Metal–organic frameworks MIL-88A with suitable synthesis conditions and optimal dosage for effective catalytic degradation of Orange G through persulfate activation
Jiumei Wangac,
Jinquan Wan*abc,
Yongwen Maabc,
Yan Wangac,
Mengjie Puac and
Zeyu Guanac
aSchool of Environment and Energy, South China University of Technology, Guangzhou 510006, China. E-mail: ppjqwan@scut.edu.cn
bState Key Lab Pulp and Paper Engineering, South China University of Technology, Guangzhou 510640, China
cThe Key Laboratory of Pollution Control and Ecosystem Restoration in Industry Clusters, Ministry of Education, South China University of Technology, Guangzhou 510006, China
First published on 15th November 2016
Abstract
MIL-88A was synthesized in diverse preparation conditions and characterized by various techniques. The catalytic performance of MIL-88A series for degrading Orange gelb (OG) through persulfate activation were also tested at temperature of 25 °C and the results were shown in the following sequence: 85 °C/2 h > 65 °C/2 h > 105 °C/2 h > 65 °C/6 h > 65 °C/12 h. MIL-88A produced at the synthesis temperature of 85 °C and in the crystallization time of 2 h (85 °C/2 h) was the best one with a removal rate of 96.4% mainly due to high SBET and much greater leach-out of Fe. An optimal dosage (0.3 g L−1) for MIL-88A in the MIL-88A/PS/OG system exists because of inhibition and promotion by fumaric acid. The filtrate experiment indicated that the catalytic activation involved heterogeneous reactions on the surface of the catalyst and homogeneous reactions in solutions. But the heterogeneous reaction occupied the principal position and the probable mechanism was obtained. Moreover, only solution pH < 4 could show a high degradation effect without the help of temperature. Comparing two different methods in the recycling experiment, the removal rate of OG decreased after the fourth run in both. Loss of active catalytic sites for Fe(III) in MIL-88A in the process of separating and sampling was responsible for activity decay.
1. Introduction
Persulfate (PS) has been widely used in disinfection and chemical analysis, among other applications. Sulfate radicals-based advanced oxidation processes have used PS as the initiator to generate sulfate radicals for degrading poisonous and harmful pollutants in aqueous solution. Sulfate radicals (SO4−˙) (E0 = 2.5–3.1 V) not only show a standard redox potential with hydroxyl radicals (˙OH) (E0 = 1.8–2.7 V), but also demonstrate higher oxidation ability and strong specificity of certain pollutants in neutral conditions.1,2 These advantages of sulfate radicals make it competitive in environmental applications. For this reason, developing a catalyst for efficient PS activation is timely and particularly important.Metal–organic frameworks (MOFs) are composed of transition metal ions and organic ligands that demonstrate special physical and chemical properties such as high surface area, controllable pore volume, multiple functionalities and high chemical resistance.3–5 Diverse researchers in academia and industry have great interest in MOFs owing to such unique qualities. Moreover, MOFs have been widely used in gas storage,6–8 catalysis,9–11 chemical analysis,12 biological medicine8,13 and photocatalysis.14 In addition, several MOFs can be produced as catalysts and adsorbents with high stability in an aqueous solution.15–18 In particular, certain adsorption materials can efficiently remove poisonous and harmful substances from polluted water including heavy metals,19 halogens,20 toxic dyes21 and drugs.22
With the development of MOFs in these fields, increasing numbers of researchers applied MOFs to water treatment with good results. It had been confirmed that MOFs containing iron,25 cobalt,23,24 manganese26 and other metal elements25 can be used as heterogeneous catalysts to activate oxidants (e.g., hydrogen peroxide, PS) for effectively degrading organic pollutants. For example, Du et al.27 and Ai et al.28 produced MIL-53 as a photocatalyst and Fenton reaction catalyst to degrade dyes. Liu et al.29 used another well-known MOF magnetic MIL-100 for the adsorptive removal of RB from aqueous solution. Bhattacharjee et al.30 also applied an Fe-based MOF, Fe-MOF-74, as the Fenton reaction catalyst to degrade phenol, and MIL-101 produced by Vu et al.31 was adopted as the catalyst in the photo-Fenton reaction as well.
