Effect of Li2O additions upon the crystal structure, sinterability and electrical properties of yttria stabilized zirconia electrolyte
Jie Xiongab,
Chengran Jiaoc,
Minfang Hand,
Wentao Yib,
Jie Mab,
Chunyan Yanb,
Weiwei Cai*a and
Hansong Cheng*a
aSustainable Energy Laboratory, Faculty of Materials Science and Chemistry, China University of Geosciences Wuhan, 388 Lumo RD, Wuhan 430074, Hubei, China. E-mail: willcai1985@gmail.com; chghs2@gmail.com; Fax: +86 027 67883736; Tel: +86 027 67883736
bCollege of Chemistry, Chemical Engineering and Material Science, Zaozhuang University, Zaozhuang 277160, Shandong, China
cSchool of Mechanical and Electronic Engineering, Zaozhuang University, Zaozhuang 277160, Shandong, China
dDepartment of Thermal Engineering, Tsinghua University, Beijing 100084, China
First published on 3rd November 2016
Abstract
The crystal structure, lattice constant, sinterability, microstructure, conductivity and cell performance of 8 mol% yttria stabilized zirconia (YSZ) with varying ratios of Li2O (n mol%, n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3), i.e., n mol% Li2OYSZ, were investigated. The results show that the Li+ can dissolve into the ZrO2 cubic lattice and cause lattice contraction due to the small radius of Li+. The YSZ retained its cubic lattice until Li2O addition of 1.7 mol%, and the cubic-to-monoclinic transition was detected in cases of Li2O content more than 1.7 mol%, leading to the increase of the lattice parameter. Li2O addition of 0.25 and 0.5 mol% decreased the sintering temperature from the typical 1400 °C to 1265 °C and 1250 °C, and promoted both the densification and electrical conductivity of the YSZ samples. The conductivities of 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples at 800 °C were as high as 0.0275 S cm−1 and 0.0313 S cm−1, which are 1.26 and 1.43 times greater than that of pure YSZ, respectively. Then the densification of the YSZ samples reduced gradually at Li2O content greater than 1 mol%. For n mol% Li2OYSZ (n ≥ 1.7) samples, a dense and pure cubic phase ceramic could be achieved at sintering temperatures above 1400 °C due to the vaporization of Li2O at such high temperatures resulting from its high vapor pressure. Solid oxide fuel cells (SOFCs) with 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ electrolyte displayed open circuit voltages higher than 1.0 V at 800 °C, indicating the absence of electronic conductivity, and meanwhile exhibited much increased maximum power densities than that of the cell with pure YSZ electrolyte, implying that inclusion of a small amount of Li2O into YSZ electrolyte could be a desirable improvement approach for zirconia-based SOFC.
1. Introduction
Yttria stabilized zirconia (YSZ) is commonly used as an electrolyte for solid oxide fuel cells (SOFC) because of its pure ionic conductivity and chemical stability both in oxidizing and reducing atmospheres.1–5 However, a major drawback of the use of zirconia-based materials is the high firing temperature required to achieve usable densification (usually exceeding 1400 °C for numerous hours6,7). To reduce the sintering temperature, various alkaline-earth metal or transition metal oxides have been studied as sintering aids to improve sinterability of YSZ ceramics, such as metal oxides of Ca,8 Co,6 Fe,7 Cu,9 Mn10 and Ni.11 Nevertheless, negative effects of these transition metal oxides on the properties of YSZ were also reported,6,8,11–14 causing usually a decrease in conductivity, or even accompanied by the zirconia phase transformation sometimes.As for lithium oxide or lithium salts, which have been used for the sintering aids of those ceramics that usually present poor densification, and encouraging results were reported.15–20 In particular, Esposito et al.21 found that samaria doped ceria (SDC) could be fully densified at 900 °C with 5 mol% Li2O as an additive, and Han et al.22 reported highly dense gadolinia doped ceria (GDC) sintered bodies with addition of 2.5 mol% Li2O were obtained at a remarkably low temperature of 800 °C, even though SDC and GDC samples free of sintering additives were normally sintered at as high as 1550 °C to get a relative density of 95%.23,24 To the best of the authors' knowledge, Li2O has scarcely been reported to be applied as a sintering aid of YSZ, and the only application on promotion of YSZ conductivity was reported by Nagaeva et al.,25 and consequently the present study was initiated to evaluate the potential of Li2O additives to decrease the sintering temperature or improve other properties of YSZ. Besides, it is necessary to know how the properties will be changed by the possible interaction between YSZ and minor Li2O additives.
