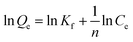Magnetic Ni/PPy nanocomposite as effective reusable adsorbent for removal of arsenite and fluoride from contaminated water†
Suneel K. Srivastava*a,
Samarpita Senapatia,
Shiv B. Singhb and
Prasanta K. Raulc
aDepartment of Chemistry, Indian Institute of Technology, Kharagpur-721302, India. E-mail: sunil111954@yahoo.co.uk
bDepartment of Metallurgical and Materials Engineering, Indian Institute of Technology, Kharagpur-721302, India
cDepartment of Chemistry, Defence Research Laboratory, Tezpur 784001, India
First published on 25th November 2016
Abstract
Magnetic nickel/polypyrrole (Ni/PPy) nanostructures have been synthesized at room temperature via an in situ oxidative polymerization of pyrrole (Py) monomers in the presence of FeCl3 oxidant in an aqueous suspension of Ni nanoflowers and characterized by XRD, FESEM, TEM, FTIR and Raman methods. Room temperature magnetic measurement shows that the magnetic properties of Ni are retained in all the nanocomposites. Ni/PPy nanostructures exhibit excellent adsorption efficiency in the removal of arsenic and fluoride from contaminated water as well as in the real ground water of Assam, India. Interestingly, Ni can be magnetically separated below its WHO prescribed toxicity value. The equilibrium data have been tested based on the Langmuir and Freundlich isotherm models. It is also found that the nanocomposite after adsorption could be regenerated by treating with acid/alkali and easily separated from the reaction mixture by the application of a magnetic field.
1. Introduction
Arsenic is one of the most toxic components in the environment due to its carcinogenic effect even at a very low (>10 μg L−1 or ppb) exposure levels (according to WHO).1 Arsenic contamination has emerged as a major public health problem in South East Asia, particularly in Bangladesh, all states in the Ganga flood plains in India, and many parts of China.1,2 Though, it may be originated from various sources, water remains one of the major sources of arsenic poisoning for the human body.3 Arsenic exists in ground water predominantly as arsenite, As(III) and arsenate, As(V).4 It is well established that arsenite is about ten times more toxic to animals and plants than arsenate due to its greater ability to form complex with certain co-enzyme.4 Fluoride constitutes another important toxic constituent leading to fluorosis in children as well as adults, when its concentration is more than 4–10 mg L−1. Fluoride is generally released into the groundwater mainly by slow dissolution of fluorine-containing rocks.5 In addition, fluoride is a persistent and non-degradable poison accumulating in the soil, plants, wild life and in humans. Therefore, it is essential to remove As(III) and F− from water to optimum level for healthy and happy life. The ability to remove toxic contaminants from aquatic environments rapidly, efficiently, and economically is an important technological challenge.6Among various methods used to remove As(III) and F− from water, adsorption process seems to be a more attractive method in terms of cost, simplicity of design and operation and high efficiency as well as low secondary pollution.6–12 In this regard, many adsorbents have been employed for the removal of As(III) and F− from water.13,14 Recently, nanomaterials have been proved to be an effective adsorbent over the traditional ones due to their larger surface area, accessible active sites and a short diffusion length.11–14
It is well known that the metallic (Fe, Co, Ni) nanoparticles exhibit larger magnetic moments compared to their oxide counterparts.15 Therefore, it is expected that these magnetic nanoparticles could lead to much better separation efficiency. Though, a very limited amount of work has been reported focusing on these elemental nanoparticles or their nanocomposites for the removal of As(III),16,17 the contempory work on F− removal is not yet reported. Recently, the removal of heavy metals have been reported by using Fe nanoparticle decorated graphene,18 MWCNT coated Fe nanoparticle,19 core/shell Fe@SiO2,20 montmorillonite supported Fe21 and Ni@C nanostructures.10
Polypyrrole (PPy) is one of the most widely investigated conducting polymers due to its low cost, good environmental stability, non-toxicity, high electrical conductivity and its ease of preparation.13 It is often used as an effective adsorbent for the cations and anions depending on the polymerisation condition and co ions present etc. PPy prepared by different methods carries charges via some of the nitrogen atoms in the polymer matrix.13 These positively charged nitrogen atoms in PPy provide a good prospect for its application as an adsorbent.22 However, the strong π* interactions between the Py main chain aggregate PPy into irregular morphology and decrease its surface area, and consequently the adsorption capacity.23 However, this drawback can be overcome by the formation of nanocomposites comprising PPy and inorganic materials, the separation of PPy nanocomposite from water medium remains another major issue in terms of its recyclability and cost effectiveness. From this perceptive, the magnetic separation technique has greater advantages because of its effective control,24 reusability, high speed, accuracy and simplicity as compared to other conventional tedious separation methods.25 Fe3O4/PPy magnetic nanocomposites have recently been reported as effective adsorbent in the removal of chromium(VI)26 and F−.13 Interestingly, no work has been reported till date on Fe, Co or Ni magnetic nanoparticles coated with PPy in harnessing for the removal of As(III) and F−.
These motivated us to focus our work on the synthesis of nickel based polypyrrole (Ni/PPy) magnetic nanostructures for the removal of As(III) and F− present in water. The choice of Ni/PPy nanocomposite in the present work is mainly guided by its economical feasibility, which involves an easy way for sorbent preparation and inexpensive chemical reagents. In our case, the main role of the magnetic kernel in the nanocomposites is only for simple separation. The preparation of Ni/PPy magnetic nanostructures involved the oxidative polymerization of pyrrole monomer on Ni nanoflowers in the presence of FeCl3 acting as oxidant. Subsequently, these Ni/PPy nanocomposites have been characterized by XRD, FTIR and TEM. Finally, these have successfully been used in the removal of arsenite and fluoride from contaminated water as well as in real ground water of Assam, India. We also made experiments to ascertain leaching of Ni, if any, from Ni/PPy nanocomposite in waste after its magnetic separation.
