThT in 10 mM sodium phosphate buffer, pH 7.0) containing a mixture of buffer and same ratio present metal, curcumin and metal–curcumin mixture, mixed thoroughly and incubated for 30 min.37 The final concentration of protein was 6.8 μM while the concentration of ThT was 30 μM. The sample solutions were excited at 450 nm (ref. 35) and the emissions were measured over the range 460–600 nm. Slit widths for both excitation and emission were kept at 5 nm. Three replicates were performed and the data were averaged.2.8. ANS fluorescence study
Exposure of hydrophobic patches in protein during the aggregation process was monitored using polarity sensitive fluorescent probe 1-anilinonapthalene-8-sulfonate (ANS).38 A stock solution of ANS was added to each aliquot of β-lg solution (both in absence and presence of metal ion and metal–curcumin mixture) so that the final ANS concentration in each aliquot was 30 μM. Typically, ANS concentration was 50 molar excess of protein concentration.44 The ANS-fluorescence intensities were measured using Shimadzu RF-5301 PC with excitation at 370 nm and scanning the emission wavelength from 400 nm to 650 nm.39,40 Slit widths were set at 5 nm for both excitation and emission. Each spectrum was blank corrected. Data points were the average of triplicate measurements.2.9. Congo red assay
The formation of aggregates by thermally exposed β-lg in absence and presence of Al(III) and Zn(II) and Al(III) + curcumin mixture or Zn(II) + curcumin mixture, can be investigated by measuring the shift in absorbance of Congo red in the region 400–650 nm. Congo red stock solution (10 mM) was prepared by dissolving the dye in 10 mM sodium phosphate buffer (pH 7.0) containing 150 mM NaCl under continuous stirring. It was then filtered with a 0.2 μm Millipore filter. A fresh working solution was prepared by diluting the stock solution 100 times.41 For this study, 250 μL (27.2 μM) aliquots of the protein solutions (containing the metal ions and curcumin) were withdrawn and mixed with 250 μL of a solution containing 40 μM Congo red solution. Final volume (2 mL) was adjusted with 10 mM sodium phosphate buffer, pH 7.0.2.10. Analysis of secondary structures by CD spectroscopy
To trace the conformational changes that metal ion and the metal ion–curcumin mixtures might induced on β-lg during thermal exposure, circular dichroism measurements were carried out on a JascoSpectropolarimeter (J-815) in the far-UV (200–250 nm) region using the rectangular cells of 1 mm path-length. Heat treated solutions having β-lg concentrations 0.25 mg mL−1 were used for far-UV CD measurements. All the spectra are average of three scans. The spectrum of 10 mM phosphate buffer (pH 7.0) as a control was subtracted from spectra of the samples. CD measurements were expressed as mean residue ellipticity in deg cm2 dmol−1. The calculation of secondary structural changes of β-lg in different systems was done by CDNN 2.1 software (Applied Photophysics Company).2.11. Transmission electron microscopy (TEM)
Aggregates obtained from heat treated solutions of β-lg with metal ions and metal ion + curcumin mixture were imaged using a high resolution transmission electron microscope (JEOL-HRTEM-2011, Tokyo, Japan) with an accelerating voltage of 80–85 kV indifferent magnifications. The sample solutions were sonicated for 60 s and diluted 50–150 times. In each case, diluted protein solution was placed on a carbon coated copper grid of mesh size 300 C (Pro Sci Tech) and after 20 s the droplet was removed with a filter paper. The sample was then stained with 3 mL of 2.0% uranyl acetate (Sigma, Steinheim Germany). The TEM grids was then allowed to dry prior to measurement and observed at a magnification of 12–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Fig. 1).
000 (Fig. 1).