These MOFs (including MIL-53, MIL-100, Fe-MOF-74, MIL-101) have been widely investigated vis-à-vis photocatalysis and Fenton reactions. But there are only a few studies on MOFs applied to PS activation for degrading organic matter. Herein, we report a kind of Fe-based MOF, MIL-88A, used as an efficient catalyst to degrade organic pollutants at room temperature (i.e., 25 °C).
Previously, the same catalyst, MIL-88A, was produced by Lin et al.32 to activate PS for degrading rhodamine B (RB).32 This synthesis method of MIL-88A is different from ours. In addition, their degradation rate of RB attained about 50% at room temperature and MIL-88A dosage was greater than ours.32 Another team prepared the same catalyst, MIL-88A, as a new photocatalyst for decolorization of methylene blue dye.33 But the degradation result was not ideal without the help of visible light. Moreover, a large number of previous studies confirmed that UV-Vis and heat could activate PS to generate sulfate radicals only by themselves. The process of sulfate radicals produced from the activation of PS by UV-Vis, heat and transition metal ions were shown in eqn (1)–(3), respectively.34–36
| Photochemical activation: S2O82− + HV → 2SO4−˙ | (1) |
| Thermal activation: S2O82− + Heat → 2SO4−˙ | (2) |
| Chemical activation: S2O82− + MN+ → SO4−˙ + M(N+1)+ + SO42− | (3) |
In this study, the MIL-88A obtained was good at degrading organics via changing preparation methods and conditions; adding a little to the system led to an OG degradation rate of 96% at room temperature. Moreover, compared to other abovementioned MOFs (including MIL-53, MIL-100, Fe-MOF-74, MIL-101), MIL-88A was produced only with the use of water as solvent, while others were made of N,N-dimethylformamide (DMF). It is well known that DMF is a recognized carcinogen, harmful to the environment and easily causes secondary pollution. Furthermore, upon using the same quality of raw materials, the MIL-88A output greatly increases and costs decline; consequently, it exhibits great potential for viability in industrial production. Therefore, DMF-free MOFs should be developed and adopted in such oxidant-activation reactions. In our study, the MIL-88A was synthesized from Fe3+ and fumaric acid in water, and the as-synthesized MIL-88A was investigated as a catalyst to activate PS, which generated sulfate radicals for degradation of representative pollutant Orange G (OG). In addition, MIL-88A was characterized via X-ray diffraction (XRD), scanning electronic microscopy (SEM) and Raman spectra as well as N2 isothermal adsorption. The effects of degradation reaction by MIL-88A synthesized in different preparation conditions (e.g., temperature, time) were also compared and analyzed. During the experiment based on effects of MIL-88A dosage, we found that MIL-88A was different from other MOFs because it had an optimum dosage: when higher or lower than the optimum dosage, the degradation effect decreased. Thus, we conducted experiments to identify the reasons for such optimal dosage. Meanwhile, effects of pH were also investigated. Regarding recycling, we used two methods to explore recycling efficiency. In order to prove that the MIL-88A/PS/OG degradation system reaction was dominated by heterogeneous factors, the filtrate degradation experiments were examined and possible mechanisms described.