2. Experimental section
2.1. Materials
Nickel chloride hexahydrate (NiCl2·6H2O) and triethanolamine were purchased from E. Merck. Ferric chloride anhydrous (FeCl3) was obtained from Ranbaxy Laboratories Limited. Hydrazine hydrate and sodium hydroxide (NaOH) were purchased from Rankem Mumbai. Acetone and silver nitrate were obtained from Merck Specialties Private Limited, Mumbai, India. Ethanol and pyrrole were procured from Hong Yang Chemical Corporation, China and SRL India respectively. All the reagents were of analytical grade and used without any further purification. Double distilled water was used in all the experiments, whenever required.2.2. Synthesis
Ni nanoflower has been prepared in presence of triethanolamine capping agent according to the method reported earlier.27 Subsequently, Ni/PPy nanocomposites were synthesized at room temperature via an in situ polymerization of pyrrole monomer in the presence of FeCl3. In this procedure, 100 μL pyrrole monomer was added to Ni nanoflower already dispersed in water and was further subjected to ultrasonication for 30 min. Subsequently, aqueous solution of FeCl3 (1.5 g FeCl3) was added drop by drop to the above mixture followed by continuous stirring for 5 h at room temperature. The final product was washed with water and ethanol several times and dried in vacuum at 60 °C. This procedure was adopted to prepare Ni/PPy with varying proportion of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py in w/w (1
Py in w/w (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
2.3. Analysis of arsenic and fluoride
The mother liquor was analyzed for residual arsenite concentration with an atomic absorption spectrometer consisting of a hollow cathode lamp (193.7 nm), slit: 0.5 nm and argon gas as carrier using hydride generation method. Before analysis of samples, the instrument was calibrated with 5 ppb, 10 ppb, 20 ppb and 30 ppb As(III) solutions. On the other hand the concentration of the corresponding residual fluoride was determined by ion selective electrode.11,132.4. Adsorption study
According to Singh et al.,28 the maximum concentration of As(III) is found to be 1000 μg L−1 in the North Eastern region of India. Contemporary fluoride levels in some parts of India is as high as 30 mg L−1.29 Therefore, we have carried out the adsorption studies using Ni/PPy adsorbent by taking the initial concentration of As(III) and fluoride corresponding to respective 100–1500 μg L−1 and 5–40 mg L−1, which can be prepared as follows:Initially, the stock solution of arsenite and fluoride were prepared by dissolving 1.32 g of arsenic(III) oxide in presence of requisite amount of NaOH and 221.0 mg of sodium fluoride in 1000 and 100 mL of double distilled water respectively. Subsequently, the working As(III) and F− solutions of appropriate strengths were freshly prepared from the respective stock solutions. Further, in order to study the adsorption of As(III), 100 mL of working As(III) solution was taken in a conical flask containing the desired amount of Ni/PPy nanocomposite adsorbent. Thereafter, it was subjected to mechanical shaking (175 rpm) for 3 hours at room temperature (25 °C). Finally, the solid components in the solution were magnetically separated and the supernatant left out was analyzed for residual As(III) by atomic absorption spectrophotometer. A similar procedure was adopted for the adsorption of F− by Ni/PPy and the concentration of the corresponding residual fluoride was determined by ion selective electrode.11,13 In addition, batch adsorption experiments were also carried out in order to investigate the effect of various parameters, like adsorbent dose, initial concentration, presence of interfering ions, pH etc.
The specific amount of arsenite or fluoride adsorbed was calculated according to the following equation:
| Qe = (C0 − Ce) × V/W, | (1) |
The performance of all 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ni/PPy (w/w) nanocomposites as adsorbents was investigated in this manner. For this purpose, 1 g L−1 of adsorbent was incubated in presence of known initial concentration of arsenite (200 μg L−1) and fluoride (10 mg L−1) in six separate flasks. Thereafter, the efficiency of Ni/PPy nanocomposite of varying concentrations was evaluated to assess the extent of As(III) or F− removal from aqueous solution.
1 Ni/PPy (w/w) nanocomposites as adsorbents was investigated in this manner. For this purpose, 1 g L−1 of adsorbent was incubated in presence of known initial concentration of arsenite (200 μg L−1) and fluoride (10 mg L−1) in six separate flasks. Thereafter, the efficiency of Ni/PPy nanocomposite of varying concentrations was evaluated to assess the extent of As(III) or F− removal from aqueous solution.