2. Experimental
The different molar ratios of lithium oxide (i.e., n mol% Li2O, where n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3) to 8 mol% Y2O3 stabilized ZrO2 (YSZ, Tosoh Corporation, Tokyo, Japan) were added into YSZ powders in the form of lithium nitrate (>99.99% pure, Baotou, China), then the powders were wet ground (in ethanol) in a polyurethane jar for 24 h. The resulting paste was then oven-dried at 70 °C and subsequently calcined at 600 °C for 2 h in air to decompose lithium nitrate into Li2O. The derived powders were denoted as n mol% Li2OYSZ, and they were characterized by differential thermal analysis and thermo-gravimetric analysis (DTA/TG, Netzsch STA 449C) in air at a heating rate of 10 °C min−1 from ambient temperature to 1300 °C. Sintering behaviors of samples were performed using a dilatometer (Netzsch DIL 402C, Germany) at a heating rate of 5 °C min−1 from room temperature to 1400 °C.Slurries for tape casting were prepared as our previous study26 by dispersing n mol% Li2OYSZ powders in mixed ethanol–butanone solvent with caster oil as dispersant, dibutyl phthalate (DBP) as plasticizer, and polyvinyl butyral (PVB) as binder. The slurries were ball milled for 48 h before tape casting using a tabletop castor (DR-150, Japan). Thereafter, tapes were dried in air at room temperature for 12 h and punched to discs with a diameter of 20 mm, then fired in air at different temperatures, varied from 700–1400 °C for 8 h. The fired discs were characterized by X-ray diffraction (XRD, PANalytical X'Pert PRO, Netherlands) with Cu Kα1 radiation over the range of 2θ from 20° to 80°. The relative densities of n mol% Li2OYSZ pellets were determined by the Archimedes drainage method. Microstructures of fired ceramic were observed by a field emission scanning electron microscope (JSM-7800F, JEOL, Japan). Two electrode electrochemical impedance spectroscopy (IM6, ZAHNER, Germany) measurements were carried out in dry air in a temperature range between 300 °C and 800 °C and over a frequency range of 0.1 Hz to 4 MHz with 10 mV as the excitation ac amplitude.
The anode pastes comprising NiO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YSZ of 50
YSZ of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% were mixed throughly and ball milled with ethylcellulose and terpineol for 24 h, then the slurry was screen printed onto the fired electrolyte disks, followed by co-firing at 1250 °C for 5 h in air. Similarly, the (La0.8Sr0.2)0.95Mn3−δ (LSM) cathode paste comprising of LSM, YSZ, ethylcellulose and terpineol was coated on the other side of electrolyte. Afterwards the disks were sintered in air at 1000 °C for 2 h to get the NiO–YSZ‖n mol% Li2OYSZ‖YSZ–LSM electrolyte-supported cells with about 150 μm thick electrolyte, and approximately 30 μm thick cathode, followed by sealing of the single cells with ceramic sealant (CERAMABOND 552, USA). And then, cell performances were evaluated at 800 °C in a humidified hydrogen with 3 vol% H2O at a rate of 70 mL min−1 on the anode side and ambient air on the cathode side. The I–V characterization was conducted on Arbin instrument (BT2000, USA).
50 wt% were mixed throughly and ball milled with ethylcellulose and terpineol for 24 h, then the slurry was screen printed onto the fired electrolyte disks, followed by co-firing at 1250 °C for 5 h in air. Similarly, the (La0.8Sr0.2)0.95Mn3−δ (LSM) cathode paste comprising of LSM, YSZ, ethylcellulose and terpineol was coated on the other side of electrolyte. Afterwards the disks were sintered in air at 1000 °C for 2 h to get the NiO–YSZ‖n mol% Li2OYSZ‖YSZ–LSM electrolyte-supported cells with about 150 μm thick electrolyte, and approximately 30 μm thick cathode, followed by sealing of the single cells with ceramic sealant (CERAMABOND 552, USA). And then, cell performances were evaluated at 800 °C in a humidified hydrogen with 3 vol% H2O at a rate of 70 mL min−1 on the anode side and ambient air on the cathode side. The I–V characterization was conducted on Arbin instrument (BT2000, USA).