 | ||
| Fig. 1 Schematic representation of Al3+ and Zn2+ induced amyloid fibrillation of heat stressed β-lg in absence and presence of curcumin at 70 °C for 2 h. | ||
3 Results and discussion
3.1. Microenvironment change of β-lg probed by intrinsic fluorescence
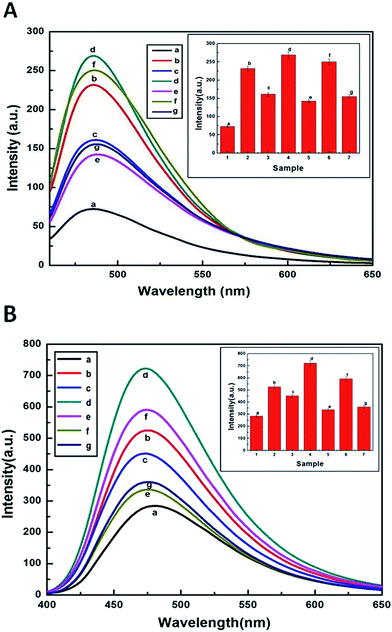
To investigate curcumin effect on the Al(III) and Zn(II)-induced conformational transition of β-lg, intrinsic fluorescence measurements were done to observe the change of the microenvironment of the fluorophore. The tryptophan emission spectra of β-lg in the wavelength region 300–400 nm with different system containing metal ions {Al(III) and Zn(II)} and curcumin–metal ion in β-lg solution at pH 7.0 have been represented in the Fig. 2A. Nativeβ-lg contains two tryptophan residues of which the Trp 19 is solely responsible for the intrinsic fluorescence property of β-lg.42 It shows the characteristic emission maxima at 332 nm upon excitation at 295 nm. Fluorescence emission spectrum of heat treated β-lg in absence and presence of metal ions β-lg showed a red shift of the emission maxima and an increase in intensity than native form (profile b, d, f Fig. 2A). The heat-induced red shift of the tryptophan fluorescence spectrum of the metal ions–β-lg systems reflects the considerable increase of the accessibility of Trp residues to the solvent, i.e. increase of the protein molecule hydrodynamic volume. Heat treated β-lg in presence of curcumin showed lower fluorescence intensity (Fig. 2A, profile c) than that of metal induced heat treated β-lg (profile d, f Fig. 2A). The lower intensity may be due to entanglement of formed fibrils into relatively unordered aggregates. Furthermore much lower intensities were observed in Al–curcumin–β-lg and Zn–curcumin–β-lg systems (profile e, g Fig. 2A) than curcumin–β-lg or β-lg–metals. The reduction of the fluorescence intensities, indeed, can be due to a fluorescence quenching in presence of both curcumin charged amino acid residues close to the Trp residues. It was interesting to note that the maximum decreased in β-lg intensity was found when it was heated with Al–curcumin mixture than Zn–curcumin.3.2. Dynamic light scattering (DLS) study: formation of smaller aggregates in curcumin containing samples
To monitor the effect of the curcumin on the formation of β-lg aggregate in the presence of Al3+ and Zn2+ ions, DLS study was employed (Fig. 2B). The scattering intensity of an aggregate is proportional to the mass and number of particles.43 Fig. 2B showed the temporal evolution of the scattering intensity of heat treated β-lg alone, in presence of Al(III) and Zn(II) and curcumin–Al(III) and curcumin–Zn(II) mixture. The data were plotted as scattered light intensity versus size of the radius. The hydrodynamic diameter of native β-lg (profile a, Fig. 2B) was ranging from 70 to 160 nm. DLS study of heated β-lg showed the formation of aggregates of size from 80 nm to 1500 nm (profile b, Fig. 2B). Again when β-lg were heated in presence of Al(III) and Zn(II) (profile d and f, Fig. 2B) generate the bigger hydrodynamic diameters up to 2500 nm. On the other hand the small aggregate of hydrodynamic diameter ∼100 nm to 1000 nm was observed when β-lg was heated with curcumin (profile c, Fig. 2B). DLS analysis also distinctly showed the presence of smaller oligomer of β-lg with the diameters ranging from only ∼50 nm to 700 nm upon heating with Al–curcumin (lower intensity) and Zn–curcumin (higher intensity) mixtures (profile e and g respectively in Fig. 2B). Therefore metal ion can promote the heat induced β-lg aggregation whereas curcumin and metal ion–curcumin mixtures have inhibitory effect for the formation of β-lg aggregates during thermal exposure at 70 °C. From the present study we may conclude metal ion–curcumin mixtures have greater inhibitory effect than curcumin on β-lg thermal aggregation and Al–curcumin mixture has highest suppressing activity.3.3. ATR-FTIR analysis revealed the enhancement of α-helical content
Information about secondary structure of protein can be obtained from the study of amide I (1600–1700 cm−1) band of ATR-FTIR spectra. Fig. 3A showed native β-lg molecule in absence of any metal and curcumin shows the amide-I band at around 1635 cm−1 (profile a) which is the characteristic features for the protein like β-lg having predominant β-sheet structure.44 It also showed that the conformational changes in the secondary structure during the unfolding and self-assembly processes of β-lg at 70 °C (profile b). The heat treated β-lg molecule showed a peak centered around 1633 cm−1. The increase in intensity of a specific band and appearance of a slight hump near 1620 cm−1 in heated β-lg were attributed due to the formation of β-sheets involved in intermolecular cross-linking (cross β-structure) between unfolded proteins and thus characterized protein aggregation.44 Again heat stressed β-lg in presence of Al(III) and Zn(II) metal ions showed more diffused band showing a peak at 1631 cm−1 and 1632 cm−1 indicating the formation of more beta structures (profile d and f, Fig. 3A). But when β-lg was heated with curcumin the observed FTIR band of lower intensity appeared at 1637 cm−1 (profile c, Fig. 3A). This different result indicated the inhibitory effect of curcumin in the β-lg aggregation. The quite different amide I bands of β-lg (1645 cm−1 and 1651 cm−1) were observed when it was heated with Al–curcumin and Zn–curcumin mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) respectively (profile e and g, Fig. 3A). These differences in amide I bands shows the major alterations to the β-lg conformations (reduction of the cross β-sheet and increase of the α-helical structure) and thus inhibiting the aggregation. Metal–curcumin mixtures thus enhance the formation α-helical structure of β-lg and in particular, the Al–curcumin mixture has more effective power in the inhibition of aggregation.