2. Experimental
2.1 Reagents and chemicals
The commercially available dye OG, fumaric acid, sodium PS, sodium hydroxide and phenanthroline were obtained from Aladdin Chemical Reagent Co. Ltd, (Shanghai China). Iron chloride hexahydrate (FeCl3·6H2O), absolute ethanol, hydrochloric acid, hydroxylamine hydrochloride, acetic acid and ammonium acetate were purchased from Sinopharm Chemical Reagent Co. Ltd, (Beijing China). All above-mentioned reagents and chemicals were analytical grade and used without further purification. The D.I. water used in the experiments was purified by Millipore Milli-Q system.2.2 Synthesis of MIL-88A
According to a previously reported procedure,37 a typical hydrothermal synthesis was performed as follows: 0.9744 g (8.4 mmol) fumaric acid and 2.2722 g (8.4 mmol) FeCl3·6H2O were added to a beaker with 42 mL D.I. water. The mixed solutions was stirred for an hour at magnetic stirrers before it was placed into a 100 mL Teflon-lined steel autoclave, then heated at different temperatures (65 °C, 85 °C, 105 °C) for several times (2 h, 6 h, 12 h). Until drops to the room temperature, the precipitates were recovered by centrifugation at 9000 rpm for 10 min. The as-synthesized solids should be washed by ethanol and water repetitively in order to remove the extra fumaric acid which did not participate in reactions. And the final precipitates were dried in a vacuum oven at 100 °C for no less than 10 h after centrifugation.2.3 Characterization of MIL-88A
The crystal structures of MIL-88A were determined by a D8 Advance (Bruker AXS) X-ray diffractometer (XRD), with a copper target tube radiation (Cu Kα1) producing X-rays at a wavelength of 0.15418 nm. Materials were placed on a quartz plate and were scanned from 5° to 30° (2θ) at scan speed of 1.2 s every step with a scan step of 0.02°. The morphology was observed by scanning electron microscope (FESEM, Quanta 200, Holland). BET surface area of MIL-88A was measured using a surface area analyzer (ASAP 2020, Micromeritics) and Raman spectroscopy was recorded by Raman spectrometer (Lab-RAM Aramis, HORIBA JOBIN Yvon).2.4 Catalytic degradation experiment of OG
OG and sodium PS solution were prepared first. Next, given quantities of PS solution and water, along with a certain OG concentration were placed in an Erlenmeyer flask. The pH drops to 2.8 or so when PS is added to the mixed solution because PS is an acidic oxidant. In this experiment, pH was not adjusted except in cases of special instructions. Next, the catalyst MIL-88A was added to the flask and then subjected to room temperature and shaking at 200 rpm. The solution was sampled at regular intervals and immediately quenched with alcohol. The compounds to be analyzed must pass through the membrane (aperture, 0.45 μm) to remove solids that could affect results. Residual dye was analyzed via the UV-Vis spectrophotometry method (Tianmei, UV2310-II) at a maximum absorption wavelength (λmax = 478 nm). Experiments were repeated at least twice in each group. The MIL-88A used in all subsequent experiments was prepared in 65 °C/2 h except in special circumstances.3. Results and discussion
3.1 Characterization of MIL-88A
Fig. 1A shows the X-ray diffraction patterns of MIL-88A, which were produced by diverse synthesis temperatures and crystallization times of 65 °C/2 h, 85 °C/2 h, 105 °C/2 h, 65 °C/6 h and 65 °C/12 h, respectively. It can be seen that the XRD intensities of these five obtained samples were different, but the strong peak position was recorded at almost 2θ = 8°, 10.4° and 12.9°, respectively, which indicated that the phase type was basically identical, but there were differences in content. This was also revealed via SEM (Fig. 2). Results again proved that different preparation conditions (e.g., temperature, pressure, pH, time) for MIL-88A XRD patterns and crystallinity were influential.37,38 Because XRD patterns of MIL-88A exhibited great agreement with results of previous studies,33,37 the MIL-88A was considered to have been successfully synthesized. | ||
| Fig. 1 XRD patterns: (A) MIL-88A synthesized in different preparation conditions (above: after soaking in the water). (B) Before reaction and after reaction (65 °C/2 h). | ||
MIL-88A morphology was examined via SEM as shown in Fig. 2. Samples presented a standard hexagonal rod-like morphology from a single sample (Fig. 2F).32,33,37 Fig. 2A–C show the SEM of MIL-88A, which was synthesized in 65 °C/2 h, 65 °C/6 h and 65 °C/12 h, respectively. Crystal growth through time was observed, while MIL-88A morphology exhibited no significant change. Many of the MIL-88A samples were in growth stage when crystallization time was short (see Fig. 2A), and most samples were already in full-growth stage in the entire visual field when crystallization time was longer (see Fig. 2B and C). In brief, with increased crystallization time, MIL-88A size became larger. Comparing the SEM of MIL-88A obtained at 105 °C/2 h in Fig. 2D with that produced at 65 °C/2 h in Fig. 2A, it was apparent that not only the size of samples became larger as synthesis temperature increased, but the surface of samples was also cracked. High temperature damaged the crystal structure of MIL-88A; consequently this type of material is not suitable for synthesis at high temperatures.