2.5. Desorption study
The reusability of the used Ni/PPy nanostructure has also been examined for As(III) as well as F− after performing the respective desorption study. For this purpose, initially a certain amount of As(III) and F− solution was separately subjected to undergo adsorption on 1 g L−1 of Ni/PPy. Subsequently, the medium was analyzed after separating the solid adsorbent by the application of a magnetic field. The recovered solid Ni/PPy adsorbent left in this process was treated with dilute acid/alkali and reused for adsorption study of the contaminants. This process was performed repeatedly to confirm the extent of reusability of Ni/PPy at room temperature (25 °C).2.6. Characterizations
Detailed characterization techniques are provided in ESI.†3. Result and discussion
Fig. 1 shows the X-ray diffraction patterns of Ni and Ni/PPy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites. The appearance of the peaks at 52.7°, 61.17°, 91.96° and 114.8° in the diffractograms can be indexed as (111), (200), (220) and (311) planes of face centered cubic type Ni (JCPDS no. 040850). In case of the Ni/PPy nanocomposites (Fig. 1(b–d)), no additional peaks could be observed when compared with pure nickel. This is due to the amorphous nature of PPy.31 It is also seen that the intensity of Ni peaks are reduced with increasing content of PPy in Ni/PPy composites.
1) composites. The appearance of the peaks at 52.7°, 61.17°, 91.96° and 114.8° in the diffractograms can be indexed as (111), (200), (220) and (311) planes of face centered cubic type Ni (JCPDS no. 040850). In case of the Ni/PPy nanocomposites (Fig. 1(b–d)), no additional peaks could be observed when compared with pure nickel. This is due to the amorphous nature of PPy.31 It is also seen that the intensity of Ni peaks are reduced with increasing content of PPy in Ni/PPy composites.
 | ||
Fig. 1 X-ray diffraction pattern of (a) Ni and Ni/PPy nanocomposites prepared using mass ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py (b) 2 Py (b) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (d) 1 1 and (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. 2. | ||
Fig. 2 shows the FESEM image of Ni/PPy composites consisting of different proportion of Ni and pyrrole monomer. It is evident that the proportion of spherical particles increases with the increasing proportion of Py. When Ni/Py weight ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the flower like nickel is nearly disappeared by the aggregation of spherical particles. FESEM also established the deposition of nanosized spherical PPy particles (dia: 100–150 nm) on the surface of nickel nanoflower.
2, the flower like nickel is nearly disappeared by the aggregation of spherical particles. FESEM also established the deposition of nanosized spherical PPy particles (dia: 100–150 nm) on the surface of nickel nanoflower.
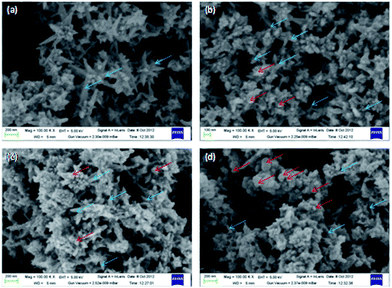 | ||
Fig. 2 FESEM image of (a) Ni nanoflower (b) Ni/PPy (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (c) Ni/PPy (1 1) (c) Ni/PPy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (d) Ni/PPy (1 1) and (d) Ni/PPy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) nanocomposites. 2) nanocomposites. | ||
FTIR (Fig. S1, ESI†) and Raman spectroscopy (Fig. S2, ESI†) further confirmed the presence of PPy in this hybrid nanostructure. Room temperature (25 °C) magnetization data in Fig. S3 and Table S1 (ESI†) show the ferromagnetic behaviour of Ni/PPy with gradually decreasing value of Ms with increasing content of PPy.
Fig. S4 (ESI†) represents the % removal of arsenite and fluoride by Ni/PPy containing varying ratio of Ni and PPy. These findings clearly show that the nanocomposite prepared using Ni and Py in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adsorbed the contaminants most efficiently due to the presence of higher concentration of PPy on the surface of Ni. Therefore, subsequent experiments have been carried out employing Ni/PPy prepared using Ni
2 adsorbed the contaminants most efficiently due to the presence of higher concentration of PPy on the surface of Ni. Therefore, subsequent experiments have been carried out employing Ni/PPy prepared using Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py as 1
Py as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 weight ratio. The adsorption ability of Ni/PPy nanocomposite has also been verified by nitrogen gas adsorption–desorption isotherms (Fig. S5, ESI†). The surface area of Ni/PPy nanocomposite (weight ratio of Ni
2 weight ratio. The adsorption ability of Ni/PPy nanocomposite has also been verified by nitrogen gas adsorption–desorption isotherms (Fig. S5, ESI†). The surface area of Ni/PPy nanocomposite (weight ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py = 1
Py = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) is found to be 47.304 m2 g−1 which suggests its higher adsorption capacity.
2) is found to be 47.304 m2 g−1 which suggests its higher adsorption capacity.
The effect of the adsorbent dosage on the removal of arsenite (200 μg L−1) and fluoride (10 mg L−1) has been studied by varying amount of adsorbent from 0.1 g L−1 to 4 g L−1 and the % removal of adsorbate with the variation of adsorbent dose are displayed in Fig. 3. These studies show that the % removal of As(III) increases from 61 to 99% corresponding to the adsorbent dose of 0.1 to 1 g L−1; no change in % removal of As(III) is observed with further increase in the dose of the adsorbent. Similarly, % removal of F− also increased from 35% to 85% corresponding to a dose of 0.1 to 2 g L−1 of Ni/PPy. The increasing adsorption at higher adsorbent dose could be ascribed to the presence of increased number of active sites available for adsorption.11,13 Accordingly, in all the subsequent experiments 1.0 g L−1 of adsorbent has been taken as the optimum dosage which gives reasonably good adsorption efficiency of As(III) and F−.
Fig. 4 shows the % removal of As(III)/F− as a function of contact time when a fixed amount of adsorbent was used. It is clearly evident that rapid adsorption takes place in the first 90 min of shaking for F− and As(III). Finally, the adsorption equilibrium is attained at 180 min, corresponding to the removal of As(III) and F− by 99 and 75% respectively. With an increase in the contact time up to 10 h, removal increased by less than 1%, which indicated that complete adsorption occurred within 3 hours. Thus, further adsorption experiments were conducted for 3 hours.