1) respectively (profile e and g, Fig. 3A). These differences in amide I bands shows the major alterations to the β-lg conformations (reduction of the cross β-sheet and increase of the α-helical structure) and thus inhibiting the aggregation. Metal–curcumin mixtures thus enhance the formation α-helical structure of β-lg and in particular, the Al–curcumin mixture has more effective power in the inhibition of aggregation.
3.4. Rayleigh light scattering (RLS) measurements
The intensity of the scattered light is extremely sensitive to the presence of large objects such as protein aggregates. We used light scattering to detect the onset of aggregation. The measurement of fluorescence intensity at 350 nm is a promising approach to monitor protein aggregation after exciting protein solution at the same wavelength.45 Rise of RLS data from a protein solution is an indication of aggregation. Fig. 3B showed change in scattering intensity measured at 350 nm after exciting the heat treated different β-lg samples at pH 7.0. Heat treated β-lg (point b) shows an increase in scattering intensity than native β-lg indicating the aggregate formation after thermal treatment. Curcumin reduces the intensity during the thermal exposure of β-lg solutions in presence of curcumin (point c). Again more intensities are observed when β-lg solutions are heated with Al(III) and Zn(II) metal ions (point d and f). Highest scattering intensity of the solution (3 fold) is observed in case of Al(III)–β-lg system suggesting the probability of highest aggregate formation as was also evident by DLS study. But interestingly, when heating the β-lg in the presence of curcumin–metal ion [Al(III) and Zn(II)] mixtures, the significant lowering of RLS intensities are observed (point e and g in Fig. 3B). Hence lower in scattering intensity of the heat treated β-lg solutions in presence of curcumin–metal ion [Al(III) and Zn(II)] mixtures support the disaggregation of β-lg. The Fig. 3B also clearly indicated that Al–curcumin mixture has highest inhibition capability than Zn–curcumin mixture on heat treated metal induced β-lg aggregation.3.5. Formation of β-lg aggregates studied by ThT assay
In order to investigate the eventual formation of cross-beta structures and their fibrillar aggregates of β-lg, ThT, a cationic benzothiazole dye, fluorescence has been widely used. ThT showed enhanced fluorescence spectra upon binding to protein assembly.46 It binds to intermolecular β-sheet present in the aggregates. In our present work, β-lg was heated at 70 °C with curcumin, metal ions and in the presence of metal–curcumin mixtures. The heat treated β-lg undergoes thermal aggregation showing almost 5 fold increase in ThT intensity upon binding with ThT (profile b, Fig. 4A). In the presence of Al(III) and Zn(II) ions (profile d and f, Fig. 4A), the sharp increase in the ThT intensities clearly show the formation of more fibrillar aggregates. In contrast, the amyloid formation of β-lg is somewhat suppressed by curcumin, as demonstrated by the decrease in ThT fluorescence intensity. Furthermore, after heat treatment of β-lg in the presence of curcumin with metal ions, the significant decrease of fluorescence intensities were observed (profile e and g, Fig. 4A). It is also noteworthy, that β-lg sample heated with Al(III)–curcumin mixture at molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has a greater reduction in the ThT fluorescence intensity compared with that in the presence of Zn(II)–curcumin mixture (1
1 has a greater reduction in the ThT fluorescence intensity compared with that in the presence of Zn(II)–curcumin mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio). These findings indicate that Al(III)–curcumin has greater ability to form a smaller number of cross-beta structures than that of Zn(II)–curcumin system. This suggest that curcumin must have stronger ability to bind with Al(III) than that of Zn(II) so that free Al(III) ions in solution decreased and thereby metal induced aggregation of β-lg inhibited. These findings are in agreement with the study of morphology of the aggregates with TEM.