We also evaluated the Raman spectra of MIL-88A as shown in Fig. 3. The spectra for MIL-88A synthesized in different conditions were similar except for Raman intensities. Although synthesis conditions varied, obtained samples were comprised of the same materials. In addition, the band at 1648 cm−1 is attributed to the symmetric vibration modes of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, which were obtained from fumaric acid (molecular formula: (COOH)CH
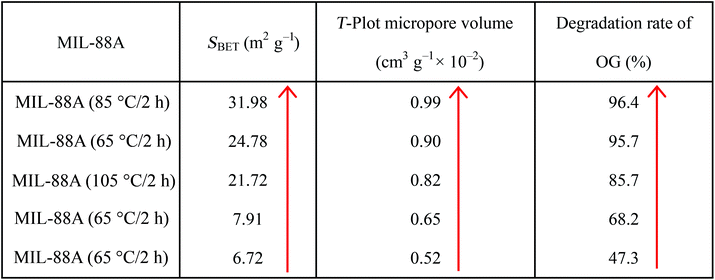
C, which were obtained from fumaric acid (molecular formula: (COOH)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(COOH)). Fig. 4 shows N2 sorption/desorption isotherms for MIL-88A prepared in 65 °C/2 h, which was considered to be a combination of the type II and III sorption isotherms. Results of BET surface area and pore volume of MIL-88A produced in different preparation conditions are displayed in Table 1. The pore size distribution (see inset in Fig. 4, BJH adsorption dV/dD pore volume) suggests that pores in MIL-88A are mesoporous with a dominant range from 5 to 80 nm and 15–18 nm account for the majority.
CH(COOH)). Fig. 4 shows N2 sorption/desorption isotherms for MIL-88A prepared in 65 °C/2 h, which was considered to be a combination of the type II and III sorption isotherms. Results of BET surface area and pore volume of MIL-88A produced in different preparation conditions are displayed in Table 1. The pore size distribution (see inset in Fig. 4, BJH adsorption dV/dD pore volume) suggests that pores in MIL-88A are mesoporous with a dominant range from 5 to 80 nm and 15–18 nm account for the majority.