3. Results and discussion
3.1 XRD studies and thermal analysis
X-ray diffractograms of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3) discs fired at 1250 °C for 8 h are presented in Fig. 1. The same sample height and flatness were carefully preserved while performing the XRD tests to reduce the experimental error. From Fig. 1b, it's noted that the diffraction peaks of samples added with Li2O shift to right (higher diffraction angles) with the increase of n in n mol% Li2OYSZ (n ≤ 1.7 mol%) compared to that of pure YSZ, indicating that the Li+ was effectively incorporated into the zirconia crystal lattice and gradually reduced the lattice parameter as shown in Fig. 2, since the ionic radius of Li+ (0.68 Å)15 is smaller than that of Zr4+ (0.84 Å).7,27 Besides, no detectable phase transition was observed for YSZ peaks when the Li2O additive amounts were less than 1.7 mol%, suggesting that small Li2O addition does not change the crystalline structure of YSZ. As the addition concentration of Li2O is increased up to n = 1.7, monoclinic phase of zirconia appeared, and it became progressively intensified with the increasing Li2O additive amounts, thus leading to the steady increase of the lattice constant. This indicates a possibility that the solid solution of cubic YSZ with Li2O is limited to below 1.7 mol%. The lattice constant calculated on the basis of Bragg equation and crystal plane indices by adopting the MDI Jade software (version 5.0) gradually decreases first and then increases with the further increase of Li2O content as indicated in Fig. 2, which is consistent with the observations that diffraction peak initially shifted toward higher angles from pure YSZ to 1.7 mol% Li2OYSZ, followed by shifting back to lower angles for samples with the Li2O addition more than 1.7 mol%. | ||
| Fig. 1 X-ray diffraction patterns of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3) pellets after sintering at 1250 °C for 8 h. | ||
XRD patterns of 1.7 mol% Li2OYSZ pellets sintered at various temperatures from 700 to 1400 °C for 8 h are shown in Fig. 3. The monoclinic phase of zirconia was not observed in Fig. 3a until the temperature is increased up to 800 °C, and the intensities of the diffraction peaks of monoclinic zirconia increase steadily with increasing temperatures from 800 to 1100 °C, presumably indicating that the incorporation of lithium oxide into zirconia crystal lattice need to overcome certain energy barrier, and high temperature provides the possibility. Accordingly, the diffraction peaks shifted to lower angles as sintering temperature increased as illustrated in Fig. 3b due to the lattice expansion caused by increasing cubic-to-monoclinic phase transition. Then the diffraction peaks of monoclinic zirconia gradually abate or even disappear with the further increase of sintering temperatures over 1100 °C, accompanied by the diffraction peaks shifting toward higher angles as a result of crystal lattice contraction. When the 1.7 mol% Li2OYSZ disc was sintered at 1400 °C, the X-ray diffraction pattern shows that the coexistence of cubic and monoclinic phases disappeared, only a cubic fluorite structure of zirconia is present on the diffractogram without a secondary phase of monoclinic zirconia. This could be attributed to the much higher vapor pressure of Li2O28 (at least three orders of magnitude higher) than that of common metal oxide additives such as Fe2O3, CoO and NiO at the same temperature. The higher the temperature is, the greater the vapor pressure of lithium oxide will be. Therefore, high sintering temperature will accelerate the vaporization of Li2O, which is responsible for the diminution and disappearance of monoclinic zirconia above 1100 °C, similar to the report that Li species could mostly vaporize in the GDC with 2.5 mol% Li2O samples sintered above 1100 °C mentioned elsewhere.28
Fig. 4 shows the TG/DTA results for 1.7 mol% Li2OYSZ precursor particles. According to the TG curve, no significant weight loss was recognized. This result implies that the nitrates were decomposed completely, and there were no organic and nitrate group residues in the precursor powders derived from calcining the mixture of YSZ and lithium nitrate at 600 °C for 2 h as previously described in Experimental section. And the DTA curve presents four major peaks: two for endotherm and the other two for exotherm. The endothermic peak at about 845 °C was likely due to the cubic-to-monoclinic phase transformation as indicated in Fig. 3a. The latter endothermic peak around 1000 °C probably corresponded to the increase of monoclinic zirconia as depicted in Fig. 3a. The broad exothermic peaks at approximate 1100 °C and 1230 °C on the DTA curve may be ascribed to the diminishment and elimination of monoclinic zirconia as shown in Fig. 3a due to the vaporization of Li2O at such high temperatures causing by its high vapor pressure. To avoid the occurring of frequent phase changes, the amounts of Li2O addition should be less than the critical point of phase transition, i.e. the solid solution limit of 1.7 mol% Li2O in YSZ.