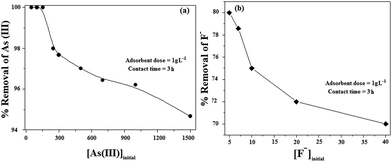
The effect of initial concentration of adsorbate on its removal is studied using 1 g L−1 of Ni/PPy nanocomposite. Fig. 5 clearly shows that the removal of As(III) is 100% upto the concentration of 200 μg L−1 and it is 98 and ∼95% at the concentrations of 500 and 1500 μg L−1 respectively. It is also noted that about 70–72% of F− is removed, when its concentration is 20–40 mg L−1. It is observed that % removal of As(III) and F− falls rapidly with increasing initial concentration of the contaminant. In all probability, the binding capacity of the adsorbent (Ni/PPy) becomes saturated at higher adsorbate concentrations and leads to the subsequent reduction in the % removal of As(III) and F−.32
The adsorption capacity of an adsorbent depends on its surface charge, which can be determined by the zero point charge (ZPC) technique, where the electrical charge density of a material surface is zero. ZPC can be calculated by plotting final pH versus initial pH of the solution, where the horizontal portion of the graph parallel to the x-axis refers to the ZPC value.33 Fig. 6 shows the plot of final pH vs. initial pH of As(III) and F− solution and the corresponding results show that the ZPC of Ni/PPy nanocomposites are 7.6 and 5.9 respectively. Such difference in the ZPC values is also in good agreement with earlier report on iron oxide and akaganéite.34,35
 | ||
| Fig. 6 Final pH versus initial pH for determination of ZPC of adsorbent in (a) As(III) and (b) F− solution. | ||
pH is one of the most important parameters influencing the removal of the contaminants. Therefore, the effect of pH (3–11) on the removal of As(III) and F− was investigated using constant adsorbent dose of 1 g L−1 at the agitation speed of 175 rpm and shaking time of 3 h; the corresponding findings are displayed in Fig. 7. It is observed that the removal of As(III) is only 70% at pH 3 and increases with increasing solution pH and shows maximum efficiency in the range of 5–9 (almost complete removal). Thereafter, the removal efficiency decreases with increasing solution pH and exhibit 96 and 90% adsorption at the pH of 9.5 and 11 respectively. The variation of As(III) removal with respect to pH of solution can be explained in terms of the speciation of arsenite species and adsorbent surface charge as follows.12,35
| H3AsO3 ↔ H2AsO3− + H+, pKa = 9.23 |
| H2AsO3− ↔ HAsO32− + H+, pKa = 12.1 |
| HAsO32− ↔ AsO33− + H+, pKa = 12.7 |
This suggests that arsenite is stable as neutral species (H3AsO3) at pH < 9, whereas, H2AsO3− stable species, when pH ranges from 9–12.35 It is anticipated that the adsorption of As(III) takes place effectively at lower pH (3–8) through specific adsorption, i.e., As(III) ions become specifically adsorbed on positively charged Ni/PPy surface sites due to short-range interactions between Ni/PPy and neutral species (H3AsO3). On the contrary, the electrostatic repulsion between the negatively charged arsenite (H2AsO3−) and negatively charged Ni/PPy surface becomes more prominent at pH > 9, which accounts for the observed reduction in the % removal of As(III).35
Fig. 7(b) shows the variation of fluoride uptake of Ni/PPy with respect to pH using the initial concentration of F− as 10 mg L−1. Interestingly, maximum 85% of F− is removed at pH 3 and subsequently it declined at higher pH exhibiting 45% removal of F− at pH 11. It may be noted that pH of the solution in our experiment has been adjusted by the addition of dilute HCl. As a result, the Ni/PPy nanocomposite is protonated forming chloride ion doped radical cation structure similar to that of polyaniline.7 According to Diarmid et al.,36 chloride ion is a fleeting dopant, which can easily be exchanged with small sized F− ions and accounts for the higher removal efficiency of F− by Ni/PPy in acidic solution. In addition, the zero point charge of the nanocomposite in acidic pH suggests that the positive surface charge of adsorbent in acidic pH attracts the F− ion electrostatically and facilitates its removal. However, in the alkaline pH range, the lower adsorption capacity of Ni/PPy is due to the competitive interaction between hydroxyl (−OH) ions and F− for the identical sorption sites available on the surface of Ni/PPy adsorbent.7,37
Many anions, such as chloride, sulfate, phosphate, iodide and iodate are usually present in water, which also compete with As(III)/F− during the adsorption. Therefore, it would be interesting to study their effect on the removal of As(III)/F− using Ni/PPy nanocomposite. Fig. 8(a) shows the effect of sulphate and phosphate anions on the removal of As(III) by Ni/PPy nanocomposite at a pH of 5.8 taking 200 μg L−1 of As(III) solution, while keeping other conditions unaltered. These findings indicate that As(III) removal decreases with the increasing concentration of these anions. Though, the removal of As(III) is 100% in absence of these ions, it is found to be 94 and 84% in 1.5 mM solution of sulfate and phosphate respectively. Such decrease in % removal of As(III) in presence of phosphate may be due to the fact that phosphate and arsenic have the similar chemical characteristics.37 Therefore, it is expected that they have similar adsorption tendency and compete for adsorption on the Ni/PPy surface.12
The effect of co-existing anions (chloride, sulfate, iodide and iodate) on fluoride adsorption has also been investigated by carrying out the adsorption studies using 10 mg L−1 of fluoride solution. These findings in Fig. 8(b) clearly show that the presence of Cl−/SO42− ions has a negligible effect on the removal of F−, though; it is slightly affected in presence of IO3−/I− ions.