3.2 OG removal by MIL-88A synthesized in diverse preparation conditions
Dosage of the catalyst MIL-88A used in this work was 0.3 g L−1, and the reaction performance of the obtained catalyst was investigated via degradation of OG. Fig. 5A exhibits the removal rates of OG as a function of time in oxidation at room temperature via MIL-88A, which was synthesized in varying preparation conditions. Before this, we also tested the adsorption of MIL-88A and removal efficiency by PS alone in the reaction system as comparisons. To this end, the MIL-88A was added to the OG solution without PS, and another experiment added PS alone. Results indicated that OG had no obvious decolorization even though MIL-88A was mixed with the reaction system for 1 h. The same results were obtained when PS was added alone, while the experiment that MIL-88A with PS was added to the OG solution together reflected a high degradation rate reaching 96%. It can draw a conclusion that MIL-88A almost had no adsorption to OG, but PS could be activated by MIL-88A to generate sulfate radicals for degrading OG. During this process, MIL-88A was also oxidized, and this could be proved by XRD showed in Fig. 1B and SEM in Fig. 2E. The intensity of XRD pattern from MIL-88A after reaction was much sharper than before reaction. And the SEM of MIL-88A after reaction showed that the surface had been corroded and oxidized by the MIL-88A/PS/OG system. So it could be speculated that with the increase of reaction cycles, the morphology of MIL-88A would be changed gradually.Fig. 5A also shows another important result that the preparation conditions of MIL-88A would have a great impact on its catalytic activity. Comparing a, b, c, d and e exhibited in Fig. 5A, the degradation rates of MIL-88A prepared in 65 °C/2 h, 65 °C/6 h, 65 °C/12 h, 85 °C/2 h and 105 °C/2 h were 95.7%, 68.2%, 47.3%, 96.4% and 85.7%, respectively, when the reaction continued for 2.5 h. The degradation ability of MIL-88A under investigation in removal of OG was found in the following order: 85 °C/2 h > 65 °C/2 h > 105 °C/2 h > 65 °C/6 h > 65 °C/12 h. It was obvious that preparation time and temperature will affect the catalytic activity of MIL-88A to a different extent. This phenomenon might be caused by BET surface area. Table 1 shows BET surface area, pore volume for the MIL-88A and OG degradation rate. Increased SBET led to increased MIL-88A activity, which indicates that the lower the SBET the lower the catalytic ability. Compared to other MOFs such as MIL-101 and MIL-53, the specific surface area of synthesized MIL-88A was relatively low;39 Horcajada et al.45 carried out a series of studies to prove that MIL-88 does not exhibit significant surface area (SBET < 30 m2 g−1). Even so, the OG removal rate along with SBET also displayed some regularity. Recently, Li et al.39 suggested that the greater the SBET, the higher the adsorption property of the adsorbent. Thus higher SBET not only benefits the adsorption property, but also catalytic performance of the contaminants. In addition, we measured the leach-out of Fe from MIL-88A synthesized in varying conditions as shown in Fig. 5B. A correlation between the OG removal rate and dissolved Fe content seemed to exist, as the higher the Fe leach-out, the greater the catalytic ability. Because the active site of MIL-88A was ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) and Fe3+, the difference of Fe content must make a difference in the OG removal rate. In other words, it was reasonable to suggest that Fe contents would also affect catalytic capability to some extent. Comparing XRD patterns of MIL-88A produced at different conditions after soaking in water (see Fig. 1A), little change was exhibited, but peak position and intensity continued to be consistent. Combined with the filtrate experiment (see Fig. 7A), it further proved that MIL-88A had relatively good stability and integrity in water. Excepting the relationship attributed to SBET and Fe, pore volume of the MIL-88A may be another cause that explains results. Catalysts with bigger pore volume (see Table 1) could have a stronger ability to attract greater quantities of PS molecules and retain them in pores, and then to activate them to generate sulfate radicals. In summary, higher SBET, a larger dissolved quantity of Fe and larger pore volume are likely the principal reasons to explain why MIL-88A synthesized at 85 °C/2 h was the most successful. Considering cost and degradation effects, the following experiments all point to MIL-88A synthesized in 65 °C/2 h.
Fe(III) and Fe3+, the difference of Fe content must make a difference in the OG removal rate. In other words, it was reasonable to suggest that Fe contents would also affect catalytic capability to some extent. Comparing XRD patterns of MIL-88A produced at different conditions after soaking in water (see Fig. 1A), little change was exhibited, but peak position and intensity continued to be consistent. Combined with the filtrate experiment (see Fig. 7A), it further proved that MIL-88A had relatively good stability and integrity in water. Excepting the relationship attributed to SBET and Fe, pore volume of the MIL-88A may be another cause that explains results. Catalysts with bigger pore volume (see Table 1) could have a stronger ability to attract greater quantities of PS molecules and retain them in pores, and then to activate them to generate sulfate radicals. In summary, higher SBET, a larger dissolved quantity of Fe and larger pore volume are likely the principal reasons to explain why MIL-88A synthesized at 85 °C/2 h was the most successful. Considering cost and degradation effects, the following experiments all point to MIL-88A synthesized in 65 °C/2 h.