1 molar ratio). These findings indicate that Al(III)–curcumin has greater ability to form a smaller number of cross-beta structures than that of Zn(II)–curcumin system. This suggest that curcumin must have stronger ability to bind with Al(III) than that of Zn(II) so that free Al(III) ions in solution decreased and thereby metal induced aggregation of β-lg inhibited. These findings are in agreement with the study of morphology of the aggregates with TEM.
3.6. ANS-fluorescence study to monitor the hydrophobicity change
The protective role of curcumin to inhibit the metal ions catalyzed thermal induced aggregation of β-lg was further confirmed by ANS fluorescence studies. Notably, molten globule state of proteins can be detected by binding of a conformation-sensitive probe, ANS to the hydrophobic region of protein that ultimately results in an increase of fluorescence intensity.47 The source of ANS interaction with β-lg may be due to both electrostatic and hydrophobic interactions.48 Fig. 4B shows the ANS fluorescence spectra of thermally exposed β-lg at pH 7.0 in phosphate buffer. In general at pH 7.0 the heat exposed β-lg showed ANS fluorescence intensity at around 480 nm. This increase in fluorescence intensity may be attributed to more access of ANS to the hydrophobic patches present in heat treated β-lg compared to native β-lg. Thus increase in hydrophobic patches enhances the protein–protein interactions leading to more thermal aggregation of β-lg.44 Again, in the presence of Al(III) and Zn(II) (Fig. 4B(d) and (f)), the fluorescence intensity considerably increases indicating the enhance of ANS biding metal-exposed hydrophobic sites. In contrast, the amyloid formation of β-lg is somewhat suppressed by curcumin, as demonstrated by the decrease in ANS fluorescence intensity. Furthermore, when Al–curcumin–β-lg and Zn–curcumin–β-lg (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixtures were heated separately, the significant lowering of fluorescence intensities compared with curcumin–β-lg system is observed. Evidently, metal ions–curcumin–β-lg have different role than the curcumin–β-lg system. The lowest fluorescence intensity was found in case of heat treated Al–curcumin–β-lg (Fig. 4B(e)) indicating minimum ANS biding hydrophobic sites are available on β-lg as compared to Zn–curcumin–β-lg (Fig. 4B(g)) and curcumin–β-lg systems. This was strongly supported by our ThT data.
3) mixtures were heated separately, the significant lowering of fluorescence intensities compared with curcumin–β-lg system is observed. Evidently, metal ions–curcumin–β-lg have different role than the curcumin–β-lg system. The lowest fluorescence intensity was found in case of heat treated Al–curcumin–β-lg (Fig. 4B(e)) indicating minimum ANS biding hydrophobic sites are available on β-lg as compared to Zn–curcumin–β-lg (Fig. 4B(g)) and curcumin–β-lg systems. This was strongly supported by our ThT data.
3.7. Congo red assay
The presence of amyloid-like fibrils can also be identified using a Congo red spectroscopic assay. CR is a colorant that binds preferentially with β-sheet structure of the aggregates but not to the native.7 CR is structurally similar to ThT and equally employed to detect protein aggregates. Binding of CR with the aggregates induces a characteristic increase in the absorption maxima from 480 nm to 490 nm. Generally, at neutral pH the heat stressed β-lg showed absorption maxima at 485 nm (profile b, Fig. 5A). But, the enhancement of CR absorption intensities was observed when β-lg was heated with metal ion of Al(III) and Zn(II) respectively (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These considerable differences in absorption may indicate different structural properties of amyloid fibrils formed with metal ions. The CR dye was trapped into the cluster of β-sheet peptide backbone of the aggregates. Again, Fig. 5A, profile c showed heat stressed β-lg in presence of curcumin has lower absorption intensity than that of β-lg. Much lower intensities were observed when β-lg was heated with metal ion–curcumin mixtures [Al–curcumin and Zn–curcumin (1
1). These considerable differences in absorption may indicate different structural properties of amyloid fibrils formed with metal ions. The CR dye was trapped into the cluster of β-sheet peptide backbone of the aggregates. Again, Fig. 5A, profile c showed heat stressed β-lg in presence of curcumin has lower absorption intensity than that of β-lg. Much lower intensities were observed when β-lg was heated with metal ion–curcumin mixtures [Al–curcumin and Zn–curcumin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)]. The lowest absorbance for Al–curcumin–β-lg system may be due to entanglement of formed fibrils into relatively unordered smaller aggregates. That means Al–curcumin thus stabilize more the monomeric and dimeric forms of the protein inhibiting the nucleation and elongation for the formation of higher aggregates. Thus Al–curcumin mixture has highest capability of suppressing thermal aggregation of β-lg than Zn–curcumin mixture.