The regeneration studies of exhausted Ni/PPy nanocomposites have been carried out at 25 °C using dilute HCl and dilute NaOH eluents. Mineral acids elute As(III) and F− from exhausted nanoparticles as arsenicouis acid (H3AsO3) and HF whereas NaOH elutes these as sodium arsenite (Na3AsO3) and NaF respectively.38 Fig. 9 displays the room temperature regeneration studies on exhausted Ni/PPy using 0.5 M each of HCl and NaOH eluents. The first cycle clearly shows 87 and 72% removal of adsorbed As(III) and F− in presence of HCl and NaOH respectively. The respective concentrations of As(III) and F− in the eluents are 135 μg L−1 (67.5%) and 55 mg L−1 (55%) after 5 cycles of the desorption. All these findings clearly demonstrated the excellent regeneration capability of the used Ni/PPy for further use. This decrease in regeneracy of the adsorbent after 5 cycles may be due to the loss of Ni/PPy through washing.25
 | ||
| Fig. 9 Effect of recycle times on regeneracy of adsorbent on the removal of (a) As(III) and (b) F− ([As(III)]initial = 200 μg L−1, [F−]initial = 10 mg L−1, adsorbent dose = 1 g L−1). | ||
As nickel is toxic (maximum limit: 70 μg L−1), we also carried out our experiments to identify the expulsion of Ni from Ni/PPy, if any, in water. For this purpose, the dispersion of Ni/PPy (100 mg L−1) was placed under magnetic field followed by the analysis of supernatant liquid for Ni at different time intervals (0, 1, 5, 10, 15, 20, 25, 30 min) by atomic absorption analysis (AAS). It is observed that the concentration of nickel in the dispersion of Ni/PPy is below the detection limit of AAS (1 μg L−1). These observations indicate that amount of Ni left after magnetic separation is much below the prescribed value of Ni specified by WHO.39 The stability of the composite has also been examined by carrying out the UV-vis study of the supernatant. The UV-vis study clearly shows the absence of characteristics peak corresponding to PPy. This result confirmed that the two components in the Ni/PPy are not disintegrated by dispersion.
The analysis of adsorption isotherm is very important to justify the interaction between adsorbate and adsorbent.40 Therefore, Langmuir and Freundlich adsorption isotherms have been applied to investigate the interaction of Ni/PPy nanocomposite with As(III)/F− during their removal at room temperature (25 °C).
Freundlich isotherm is used to describe adsorption which is not restricted to monolayer formations and takes place in heterogeneous surfaces.40,41 The Freundlich isotherm can be expressed as
| Qe = KfCe1/n |
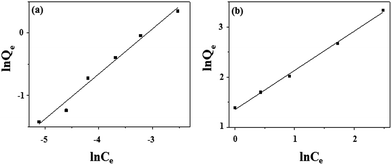
where Kf and n are the constants related to the extent of adsorption and degree of nonlinearity between solution concentration and adsorption respectively.7,8,40 Fig. 10 shows the corresponding isotherm, when ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe versus ln
Qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce values are plotted for the adsorption of As(III) and F− on Ni/PPy. A smaller value of 1/n (<1) in Table 1 suggested that the adsorption processes involve the formation of relatively stronger bond between the adsorbate and absorbent and are favourable.41
Ce values are plotted for the adsorption of As(III) and F− on Ni/PPy. A smaller value of 1/n (<1) in Table 1 suggested that the adsorption processes involve the formation of relatively stronger bond between the adsorbate and absorbent and are favourable.41
| Contaminant | Freundlich | Langmuir | ||
|---|---|---|---|---|
| n | r2 | Qm | r2 | |
| Arsenite | 1.3891 | 0.99228 | 2.6369 | 0.96852 |
| Fluoride | 1.2794 | 0.99646 | 67.7048 | 0.77382 |
Unlike Freundlich isotherm, Langmuir isotherm is based on the assumption that the structure of the adsorbent is homogeneous and all the sites are energetically equivalent.40,41 The isotherm can be described as follows:
where, Qe and Ce have the usual meaning, b is a constant related to the free energy of adsorption (L mg−1), and Qm is the maximum adsorption capacity (mg g−1). Fig. 11 represents the plot of Ce/Qe versus Ce for As(III) and F− adsorbed on Ni/PPy surface. The adsorption data obtained from these isotherms are recorded in Table 1. It clearly shows that the maximum adsorption capacity of As(III) and F− corresponds to 2.63 and 67.70 mg g−1 respectively. The value of correlation coefficients (r2) obtained from the linearized plots in both equations indicated better fitting of experimental equilibrium adsorption data with the Freundlich model and adsorption of As(III)/F− by Ni/PPy nanocomposite is not restricted to monolayer of the adsorbent surface.37 The comparisons have been made between our prepared Ni/PPy and previously reported other adsorbents for the removal of arsenic (Table 2) and fluoride (Table 3).