 | ||
| Fig. 4 Differential thermal analysis (DTA) and thermo-gravimetric (TG) curves for 1.7 mol% Li2OYSZ precursor particles. | ||
3.2 Sintering behaviors and microstructural characterization
The plots in Fig. 5 show the linear shrinkage (ΔL/L0) and shrinkage rate (d(ΔL/L0)/dt) as a function of temperature for YSZ with various Li2O levels. It is clear that the small content of Li2O addition shifts the onset of sintering to low temperature range and decreases the maximum shrinkage rate temperature (Tmax), which is in the following order: Tmax0.5% (∼1193 °C) > Tmax0.25% (∼1203 °C) > Tmax1% (∼1244 °C) > Tmax1.5% (∼1286 °C) > Tmax0% (∼1292 °C) > Tmax1.7% (∼1373 °C). Moreover, 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples nearly finished shrinkage at about 1265 °C and 1250 °C, respectively, while the YSZ sintering shrinkage was still going on even at 1350 °C. The results demonstrate that the Li2O addition can remarkably reduce sintering temperature. Among these addition levels, 0.5 mol% Li2O is the most effective additive amount for enhancing the densification of YSZ, and the difference of Tend (the shrinkage end temperature) values between the YSZ and 0.5 mol% Li2OYSZ is about 150 °C.Fig. 6 depicts the cross-sectional microstructural morphology of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3) pellets sintered at 1250 °C. Porous structure with large holes were observed for the Li2O free YSZ sample, and the density obviously improved as Li2O content increased, only few pinholes were observed in 0.25 mol% Li2OYSZ. A full dense microstructure was obtained in the 0.5 mol% Li2OYSZ sample, which may be attributed to the liquid-phase sintering effect caused by lithium oxide that typically has a feature of achieving dense ceramics with small grains.29 The grain size of 0.5 mol% Li2OYSZ calcined at 1250 °C is about 100–200 nm, whereas YSZ sintered at the same temperature has an average size of around 300 nm as shown in Fig. 7, demonstrating that the liquid sintering mechanism dominates the process of 0.5 mol% Li2OYSZ densification. The result coincides with the observation that the incorporation of lithium salts into ceria-based ceramics could suppress the grain growth and achieve dense ceramics with fine grains, which has been reported in other literature.30 As the content of Li2O was above 0.5 mol%, the porosity increased, and a sharp decrease of densification was observed as Li2O increased over 1.7 mol% owing to the cubic-to-monoclinic phase transition. These observations are in good agreement with what we have illustrated in Fig. 5, which indicates that Li2O can lower the sintering temperature of YSZ ceramic effectively from 1400 °C to 1250 °C.
