A highly sensitive flexible SnS thin film photodetector in the ultraviolet to near infrared prepared by chemical bath deposition
Abstract
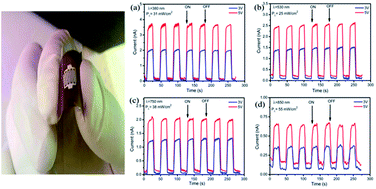
This study involves a novel fabrication of a high performance, low cost and flexible (SnS nanoflake based) photodetector on a polyethylene terephthalate (PET) substrate by using chemical bath deposition. The photoresponse properties of the fabricated SnS nanostructure in the ultraviolet (UV) to near infrared (NIR) region were studied. The photodetector was subjected to illumination from four light emitting diodes (LEDs) characterized by spectra peaks of 380, 530, 750 and 850 nm, respectively. The photodetector exhibited a good photoresponse, fast response and high reproducibility with time. The sensitivity values of the photodetector were determined to be approximately 2990, 1604, 2591, and 446 for wavelengths of 380, 530, 750, and 850 nm at a bias voltage of 3 V. At the bias voltage of 5 V, the sensitivity values of 2575, 1262, 1635 and 295 were recorded for 380, 530, 750 and 850 nm respectively. The photodetector also manifests fast photoresponse for the illumination wavelengths. Based on the abovementioned results, in addition to its low cost, flexibility, and non-toxic nature, the SnS photodetector is a promising photoelectronic device that is effectively applicable over the UV-vis-NIR range.

 Please wait while we load your content...
Please wait while we load your content...