| Type of adsorbent | Maximum amount adsorbed (mg g−1) | Reference |
|---|---|---|
| Magnetic iron oxide/CNT composites | 8.13 mg | 9 |
| Fe3O4 wheat saw | 3.9 | 42 |
| Fe3O4 nanomaterials | 8.2 | 43 |
| Fe2O3 nanomaterial | 1.25 | 43 |
| Cellulose@iron oxide nanoparticles | 23.16 | 12 |
| Cellulose@nanoscale zerovalent iron | 20.1 | 43 |
| Fe | 1.50 | 44 |
| Iron hydroxide coated alumina | 7.65 | 43 |
| Ni/PPy | 2.64 | Our case |
| Type of adsorbent | Maximum amount adsorbed (mg g−1) | Reference |
|---|---|---|
| Mg–Al-LDH nanoflake impregnated magnetic alginate beads | 32.4 | 45 |
| Activated alumina | 16.34 | 46 |
| Hydrous-manganese oxide-coated alumina | 7.09 | 47 |
| Acid–base treated raw laterite | 11.8 | 48 |
| Synthetic nano-hydroxyapatite | 4.575 | 49 |
| Synthetic hydroxyapatite | 0.295–0.489 | 49 |
| Nano-AlOOH | 3.259 | 50 |
| CaO modified activated alumina | 101.01 | 51 |
| MnO2 modified activated alumina | 10.18 | 51 |
| Alumina of alkoxide nature | 2.0 | 52 |
| Li2Al–CLDH | 128 | 53 |
| Li–Al layered double hydroxides | 46.53 | 54 |
| Nano-alumina | 14.0 | 55 |
| Al2O3/CNTs | 28.7 | 55 |
| Ni/PPy | 67.70 | Our case |
Considering the practical application of Ni/PPy nanostructures, its adsorption capacity has also been tested for As(III) and F− removal in contaminated groundwater from Nagaon and Karbi angling, Assam, India respectively. The chemical analyses of groundwater of these two places are displayed under ESI as Tables S2 and S3.† These tables show that the ground water contains 195 ppb of arsenic and 7 ppm of fluoride which are much higher than the permissible limit of WHO. However, using Ni/PPy in the dose of 1 g L−1 the concentration of arsenic can be reduced to 8 μg L−1 and that of fluoride became 1.5 mg L−1 without adjusting the pH of this water. These reduced concentrations are within the WHO specified permissible limit for arsenic and fluoride. Therefore, the real groundwater trial demonstrated that our prepared Ni/PPy can efficiently be used for the treatment of arsenic and fluoride contaminated groundwater.
4. Conclusion
A simple method has been used to successfully prepare Ni/PPy nanocomposites at room temperature. XRD, TEM, FTIR, Raman studies confirmed the deposition of spherical PPy nanoparticles on the surface of Ni nanoflowers. The measurement of room temperature (25 °C) magnetization data confirmed that the magnetic property of Ni is retained in its nanocomposites. Subsequently, these nanocomposites have been used as an adsorbent in the removal of As(III) and F− from the contaminated water and the corresponding results show that Ni/PPy prepared using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ni
2 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py acts as the most efficient adsorbent for As(III) and F−. The effect of adsorbent dose, initial concentration of As(III) and F−, pH and presence of co-ions have been investigated. The plots in the Langmuir isotherm indicate that the maximum adsorption capacity of Ni/PPy (1
Py acts as the most efficient adsorbent for As(III) and F−. The effect of adsorbent dose, initial concentration of As(III) and F−, pH and presence of co-ions have been investigated. The plots in the Langmuir isotherm indicate that the maximum adsorption capacity of Ni/PPy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for As(III) and F− corresponds to 2.64 and 67.71 mg g−1 respectively. These Ni/PPy adsorbents can easily be separated due to their magnetic property and reused after eluting with HCl/NaOH. The regeneration of the adsorbent is found to be 67.5% in As(III) using HCl eluent and 55% for F− in presence of NaOH eluent even after 5 cycles. Thus synthesized Ni/PPy nanocomposite also exhibit excellent efficiency for the removal of As(III) and F− present in real ground water. The comparison of our work with other existing adsorbents showed that Ni/PPy is more efficient in the removal of arsenic and fluoride.
2) for As(III) and F− corresponds to 2.64 and 67.71 mg g−1 respectively. These Ni/PPy adsorbents can easily be separated due to their magnetic property and reused after eluting with HCl/NaOH. The regeneration of the adsorbent is found to be 67.5% in As(III) using HCl eluent and 55% for F− in presence of NaOH eluent even after 5 cycles. Thus synthesized Ni/PPy nanocomposite also exhibit excellent efficiency for the removal of As(III) and F− present in real ground water. The comparison of our work with other existing adsorbents showed that Ni/PPy is more efficient in the removal of arsenic and fluoride.
Acknowledgements
The authors are thankful to Professor A. R. Kulkarni, Department of Metallurgical Engineering and Material Sciences, IIT Mumbai, India for Raman spectra of the samples. Experimental facilities and financial assistance from Department of Chemistry/Central Research Facility of IIT Kharagpur and CSIR (09/081(1007)/2009-EMR-I dt. 13.11.2009)/DRDO are greatly acknowledged.References
- M. A. Hossain, M. M. Rahman, M. Murrill, B. Das, B. Roy, S. Dey, D. Maity and D. Chakraborti, Sci. Total Environ., 2013, 463–464, 1217–1224 CrossRef CAS PubMed
.
- A. Pal, U. K. Chowdhury, D. Mondol, B. Das, B. Nayak, A. Ghosh, S. Maity and D. Chakraborti, Environ. Sci. Technol., 2009, 43, 3349–3355 CrossRef CAS PubMed
.