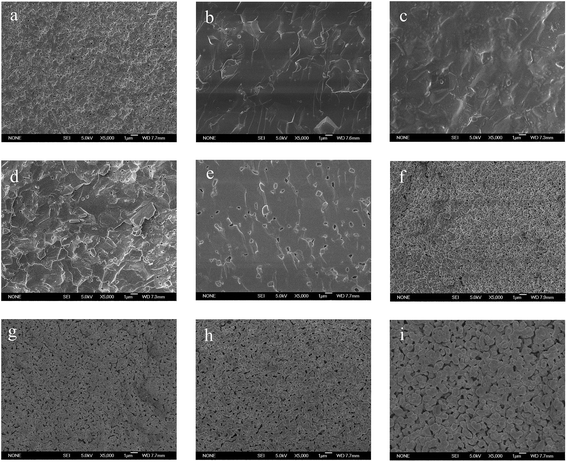 | ||
| Fig. 6 Cross-sectional SEM images of n mol% Li2OYSZ pellets calcined at 1250 °C, (a) n = 0; (b) n = 0.25; (c) n = 0.5; (d) n = 1; (e) n = 1.5; (f) n = 1.7; (g) n = 2; (h) n = 2.5 and (i) n = 3. | ||
Variation of full density temperatures of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3) pellets as a function of Li2O dopant levels are illustrated in Fig. 8. When 0.25 mol% Li2O was added to YSZ, the full density temperatures decreased considerably from 1400 °C to 1265 °C, and 0.5 mol% Li2OYSZ seems to be the most preferred alternative with the lowest densification temperature required around 1250 °C among all the doping levels. As the concentration of Li2O increased from 0.5 to 3 mol%, the full density temperatures increased rapidly from 1250 °C to 1550 °C. Afterwards, the phase inspection was carried out for these dense ceramic discs with Li2O content more than 2 mol%, and the XRD patterns are exhibited in Fig. 9. What is different from the diffractogram of n mol% Li2OYSZ (n ≥ 2) calcined at 1250 °C as shown in Fig. 1 is that only a pure cubic zirconia phase remained without the occurring of a secondary phase of monoclinic zirconia for n mol% Li2OYSZ (n ≥ 2) samples sintered above 1500 °C as a result of Li2O vaporization at elevated temperatures. The results match what we have illustrated in Fig. 3 for 1.7 mol% Li2OYSZ sintered at 1400 °C. So the high vapor pressure of Li2O ensures that Li2O can be developed as a non-residual sintering additive.
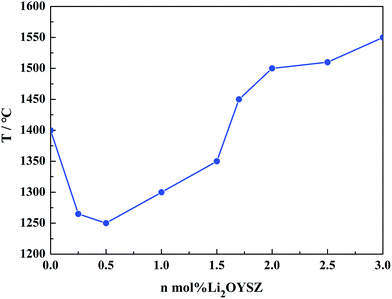 | ||
| Fig. 8 Variation of full density temperatures of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7, 2, 2.5, 3) pellets as a function of Li2O dopant levels. | ||
 | ||
| Fig. 9 X-ray diffractograms of n mol% Li2OYSZ (n = 2, 2.5, 3) discs calcined at 1500–1550 °C for 8 h. | ||
3.3 Electrical properties and cell performance
Fig. 10 shows the bulk, grain boundary and total electrical conductivity of fully dense doped YSZ as a function of the amount of Li2O at temperatures of 300–800 °C. Fig. 10a presents that the grain boundary conductivities of n mol% Li2OYSZ are always lower than that of the bulk below 400 °C, and then the bulk and grain boundary conductivity tend to be equally matched at 450 °C. Subsequently, the bulk and grain boundary conductivity followed a reverse pattern with the further increase of temperature above 500 °C, i.e. the grain boundary conductivity of n mol% Li2OYSZ is higher than that of the bulk. The space charge model of acceptor-doped zirconia proposed by Guo et al.31 may account for the phenomenon. According to the model, a grain boundary consists of a grain-boundary core and two adjacent space-charge layers. The grain-boundary core of acceptor-doped zirconia is positively charged, oxygen vacancies are therefore depleted in the space-charge layer as a result of the strong Coulomb repulsive force between positively charged oxygen vacancies and grain-boundary core, leading to the lower grain boundary conductivities.32 However, the block effect of grain-boundary core became nondominant above 450 °C owing to increasing oxygen vacancies were sufficiently mobile to transport across the grain boundaries at elevated temperatures, resulting in the enhancement of contribution of grain boundary conductivities. Moreover, the bulk, grain boundary and total electrical conductivity firstly increased from pure YSZ to 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ. However, further increase in the Li2O addition levels led to a decrease in the conductivities for 1 mol% Li2OYSZ, 1.5 mol% Li2OYSZ and 1.7 mol% Li2OYSZ. This may be explained using the equation:
 | (1) |
 defect for the charge compensation.25 On one hand, the negatively charged
defect for the charge compensation.25 On one hand, the negatively charged  will be attracted to the positively charged grain-boundary core, which caused the enrichment of
will be attracted to the positively charged grain-boundary core, which caused the enrichment of  around the grain boundary and neutralized the positive electricity field of grain-boundary core,28,31,33 thus weakened the repulsive force of grain-boundary core to oxygen vacancies, therefore the grain boundary conductivity increased after Li2O doping. On the other, the negatively charged
around the grain boundary and neutralized the positive electricity field of grain-boundary core,28,31,33 thus weakened the repulsive force of grain-boundary core to oxygen vacancies, therefore the grain boundary conductivity increased after Li2O doping. On the other, the negatively charged  and positively charged
and positively charged  point defects tend to cluster due to the electrostatic attraction, and this tendency increases at the higher addition level leading to the formation of
