Biocompatible titanate nanotubes with high loading capacity of genistein: cytotoxicity study and anti-migratory effect on U87-MG cancer cell lines
Abstract
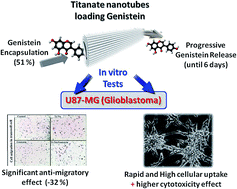
Titanate nanotubes (Ti-Nts) have proved to be a potential candidate for drug delivery due to their large surface change and higher cellular uptake as a direct consequence of their tubular shape. Ti-Nts were assessed for their safety, their kinetics of cellular uptake on U87-MG cell line and for genistein loading efficiency. No cytotoxic effect was observed under higher empty Ti-Nts concentrations up to 100 μg mL−1. The multiwalled tubular morphology was found to be an important parameter promoting high drug loading. The Ti-Nts could achieve higher genistein drug-loading content (25.2%) and entrapment efficiency (51.2%) leading to a controlled drug release as well as a higher cellular uptake of genistein-loaded-Ti-Nts which induces higher cytotoxicity and significant anti-migratory effect on U87-MG human glioblastoma astrocytoma, promising efficient antitumor activity.


 Please wait while we load your content...
Please wait while we load your content...