- H.-T. Fan, X. Fan, J. Li, M. Guo, D. Zhang, F. Yan and T. Sun, Ind. Eng. Chem. Res., 2012, 51, 5216–5223 CrossRef CAS
.
- R. Ansari, J. Feizy and A. F. Delavar, E-J. Chem., 2008, 5, 853–863 CrossRef CAS
.
- D. Banks, C. Reimann, O. Røyset, H. Skarphagen and O. M. Sæther, Appl. Geochem., 1995, 10, 1–16 CrossRef CAS
.
- Z. Liu, H. Wang, C. Liu, Y. Jiang, G. Yu, X. Mu and X. Wang, Chem. Commun., 2012, 48, 7350–7352 RSC
.
- M. Karthikeyan, K. K. Satheeshkumar and K. P. Elango, J. Hazard. Mater., 2009, 167, 300–305 CrossRef CAS PubMed
.
- M. Karthikeyan, K. K. Satheeshkumar and K. P. Elango, J. Hazard. Mater., 2009, 163, 1026–1032 CrossRef CAS PubMed
.
- J. Ma, Z. Zhu, B. Chen, M. Yang, H. Zhou, C. Li, F. Yu and J. Chen, One-pot, J. Mater. Chem. A, 2013, 1, 4662–4666 CAS
.
- Y. Ni, L. Jin, L. Zhang and J. Hong, J. Mater. Chem., 2010, 20, 6430–6436 RSC
.
- P. K. Raul, R. R. Devi, I. M. Umlong, S. Banerjee, L. Singh and M. Purkait, J. Nanosci. Nanotechnol., 2012, 12, 3922–3930 CrossRef CAS PubMed
.
- X. Yu, S. Tong, M. Ge, J. Zuo, C. Cao and W. Song, J. Mater. Chem. A, 2013, 1, 959–965 CAS
.
- M. Bhaumik, T. Y. Leswifi, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 186, 150–159 CrossRef CAS PubMed
.
- Z. Zhao, J. Liu, F. Cui, H. Feng and L. Zhang, J. Mater. Chem., 2012, 22, 9052–9057 RSC
.
- A.-H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed
.
- S. R. Kunel, B. Manning, L. Charlet and H. Choi, Environ. Sci. Technol., 2005, 39, 1291–1298 CrossRef
.
- C. Su and R. Puls, Environ. Sci. Technol., 2001, 35, 4562–4568 CrossRef CAS PubMed
.
- H. Jabeen, V. Chandra, S. Jung, J. W. Lee, K. S. Kim and S. B. Kim, Nanoscale, 2011, 3, 3583–3585 RSC
.
- H. Wang, N. Yan, Y. Li, X. Zhou, J. Chen, B. Yu, M. Gong and Q. Chen, J. Mater. Chem., 2012, 22, 9230–9236 RSC
.
- Y. Li, Z. Jin, T. Li and Z. Xiu, Sci. Total Environ., 2012, 421–422, 260–266 CrossRef CAS PubMed
.
- P. Wu, S. Li, L. Ju, N. Zhu, J. Wu, P. Li and Z. Dang, J. Hazard. Mater., 2012, 219–220, 283–288 CrossRef CAS PubMed
.
- X. Zhang, R. Bai and Y. W. Tong, Sep. Purif. Technol., 2006, 52, 161–169 CrossRef CAS
.
- J. Mansouri and R. P. Burford, J. Membr. Sci., 1994, 87, 23–34 CrossRef CAS
.
- S. R. Rudge, T. L. Kurt, C. R. Vessely, L. G. Catterall and D. L. Williamson, Biomaterials, 2000, 21, 1411–1420 CrossRef CAS PubMed
.
- S. Senapati, S. K. Srivastava and S. B. Singh, Nanoscale, 2012, 4, 6604–6612 RSC
.
- Y. Wang, B. Zou, T. Gao, X. Wu, S. Lou and S. Zhou, J. Mater. Chem., 2012, 22, 9034–9040 RSC
.
- S. Senapati, S. K. Srivastava, S. B. Singh and K. Biswas, Cryst. Growth Des., 2010, 10, 4069–4075 Search PubMed
.
- A. K. Singh, Arsenic Contamination in Groundwater of North Eastern India, in Proceedings of National seminar on Hydrology with focal theme on “WaterQuality” held at National Institute of Hydrology, Roorkee, November 22–23 2004 Search PubMed
.
- S. V. Ramanaiah, S. Venkatamohan, B. Rajkumar and P. N. Sarma, J. Environ. Sci. Eng., 2006, 48, 129–134 CAS
.
- I. Abe, S. Iwasaki, T. Tokimoto, N. Kawasaki, T. Nakamura and S. Tanada, J. Colloid Interface Sci., 2004, 275, 35–39 CrossRef CAS PubMed
.
- P. Xu, X. Han, C. Wang, D. Zhou, Z. Lv, A. Wen, X. Wang and B. Zhang, J. Phys. Chem. B, 2008, 112, 10443–10448 CrossRef CAS PubMed
.
- G. Z. Memon, M. I. Bhanger and M. Akhtar, Pak. J. Anal. Environ. Chem., 2009, 10, 14–18 CAS
.
- L. Wang and J. Li, BioResources, 2013, 8, 2521–2536 Search PubMed
.
