Gold nanoparticles supported on nanoscale amine-functionalized MIL-101(Cr) as a highly active catalyst for epoxidation of styrene†
Abstract
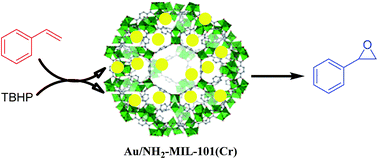
In this study, well dispersed gold nanoparticles (Au Nps) were embedded on an amine-functionalized nanoscale metal–organic framework MIL-101(Cr) via a simple solution approach. The resulting Au/NH2-MIL-101(Cr) exhibited a high isoelectric point (IEP) value with Brønsted basic sites that played an important role in determining the size and dispersion of Au Nps onto its matrix. In addition, the uniform nanomorphology of the catalyst provides the advantage of its good dispersion in aqueous media. The synthesized Au/NH2-MIL-101(Cr) catalyst was successfully characterized by different analytical techniques like powder X-ray diffraction, X-ray photoelectron spectroscopy, field emission scanning electron microscopy, transmission electron microscopy, Brunauer–Emmett–Teller (BET) surface area analysis, Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), zeta potential analysis and atomic absorption spectroscopy (AAS). The catalytic activity of the synthesized material was explored for the epoxidation of styrene using tert-butyl hydroperoxide as oxidant. The catalyst could easily be recovered and recycled up to the fourth catalytic cycle without any significant change in its catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...