1)]. The lowest absorbance for Al–curcumin–β-lg system may be due to entanglement of formed fibrils into relatively unordered smaller aggregates. That means Al–curcumin thus stabilize more the monomeric and dimeric forms of the protein inhibiting the nucleation and elongation for the formation of higher aggregates. Thus Al–curcumin mixture has highest capability of suppressing thermal aggregation of β-lg than Zn–curcumin mixture.
3.8. Secondary structural changes of β-lg studied by CD spectroscopy
Far-UV CD spectroscopy was used to analyze the conformational change of β-lg during the fibril formation in the absence and presence of metal ions, curcumin, metal–curcumin at concentrations ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) when heated at 70 °C at pH 7.0. The native β-lg (profile a, Fig. 5B) shows a negative signal around 216 nm which is characteristic for the β-sheet structure of the protein along with a very small minimum at 207 nm, also indicating some α-helical structure.49 The secondary structural elements of different β-lg systems. After heat treatment at 70 °C, the far-UV CD spectrum of β-lg shows a significant change in the peak position (profile b, c, Fig. 5B). But the thermal exposure in presence of curcumin (profile c, Fig. 5B) displays significant decrease in intensity with a shift in band positions similar to the native. This signifies that the conformation conversion of β-lg is inhibited by curcumin from a physiological unfolded random coil to a folded β-sheet. While in presence of Al(III) and Zn(II), CD spectra shows a decrease of MRE values and small band at 217 nm along with a very small band at 207 nm indicating again structural changes induced by metal ions (profile d and f, Fig. 5B). In the presence of Al(III)–curcumin and Zn(II)–curcumin mixture, CD spectra show an increase in the negative band at 215 nm, which indicates a structural change into β-sheet. The results suggest that the combined effect of metal ions–curcumin could prevent β-lg from converting into a folded conformation more effectively than curcumin without metal ions. Table 1 showed of the secondary structure contents of heat treated β-lg in the presence different situations. CD results clearly indicates the effect was more pronounced for β-lg treated with curcumin–Al(III) mixture than curcumin–Zn(II) mixture and bare curcumin. The calculation of secondary structural changes of β-lg in different systems was shown in Table 1.
1) when heated at 70 °C at pH 7.0. The native β-lg (profile a, Fig. 5B) shows a negative signal around 216 nm which is characteristic for the β-sheet structure of the protein along with a very small minimum at 207 nm, also indicating some α-helical structure.49 The secondary structural elements of different β-lg systems. After heat treatment at 70 °C, the far-UV CD spectrum of β-lg shows a significant change in the peak position (profile b, c, Fig. 5B). But the thermal exposure in presence of curcumin (profile c, Fig. 5B) displays significant decrease in intensity with a shift in band positions similar to the native. This signifies that the conformation conversion of β-lg is inhibited by curcumin from a physiological unfolded random coil to a folded β-sheet. While in presence of Al(III) and Zn(II), CD spectra shows a decrease of MRE values and small band at 217 nm along with a very small band at 207 nm indicating again structural changes induced by metal ions (profile d and f, Fig. 5B). In the presence of Al(III)–curcumin and Zn(II)–curcumin mixture, CD spectra show an increase in the negative band at 215 nm, which indicates a structural change into β-sheet. The results suggest that the combined effect of metal ions–curcumin could prevent β-lg from converting into a folded conformation more effectively than curcumin without metal ions. Table 1 showed of the secondary structure contents of heat treated β-lg in the presence different situations. CD results clearly indicates the effect was more pronounced for β-lg treated with curcumin–Al(III) mixture than curcumin–Zn(II) mixture and bare curcumin. The calculation of secondary structural changes of β-lg in different systems was shown in Table 1.