- E. A. Deliyanni, D. N. Bakoyannakis, A. I. Zouboulis and K. A. Matis, Chemosphere, 2003, 50, 155–163 CrossRef CAS PubMed
.
- X. Guo and F. Chen, Environ. Sci. Technol., 2005, 39, 6808–7163 CrossRef CAS PubMed
.
- A. G. M. Diarmid and A. J. Epstein, Faraday Discuss. Chem. Soc., 1989, 88, 317–332 RSC
.
- V. Aravind and K. P. Elango, Indian J. Chem. Technol., 2006, 13, 476–483 CAS
.
- N. Viswanathan, C. S. Sundaram and S. Meenakshi, J. Hazard. Mater., 2009, 161, 423–430 CrossRef CAS PubMed
.
- S. Senapati, S. K. Srivastava, S. B. Singh and H. N. Mishra, J. Mater. Chem., 2012, 22, 6899–6906 RSC
.
- L. Wang, J. Li, Q. Jianga and L. Zhao, Dalton Trans., 2012, 41, 4544–4551 RSC
.
- A. K. Yadav, C. P. Kaushik, A. K. Haritash, A. Kansal and N. Rani, J. Hazard. Mater., 2006, 128, 289–293 CrossRef CAS PubMed
.
- P. N. Dave and L. V. Chopda, J. Nanotechnol., 2014, 2014, 1–14 CrossRef
.
- S. Zhou, D. Wang, H. Sun, J. Chen, S. Wu and P. Na, Water, Air, Soil Pollut., 2014, 225, 1945 CrossRef
.
- T. Viswanathan, G. Gunawan, S. Bourdo, V. Saini, J. Moran, L. Pack and S. Owen, J. Macromol. Sci., Part A: Pure Appl.Chem., 2011, 48, 348–354 CrossRef CAS
.
- C. Gao, X.-Y. Yu, T. Luo, Y. Jia, B. Sun, J.-H. Liu and X.-J. Huang, J. Mater. Chem. A, 2014, 2, 2119–2128 CAS
.
- Y. Ku and H. M. Chiou, Water, Air, Soil Pollut., 2002, 133, 349–360 CrossRef CAS
.
- S. M. Maliyekkal, A. K. Sharma and L. Philip, Water Res., 2006, 40, 3497–3506 CrossRef CAS PubMed
.
- A. Maiti, J. K. Basu and S. De, Desalination, 2011, 265, 28–36 CrossRef CAS
.
- S. Gao, J. Cui and Z. G. Wei, J. Fluorine Chem., 2009, 130, 1035–1041 CrossRef CAS
.
- S. Gao, R. Sun, Z. G. Wei, H. Y. Zhao, H. X. Li and F. Hu, J. Fluorine Chem., 2009, 130, 550–556 CrossRef CAS
.
- L. M. Camacho, A. Torres, D. Saha and S. Deng, J. Colloid Interface Sci., 2010, 349, 307–313 CrossRef CAS PubMed
.
- S. P. Kamble, G. Deshpande, P. P. Barve, S. Rayalu, N. K. Labhsetwar, A. Malyshew and B. D. Kulkarni, Desalination, 2010, 264, 15–23 CrossRef CAS
.
- J. Zhou, Y. Cheng, J. Yu and G. Liu, J. Mater. Chem., 2011, 21, 19353–19361 RSC
.
- T. Zhang, Q. Li, H. Xiao, H. Lu and Y. Zhou, Ind. Eng. Chem. Res., 2012, 51, 11490–11498 CrossRef CAS
.
- V. Tomar and D. Kumar, Chem. Cent. J., 2013, 7, 51 CrossRef PubMed
.
Footnote |
† Electronic supplementary information (ESI) available: 1.1. Characterizations, Fig. S1 FTIR spectra of (a) Ni nanoflower and Ni/PPy nanocomposite prepared using mass ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py (b) 2 Py (b) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (d) 1 1 and (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Fig. S2 Raman study of Ni/PPy nanocomposite prepared using mass ratio of Ni 2. Fig. S2 Raman study of Ni/PPy nanocomposite prepared using mass ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py (a) 1 Py (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (b) 1 2, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (c) 2 1 and (c) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. S3 room temperature magnetic property of Ni/PPy nanocomposite prepared using mass ratio of Ni 1. Fig. S3 room temperature magnetic property of Ni/PPy nanocomposite prepared using mass ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Py (a) 2 Py (a) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,(b) 1 1,(b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (c) 1 1 and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (inset shows the corresponding magnified image of the hysteresis loop). Fig. S4 removal efficiency of (a) As(III) and (b) F− by Ni/PPy (1 2 (inset shows the corresponding magnified image of the hysteresis loop). Fig. S4 removal efficiency of (a) As(III) and (b) F− by Ni/PPy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1 2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Fig. S5 nitrogen adsorption–desorption isotherm of Ni/PPy (1 2). Fig. S5 nitrogen adsorption–desorption isotherm of Ni/PPy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Table S1: magnetic property of the Ni/PPy nanocomposite. Table S2: chemical analysis of real ground water of Nagaon, Assam, India. Table S3: chemical analysis of real ground water of Karbiangling, Assam, India. See DOI: 10.1039/c6ra24531e 2). Table S1: magnetic property of the Ni/PPy nanocomposite. Table S2: chemical analysis of real ground water of Nagaon, Assam, India. Table S3: chemical analysis of real ground water of Karbiangling, Assam, India. See DOI: 10.1039/c6ra24531e |
| This journal is © The Royal Society of Chemistry 2016 |