3.3 Effect of fumaric acid and possible mechanism
There was no doubt that MIL-88A dosage was an important factor in activation reactions. Generally speaking, the greater the amount of MIL-88A catalyst added, the better the degradation effect.32 In this study, we also observed the unusual phenomenon of an optimal MIL-88A dosage for OG removal as shown in the inset of Fig. 6A. When the MIL-88A loading arrived at 0.3 g L−1, the degradation effect was the best; higher or lower dosages reduced removal efficiency. In order to determine the cause for this result, we conducted many experiments, and finally agreed that it must be caused by the fumaric acid existing in the MIL-88A/PS/OG system. First, as one of the raw materials in the preparation of MIL-88A, there was an excess of fumaric acid; it was also inevitable that a small amount of fumaric acid would continue to exist even though lots of water and ethanol were used to repeatedly wash the prepared solids. Second, in the MIL-88A/PS/OG system, pH dropped when PS was added; with the decrease in pH, some of the MIL-88A disintegrated because it had become more unstable under acidic conditions than neutral, and then fumaric acid content increased.To measure the dissolved quantity of fumaric acid, different dosages (0.02 g, 0.03 g and 0.05 g) of MIL-88A were stirred into the solution with different environments (pH = 2.8 and ambient pH). In this experiment, we added three different quantities of solids (0.02 g, 0.03 g and 0.05 g) to the water whose pH value should be adjusted to 2.8 to simulate the reaction system, and the ambient pH was considered for comparison. Then the fumaric acid concentration was measured via the method reported in the literature.40 As shown in Fig. 6A, with the increase in MIL-88A dosage, fumaric acid content rose, which indicates that the greater the dosages added, the greater the MIL-88A decomposition. Comparisons of pH = 2.8 and ambient pH at the same dosage conditions appear in Fig. 7B and 6A. The iron content detected in the acid solution was much larger than the aqueous, and it was similar to the MIL-88A/PS/OG system. The fumaric acid, detected in the acid solution, was almost twice that found in the aqueous solution; finally the curve leveled off. In summary, MIL-88A was partly dissolved in the acid solution as well as the MIL-88A/PS/OG system.
Fumaric acid dosages of 5 mg L−1, 10 mg L−1, 20 mg L−1 and 50 mg L−1 were selected to examine the effect of fumaric acid on OG removal. Corresponding decolorization efficiencies were displayed in Fig. 6B. As the dosage increased from 0 mg L−1 to 10 mg L−1, the decolorizing effect obviously improved. When the fumaric acid dosage exceeded 10 mg L−1, such as 20 mg L−1 and 50 mg L−1, the decolorizing rate rapidly declined. This phenomenon might be caused by the promotion (eqn (4)) and inhibition (eqn (5)) of fumaric acid.
Fumaric acid + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) → Fe(III) → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) +… Fe(II) +…
| (4) |
| Fumaric acid + SO4−˙ → SO42− +… | (5) |
Previous studies reported the reaction between S2O82− and SO4−˙.41,42 The most representative formula by Liu et al.41 describing the relationship of Fe(III)/Fe(II) on the surface of MIL-88A (see inset in Fig. 2F) follows:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) + S2O82− → Fe(III) + S2O82− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) + S2O8−˙ Fe(II) + S2O8−˙
| (6) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) + S2O82− → Fe(II) + S2O82− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) + SO4−˙ + SO42− Fe(III) + SO4−˙ + SO42−
| (7) |
Fe(III) on the surface of MIL-88A could be converted into Fe(II) quickly and then activated S2O82− to generate SO4−˙ (see eqn (6) and (7)). Fumaric acid in this degradation reaction had functions of a complexing agent like EDTA, which was investigated by Liang et al.43 in an experiment to determine whether Fe3+ combined with EDTA could degrade trichloroethylene. When the content of fumaric acid was at a low level, the reaction of eqn (4) was stronger than eqn (5). At this time, fumaric acid played an important role in accelerating Fe(III) turn into Fe(II) and improved reaction rate subsequently. While fumaric acid was at a higher concentration, eqn (5) presents an even greater advantage. By this time, fumaric acid as an organic competed SO4−˙ with OG and it must reduce the speed of OG decolorization. This could be used to explain why the greater the amount of MIL-88A added, the worse the results.