| β-lg | % α-helix | % β-sheet | % β-turn | % random coil |
|---|---|---|---|---|
| Native | 16.844 | 25.798 | 17.908 | 39.539 |
| Heated-β-lg | 14.007 | 27.844 | 17.827 | 40.323 |
| Curcumin–β-lg | 13.694 | 27.98 | 17.751 | 40.406 |
| Al–β-lg | 12.67 | 28.85 | 17.92 | 40.5 |
| Al–curcumin–β-lg | 15.065 | 27.1 | 18.009 | 39.74 |
| Zn–β-lg | 13.361 | 28.235 | 17.899 | 40.504 |
| Zn–curcumin–β-lg | 14.85 | 27.315 | 17.78 | 39.969 |
3.9. Morphological studies with TEM images
The morphological characteristics of the aggregates of β-lg under different conditions were analyzed by TEM. Prior to taking the TEM images, β-lg (10 μM) was heated in the absence and presence of Al(III), Zn(II), curcumin, Al(III)–curcumin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Zn(II)–curcumin (1
1) and Zn(II)–curcumin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures at 70 °C for 2 h at neutral pH (Fig. 6). In Fig. 6A, the control sample (β-lg alone heated) shows a characteristic fibrillation process of β-lg which is in agreement with the results obtained in previous studies. Completely amyloid fibrillar morphology was revealed when β-lg was heated in the presence of metal ions Al(III) (Fig. 6B) whereas heating with Zn(II) yielded β-lg aggregates with lesser fibrillar network (Fig. 6C). TEM images thus showed that Al(III) accelerated more fibrillation than Zn(II) under identical conditions. Intriguingly, in the presence of curcumin, β-lg formed fewer and sheet like aggregates as shown in Fig. 6D. Thus curcumin retarded the fibrillation of β-lg. Interestingly, the Al(III)–curcumin and Zn(II)–curcumin (1
1) mixtures at 70 °C for 2 h at neutral pH (Fig. 6). In Fig. 6A, the control sample (β-lg alone heated) shows a characteristic fibrillation process of β-lg which is in agreement with the results obtained in previous studies. Completely amyloid fibrillar morphology was revealed when β-lg was heated in the presence of metal ions Al(III) (Fig. 6B) whereas heating with Zn(II) yielded β-lg aggregates with lesser fibrillar network (Fig. 6C). TEM images thus showed that Al(III) accelerated more fibrillation than Zn(II) under identical conditions. Intriguingly, in the presence of curcumin, β-lg formed fewer and sheet like aggregates as shown in Fig. 6D. Thus curcumin retarded the fibrillation of β-lg. Interestingly, the Al(III)–curcumin and Zn(II)–curcumin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture substantially inhibited the fibrillation of β-lg (Fig. 6E and F). The TEM pictures of these two systems reveal the formation of only a few amorphous aggregates (Fig. 6F) instead of fibrillar aggregates. These findings clearly demonstrate that Al(III)–curcumin and Zn(II)–curcumin shows a strong inhibitory effect on the metal ions induced thermal aggregation of β-lg compared to curcumin itself.
1) mixture substantially inhibited the fibrillation of β-lg (Fig. 6E and F). The TEM pictures of these two systems reveal the formation of only a few amorphous aggregates (Fig. 6F) instead of fibrillar aggregates. These findings clearly demonstrate that Al(III)–curcumin and Zn(II)–curcumin shows a strong inhibitory effect on the metal ions induced thermal aggregation of β-lg compared to curcumin itself.
4 Conclusions
The present studies provide the direct evidence of the anti fibrillogenic property of curcumin on Al(III) and Zn(II) catalysed aggregation of β-lg at pH 7.0 when thermally exposed at 70 °C. The Al(III)–curcumin and Zn(II)–curcumin mixtures have similar binding affinity to the monomeric β-lg like curcumin. However, the Al(III) and Zn(II) mixtures provide more improved inhibition to β-lg fibrillar aggregate formation. From the present study we may conclude metal ion–curcumin mixtures have greater inhibitory effect than curcumin on β-lg thermal aggregation and Al–curcumin mixture has highest suppressing activity. These results may have implications in understanding the molecular mechanism of action of antioxidant-metal mixtures in protein misfolding diseases.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
Financial support of University Grant Commission (UGC)-CAS-II and DST-PURSE-II Program of Jadavpur University, Kolkata are greatly acknowledged. Sampa Pal, SRF, is a recipient of UGC-BSR Research Fellowship in Science and for the Meritorious Student. The authors wish to acknowledge Prof S. C. Bhattacharya, Department of Chemistry, Jadavpur University for providing the DLS instrumental facility. The authors wish to acknowledge Prof. P. S. Dastidar of Organic Chemistry Division Indian Association for Cultivation of Science (IACS) and Prof Dipankar Chakraborty, Department of Applied Chemistry, Kolkata University for providing the TEM instrumental facility. Authors also wish to acknowledge Dr. Dipankar Mandal, Department of Physics, Jadavpur University, for providing the FTIR instrumental facility.References
- C. J. Pike, A. J. Walencewicz, C. G. Glabe and C. W. Cotman, Brain Res., 1991, 563, 311–314 CrossRef CAS PubMed.