point defects tend to cluster due to the electrostatic attraction, and this tendency increases at the higher addition level leading to the formation of  defect complex.34–36 In 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples, the concentration and transition probability of oxygen vacancy increased, which leads to the gradual increase in the bulk and grain boundary conductivity, and accordingly promoted the total conductivity. However, on further increase of the Li+ doping (n > 0.5), more and more oxygen vacancies were bounded in the defect complex and difficult to migrate, thereby the effective concentration and transition probability of oxygen vacancy decreased, consequently resulting in low bulk and grain boundary conductivity, so does the total electrical conductivity as shown in Fig. 10b. It's also noted that the total electrical conductivity of YSZ was 0.0219 S cm−1 at 800 °C, which is comparable to the report in other literature of 0.0212 S cm−1.37 The conductivities of 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples at 800 °C were improved significantly, reaching as high as 0.0275 S cm−1 and 0.0313 S cm−1, which are 1.26 and 1.43 times greater than that of lithium free YSZ, respectively, suggesting that small contents of Li2O doped YSZ could be a potential and excellent electrolyte material for SOFCs.
defect complex.34–36 In 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples, the concentration and transition probability of oxygen vacancy increased, which leads to the gradual increase in the bulk and grain boundary conductivity, and accordingly promoted the total conductivity. However, on further increase of the Li+ doping (n > 0.5), more and more oxygen vacancies were bounded in the defect complex and difficult to migrate, thereby the effective concentration and transition probability of oxygen vacancy decreased, consequently resulting in low bulk and grain boundary conductivity, so does the total electrical conductivity as shown in Fig. 10b. It's also noted that the total electrical conductivity of YSZ was 0.0219 S cm−1 at 800 °C, which is comparable to the report in other literature of 0.0212 S cm−1.37 The conductivities of 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples at 800 °C were improved significantly, reaching as high as 0.0275 S cm−1 and 0.0313 S cm−1, which are 1.26 and 1.43 times greater than that of lithium free YSZ, respectively, suggesting that small contents of Li2O doped YSZ could be a potential and excellent electrolyte material for SOFCs.
 | ||
| Fig. 10 (a) Bulk (open symbols), grain boundary (filled symbols) conductivity and (b) total electrical conductivity of doped YSZ as a function of Li2O contents at temperatures of 300–800 °C. | ||
Fig. 11 shows the electrical conductivity of YSZ with different amounts of Li2O as a function of temperature. From the Arrhenius plots, the activation energies of n mol% Li2OYSZ both in the medium-low temperature range and the medium-high temperature range can be achieved, and the detailed values are summarized in Table 1. It is noted that the activation energies of n mol% Li2OYSZ are ranked as follows: 0.5 mol% Li2OYSZ < 0.25 mol% Li2OYSZ < YSZ < 1 mol% Li2OYSZ < 1.5 mol% Li2OYSZ < 1.7 mol% Li2OYSZ, revealing exactly the reversed change trend with the conductivity, which indicates content of Li2O dopant plays an important role in determination of total conductivity.
| Temperature range | Activation energies of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7)/eV | |||||
|---|---|---|---|---|---|---|
| n = 0 | n = 0.25 | n = 0.5 | n = 1 | n = 1.5 | n = 1.7 | |
| Medium-low | 1.188 | 1.171 | 1.169 | 1.189 | 1.190 | 1.245 |
| Medium-high | 0.860 | 0.855 | 0.852 | 0.864 | 0.872 | 0.877 |
The electrochemical performance of n mol% Li2OYSZ (n = 0, 0.25, 0.5, 1, 1.5, 1.7) as electrolyte materials was evaluated in NiO–YSZ‖n mol% Li2OYSZ‖YSZ–LSM electrolyte-supported cells. Characteristics of cells with different composite electrolytes measured in 3% H2O humidified H2 fuel and ambient air oxidant at 800 °C is given in Fig. 12. The measured open circuit voltage (OCVs) of the cells with Li2O doped YSZ electrolytes were all above 1.0 V, indicating the absence of unfavorable electronic conduction in the Li2O doped YSZ electrolytes under the temperature and oxygen pressure employed in this study. The 0.5 mol% Li2OYSZ demonstrated the highest output power among all the compositions studied, and as expected the maximum power densities are ranked in the following order: 0.5 mol% Li2OYSZ > 0.25 mol% Li2OYSZ > YSZ > 1 mol% Li2OYSZ > 1.5 mol% Li2OYSZ > 1.7 mol% Li2OYSZ, revealing that the cell performance has a lot to do with the observed electrical conductivities. The peak power densities of cells equipped with 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ reached as high as 0.106 and 0.122 W cm−2, respectively, exhibiting much better performance than that of the cell using lithium oxide free YSZ electrolyte, thereby demonstrating that a small amount of Li2O addition into YSZ electrolyte could be a desirable improvement approach for zirconia-based SOFC.