To study this experiment of whether the catalytic reaction was homogeneous or heterogeneous, filtrate experiments were conducted in the following steps. First, a certain amount of MIL-88A was added to the aqueous solution, which used sulfuric acid and sodium hydroxide to adjust pH to 2.8 to simulate reaction conditions. The solution was then placed in the shaker for 2.5 h at room temperature. Afterward, solids were separated from the solution and liquids remaining were used to degrade OG with PS. Another experiment in which pH was unregulated served as the comparison. As results show in Fig. 7A, when the reaction ran for 2.5 h, the acid and water soaking liquids exhibited obvious differences in OG degradation, with removal rates of 39.8% and 7.1%, respectively. It was verified again that part of the MIL-88A was decomposed under acidic conditions as well as the MIL-88A/PS/OG system. The Fe3+ dissolved from MIL-88A in the acidic environment should be the major factor responsible for this activation. The PS mechanism activated by Fe3+/Fe2+ in the MIL-88A/PS/OG system is also exhibited in eqn (8) and (9):32,41
| Fe3+ + S2O82− → Fe2+ + S2O8−˙ | (8) |
| Fe2+ + S2O82− → Fe3+ + SO4−˙ + SO42− | (9) |
In addition, Fig. 7B showed the dissolved quantity of iron. Whether the catalyst was soaked in acid conditions or in the reaction process of the MIL-88A/PS/OG system, the dissolved iron was about 10 mg L−1 and it was relatively small, so it led to a partly degraded rate of OG compared with catalysis of solid MIL-88A with a removal rate that reached 96%. In brief, the catalytic activation involved heterogeneous reactions on the MIL-88A surface and homogeneous reactions in solutions, but it should be pointed out that the heterogeneous reaction occupied the leading position. The probable mechanism that displayed the effect of fumaric acid and heterogeneous or homogeneous reaction was illustrated in Fig. 8.
3.4 Impact of pH on OG removal
The pH solution also played a significant role in this activation. pH of 2.8, 3.0, 4.0, 5.0, 7.0 and 9.0 were investigated in the MIL-88A/PS/OG system to compare the OG removal rate; results appear in Fig. 9. Before our study, Lin et al.32 indicated that MIL-88A could also effectively activate the PS at a higher pH. For example, when the solution pH increased to 9.0 and reaction temperature was raised to 40 °C, their removal efficiency of RB could still reach >50%. After we repeated this experiment many times, we determined that when the solution pH > 5.0, the OG removal rate was only about 25% at room temperature as the reaction continued for more than 4 h. Thus, it was reasonable to conclude that without the help of temperature, the reaction activated by MIL-88A only exhibits conspicuous removal efficiency in an acidic environment of pH < 4. It can be inferred that the main reason Lin et al.32 obtained such a high removal rate at an alkaline pH was because of the temperature,32 which contributed much to the activation reaction as shown in eqn (2).36 Moreover, as Fig. 9 shows, the final OG removal rate at pH = 2.8 was similar to pH = 3 when the reaction ran for 4.5 h. But while pH increased from 3 to 4, the degradation rate declined rapidly and reached about 40%. This phenomenon might result from the instability of PS, which decomposed by itself at a high pH. When at low pH values, PS stability was relatively stronger stability, which could further reduce the OG removal efficiency as reported by Guo et al.23 and Sun et al.44 | ||
Fig. 9 Impact of pH on removal of OG. Experimental conditions: [OG] = 0.1 mmol L−1, OG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS = 1 PS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, MIL-88A dosage = 0.3 g L−1, T = 25 °C. 60, MIL-88A dosage = 0.3 g L−1, T = 25 °C. | ||
3.5 Reusability of MIL-88A via different recycling methods