- K. Ono, R. Takahashi, T. Ikeda, M. Mizuguchi, T. Hamaguchi and M. Yamada, Biochim. Biophys. Acta, Mol. Basis Dis., 2014, 1842, 646–653 CrossRef CAS PubMed.
- L. Huang, X. Liu, B. Cheng and K. Huang, Arch. Biochem. Biophys., 2015, 568, 46–55, DOI:10.1016/j.abb.2015.01.007.
- Y. R. Chen, C. L. Ni, H. P. Shi, H. M. Yu and Y. C. Chang, FASEB J., 2011, 25, 1390–1401 CrossRef PubMed.
- C. Haass and D. J. Selkoe, Nat. Rev. Mol. Cell Biol., 2007, 8, 101–112 CrossRef CAS PubMed.
- S. Pal, S. Maity, S. Sardar, J. Chakraborty and U. C. Halder, Int. J. Biol. Macromol., 2016, 84, 121 CrossRef CAS PubMed.
- S. Maity, S. Sardar, S. Pal, H. Parvej, J. Chakraborty and U. C. Halder, RSC Adv., 2016, 6, 74409–74417 RSC.
- J. D. Schmit, K. Ghosh and K. Dill, Biophys. J., 2011, 100, 450–458 CrossRef CAS PubMed.
- S. S. Leal, H. M. Botelho and C. M. Gomes, Coord. Chem. Rev., 2012, 256, 2253–2270 CrossRef CAS.
- G. Navarra, A. Tinti, M. Di Foggia, M. Leone, V. Militello and A. Torreggiani, J. Inorg. Biochem., 2014, 137, 64–73 CrossRef CAS PubMed.
- A. Stirpe, B. Rizzuti, M. Pantusa, R. Bartucci, L. Sportelli and R. Guzzi, Eur. Biophys. J., 2008, 37, 1351–1360 CrossRef CAS PubMed.
- P. W. Mantyh, J. R. Ghilardi, S. Rogers, E. De Master, C. J. Allen and E. R. Stimson, J. Neurochem., 1993, 61, 1171–1174 CrossRef CAS PubMed.
- A. I. Bush, W. H. Pettingell, G. Multhaup, M. Paradis, J. P. Vonsattel and J. F. Gusella, Science, 1994, 265, 1464–1467 CAS.
- H. V. Iyer and T. M. Przybycien, J. Colloid Interface Sci., 1996, 177, 391 CrossRef CAS.
- C. M. Bryant and D. J. McClements, Trends Food Sci. Technol., 1998, 9, 143 CrossRef CAS.
- W. L. Chen, M. T. Hwang, C. Y. Liau, J. C. Ho, K. C. Hong and S. J. Mao, J. Dairy Sci., 2005, 88, 1618 CrossRef CAS PubMed.
- S. Brownlow, J. H. M. Cabral, R. Cooper, D. R. Flwoer, S. J. Yewdall, A. C. T. North and L. Sawyer, Structure, 1997, 5, 481 CrossRef CAS PubMed.
- P. F. Fox, M. Sweeney and L. H. Paul, Advanced Dairy Chemistry-1. Proteins, Kluwer Academic/Plenum Publishers, New York, 3rd edn, 2003 Search PubMed.
- T. V. Burova, Y. Choiset, V. Tran and T. Haertlé, Protein Eng., 1998, 11, 1065 CrossRef CAS PubMed.
- G. Navarra, D. Giacomazza, M. Leone, F. Librizzi, V. Militello and P. L. San Biagio, Eur. Biophys. J., 2009, 38, 437 CrossRef CAS PubMed.
- G. Navarra, M. Leone and V. Militello, Biophys. Chem., 2007, 131, 52 CrossRef CAS PubMed.
- R. Carrotta, L. Arleth, J. S. Pedersen and R. Bauer, Biopolymers, 2003, 70, 377 CrossRef CAS PubMed.
- S. M. Fitzsimons, D. M. Mulvihill and E. R. Morris, Food Hydrocolloids, 2007, 21, 638 CrossRef CAS.