4. Conclusions
In the present study, the effects of small amounts (≤3 mol%) of Li2O addition on the crystal characteristics, sintering behaviours, micro morphology, electrical properties and electrochemical performance of 8 mol% Y2O3 stabilized ZrO2 were carried out. The increased Li2O addition levels induced the gradual lattice contraction because of the smaller ionic radius of Li+ compared with that of Zr4+, and the solid solution limit of Li2O in 8 mol% Y2O3 stabilized ZrO2 is 1.7 mol%, above which phase transition from cubic to monoclinic occurred, accompanied by the lattice expansion. By adding 0.25 and 0.5 mol% Li2O, the densification temperature of YSZ was reduced from the conventional temperature of exceeding 1400 °C to 1265 °C and 1250 °C, respectively. High electrical conductivities of 0.0275 S cm−1 and 0.0313 S cm−1 at 800 °C were obtained for fully dense 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ samples, respectively. SOFCs using 0.25 mol% Li2OYSZ and 0.5 mol% Li2OYSZ as electrolytes displayed excellent output power densities. These results suggest that small amounts of lithium oxide addition are promising for use in low temperature preparation of zirconia-based electrolytes for solid oxide fuel cells with excellent electrochemical performance.Acknowledgements
We are greatly grateful to the financial supports from the State Key Development Program for Basic Research of China (2012CB215404), the National Natural Science Foundation of China (No. 21233006, 21473164, 21503197 and 51261120378) and Fundamental Research Funds for the Central University, China University of Geosciences (Wuhan) (No. CUG150603, CUG150615, CUG150627 and CUGL150802).References
- K. Keizer, A. J. Burggraaf and G. With, J. Mater. Sci., 1982, 17, 1095 CrossRef CAS.
- B. Li, J. Zhang, T. Kaspar, V. Shutthanandan, R. C. Ewing and J. Lian, Phys. Chem. Chem. Phys., 2013, 15, 1296 RSC.
- T. Tatte, M. Part, R. Talviste, K. Hanschmidt, K. Utt, U. Maeorg, I. Jogi, V. Kiisk, H. Mandar, G. Nurk and P. Rauwel, RSC Adv., 2014, 4, 17413 RSC.
- M. F. Han, X. L. Tang, H. Y. Yin and S. P. Peng, J. Power Sources, 2007, 165, 757 CrossRef CAS.
- E. Drozdz, RSC Adv., 2016, 6, 84752 RSC.
- G. S. Lewis, A. Atkinson and B. C. H. Steele, J. Mater. Sci. Lett., 2001, 20, 1155 CrossRef CAS.
- H. B. Gao, J. Liu, H. Y. Chen, S. Li, T. M. He, Y. Ji and J. D. Zhang, Solid State Ionics, 2008, 179, 1620 CrossRef CAS.
- Z. G. Lv, R. S. Guo, P. Yao and F. Y. Dai, Mater. Des., 2007, 28, 1399 CrossRef CAS.
- J. L. Shi, T. S. Yen and H. Schubert, J. Mater. Sci., 1997, 32, 1341 CrossRef CAS.
- T. S. Zhang, S. H. Chan, W. Wang, K. Hbaieb, L. B. Kong and J. Ma, Solid State Ionics, 2009, 180, 82 CrossRef CAS.
- T. S. Zhang, Z. H. Du, S. Li, L. B. Kong, X. C. Song, J. Lu and J. Ma, Solid State Ionics, 2009, 180, 1311 CrossRef CAS.
- C. C. Appel, N. Bonanos, A. Horsewell and S. Linderoth, J. Mater. Sci., 2001, 36, 4493 CrossRef CAS.
- S. Linderoth, N. Bonanos, K. V. Jensen and J. B. Bilde-Sørensen, J. Am. Ceram. Soc., 2001, 84, 2652 CrossRef CAS.
- C. Bowen, S. Ramesh, C. Gill and S. Lawson, J. Mater. Sci., 1998, 33, 5103 CrossRef CAS.
- D. W. Yuan, S. F. Wang, W. Huebner and G. Simkovich, J. Mater. Res., 1993, 8, 1675 CrossRef CAS.
- J. D. Nicholas and L. C. De Jonghe, Solid State Ionics, 2007, 178, 1187 CrossRef CAS.
- C. L. Tsai, M. Kopczyk, R. J. Smith and V. H. Schmidt, Solid State Ionics, 2010, 181, 1083 CrossRef CAS.
- H. Naghib-zadeh, C. Glitzky, I. Dörfel and T. Rabe, J. Eur. Ceram. Soc., 2010, 30, 81 CrossRef CAS.
- A. Sayyadi-Shahraki, E. Taheri-Nassaj, S. A. Hassanzadeh-Tabrizi and H. Barzegar-Bafrooei, J. Alloys Compd., 2014, 597, 161 CrossRef CAS.
- S. M. Jamil, M. H. Dzarfan Othman, M. A. Rahman, J. Jaafar, A. F. Ismail and M. A. Mohamed, RSC Adv., 2015, 5, 58154 RSC.
- V. Esposito, M. Zunic and E. Traversa, Solid State Ionics, 2009, 180, 1069 CrossRef CAS.
- M. F. Han, Z. Liu, S. Zhou and L. Yu, J. Mater. Sci. Technol., 2011, 27, 460 CAS.
- K. Maca, J. Cihlar, K. Castkova, O. Zmeskal and H. Hadraba, J. Eur. Ceram. Soc., 2007, 27, 4345 CrossRef CAS.
- K. Maca, V. Pouchly and P. Zalud, J. Eur. Ceram. Soc., 2010, 30, 583 CrossRef CAS.
- N. Y. Nagaeva, A. A. Surin, L. A. Blaginina and V. P. Obrosov, Glass Ceram., 2008, 65, 199 CrossRef CAS.
- J. Xiong, C. R. Jiao, M. F. Han, W. T. Yi, J. Ma and C. Y. Yan, RSC Adv., 2015, 5, 87477 RSC.
- A. J. Feighery and J. T. S. Irvine, Solid State Ionics, 1999, 121, 209 CrossRef CAS.
- T. L. Zhu, Y. Lin, Z. B. Yang, D. Su, S. G. Ma, M. F. Han and F. L. Chen, J. Power Sources, 2014, 261, 255 CrossRef CAS.
- X. H. Zhao, M. J. Wang, Q. L. Zhang, H. Yang, L. Hu and D. Yu, Mater. Lett., 2014, 122, 9 CrossRef CAS.
- M. Chen, H. Zhang, L. Fan, C. Wang and B. Zhu, Int. J. Hydrogen Energy, 2014, 39, 12309 CrossRef CAS.
- X. Guo and R. Waser, Prog. Mater. Sci., 2006, 51, 151 CrossRef CAS.
- X. Guo, W. Sigle and J. Maier, J. Am. Ceram. Soc., 2003, 86, 77 CrossRef CAS.
- X. Guo and R. Waser, Solid State Ionics, 2004, 173, 63 CrossRef CAS.
- P. P. Dholabhai, J. B. Adams, P. Crozier and R. Sharma, Phys. Chem. Chem. Phys., 2010, 12, 7904 RSC.
- Y. Lin, S. M. Fang, D. Su, K. S. Brinkman and F. L. Chen, Nat. Commun., 2015, 6, 6824 CrossRef CAS PubMed.
- M. P. Delmo, S. Yamamoto, S. Kasai, T. Ono and K. Kobayashi, Nature, 2009, 457, 1112 CrossRef CAS PubMed.
- X. D. Xu, C. A. Tian, Q. Y. Yin and J. H. Cheng, Bull. Chin. Silic. Soc., 2011, 30, 593 CAS.
| This journal is © The Royal Society of Chemistry 2016 |