- L. N. Arnaudov, R. de Vries, H. Ippel and C. P. M. van Mierlo, Biomacromolecules, 2003, 4, 1614 CrossRef CAS PubMed.
- P. Relkin, Int. J. Biol. Macromol., 1998, 22, 59 CrossRef CAS PubMed.
- R. Guzzi, B. Rizzuti, C. Labate, B. Zappone and M. P. De Santo, Biomacromolecules, 2015, 16, 1794 CrossRef CAS PubMed.
- J. E. Galvin, N. Pandey, J. Strider, W. C. Nolan and S. X. Yan, Acta Neuropathol., 2008, 115, 479 CrossRef PubMed.
- R. A. Sharma, A. J. Gescher and W. P. Steward, Eur. J. Cancer, 2005, 41, 1955 CrossRef CAS PubMed.
- S. Mishra and K. Palanivelu, Ann. Indian Acad. Neurol., 2008, 11, 13 CrossRef PubMed.
- S. S. Wang, K.-N. Liu and W.-H. Lee, Biophys. Chem., 2009, 144, 78 CrossRef CAS PubMed.
- J. Chakraborty, N. Das, K. P. Das and U. C. Halder, Int. Dairy J., 2009, 19, 43 CrossRef CAS.
- T. Jiang, L. Wang, S. Zhang, P.-C. Sun, C.-F. Ding, Y.-Q. Chu and P. Zhou, J. Mol. Struct., 2011, 1004, 163 CrossRef CAS.
- X.-Z. Zhao, T. Jiang, L. Wang, H. Yang, S. Zhang and P. Zhou, J. Mol. Struct., 2010, 984, 316 CrossRef CAS.
- W. W. Yu, E. Chang, J. C. Falkner, J. Zhang, A. M. Sl-Somali, C. M. Sayes, J. Johns, R. Drezek and V. L. Colvin, J. Am. Chem. Soc., 2007, 129, 2871 CrossRef CAS PubMed.
- V. Bannerjee and K. P. Das, Colloids Surf., B, 2012, 92, 142 CrossRef PubMed.
- M. R. Nilsson, Methods, 2004, 34, 151 CrossRef CAS PubMed.
- J. M. Khan, S. K. Chaturvedi, S. K. Rahman, M. Ishtikhar, A. Qadeer, E. Ahmad and R. H. Khan, Soft Matter, 2014, 10, 2591 RSC.
- G. V. Semisotnov, N. A. Radionava, O. I. Razgulyaev, V. N. Uversky, A. F. Gripas and R. I. Gilmanshin, Biopolymers, 1991, 31, 119 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, Singapore, 3rd edn, 2006, vol. I, p. 230 Search PubMed.
- J. M. Khan, S. K. Chaturvedi, S. K. Rahman, M. Ishtikhar, A. Qadeer, E. Ahmad and R. H. Khan, Soft Matter, 2014, 10, 2591 RSC.
- S. A. Hudson, H. Ecroyd, T. W. Kee and J. A. Carver, FEBS J., 2009, 276, 5960 CrossRef CAS PubMed.
- P. Busti, C. A. Gatti and N. J. Delorenzi, Int. J. Biol. Macromol., 1998, 23, 143–148 CrossRef CAS PubMed.
- A. Lomakin, G. B. Benedek and D. B. Teplow, Methods Enzymol., 1999, 309, 429–459 CAS.
- S. Sardar, S. Pal, S. Maity, J. Chakraborty and U. C. Halder, Int. J. Biol. Macromol., 2014, 69, 137 CrossRef CAS PubMed.
- J. M. Khan, A. Qadeer, S. K. Chaturvedi, E. Ahmad, S. A. Abdul Rehman, S. Gourinath and R. H. Khan, PLoS One, 2012, 7, e29694 CAS.
- A. Naeem, K. A. Khan and R. H. Khan, Arch. Biochem. Biophys., 2004, 432, 79 CrossRef CAS PubMed.
- D. Matulis, C. Wu, T. Van Pham, C. Guy and R. Lovrien, J. Mol. Catal. B: Enzym., 1999, 7, 21–36 CrossRef CAS.
- M. Collini, L. D'Alfanso, H. Molinari, L. Ragona, M. Catalano and G. Baldini, Protein Sci., 2003, 12, 1596 CrossRef CAS PubMed.
- C. Bahttacharjee, S. Saha, A. Biswas, M. Kundu, L. Ghosh and K. P. Das, Protein J., 2005, 24, 27 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24570f |
| This journal is © The Royal Society of Chemistry 2016 |