Considering catalytic materials such as MIL-88A, recyclability is an important measure to appraise its cost/practical value. Two typical methods had been tested to illustrate MIL-88A recyclability in this experiment. The first method consisted of adding OG directly when one reaction was finished and repeating this operation three times. In the second method, solids from the MIL-88A/PS/OG system that had finished the degradation reaction every time were separated, and the solids were then added to a new system. Results of different experimental operations appear in Fig. 10A and B. As seen in Fig. 10A, the final degradation rate had no significant difference after four recycles, but the removal rate at different cycles of the same period was changed. Taking the second point of every cycle, for example, and simulating the linear diagram in accordance with linear function (y = kx + b) is displayed in the inset of Fig. 10A. The parameter k for each cycle was 0.0127, 0.02335, 0.0187 and 0.0128, respectively, and ranked as follows: k2nd > k3rd > k4th > k1st. The removal rate of OG showed a trend of increasing from the first to second and then decreasing from second to fourth. This might be attributed to a large amount of SO4−˙ remaining when the first reaction was completed; afterward, once new pollutants OG were added to this MIL-88A/PS/OG system, the previously existing SO4−˙ would immediately combine with the OG and this contributed to a higher degradation efficiency. But later, with the PS decomposing constantly and the loss of MIL-88A in every sample as well as partial dissolution, the removal rate gradually declined. The inset of Fig. 7B shows the Fe leach-out during the recycle experiment. We found that the dissolved quantity of iron remained at a stable level when the recycle experiment was carried out, again indicating that MIL-88A would be partly dissolved in the MIL-88A/PS/OG system, which caused a decline in OG removal.Results of the second method shown in Fig. 10B implied that the degradation rates decreased with progressive cyclic numbers—95.7%, 75.3%, 54.3% in the first three cycles, respectively, and only 37.9% at the fourth recycling. This result must be caused by serious loss of active catalytic sites for Fe(III) from MIL-88A in the process of separation and sampling every time, which weakened the OG removal effect. Comparing these two methods, we found that the first method showed a better recycling effect than the second, which resulted in significant loss of MIL-88A. To some extent, MIL-88A could exhibit recyclability in activating PS to degrade the organics without additional renovation.
4. Conclusions
In brief, MIL-88A as a catalyst with advantages of high efficiency, low input, easy synthesis and environmental friendliness is of great interest for water treatment. In this study, the MIL-88A synthesized in diverse preparation conditions was characterized by various techniques including XRD, SEM, RAMAN and BET. A spectral analysis and comparison for the diverse MIL-88A was also given. Moreover, the catalyst MIL-88A with the change of preparation temperature and crystallization time exhibited a highly efficient degradation performance to OG at room temperature and it produced in 85 °C/2 h was the best one mainly due to the high SBET and much more leach-out of Fe. Unlike other MOFs, what's particular for MIL-88A was that there exists an optimal dosage (0.03 g) for the MIL-88A/PS/OG system because of the inhibition and promotion by fumaric acid. And then the effect of fumaric acid as one raw material of MIL-88A for removal of OG was considered, the dosage of fumaric acid less than 10 mg L−1 would advance the reaction, while the activity would be inhibited when it arrived at 20 mg L−1. Furthermore, the filtrate experiment had supported that the catalytic activation involved heterogeneous reaction on the surface of the catalyst and homogeneous reaction in solutions, but the heterogeneous reaction occupied the main position and the possible mechanism was also obtained. At the same time, the iron content which was detected at acid environment or in the system of MIL-88A/PS/OG were about 10 mg L−1 and it was relatively small. The effect of pH for the catalytic activity was also investigated, and the results showed that the lower the pH value was, the better the degrading effect was. Without the help of temperature, MIL-88A could hardly activate PS as pH was higher than neutral pH because of the self-discomposed of PS under this condition. What's more, two different methods for recycle experiment had been compared to illustrate the recyclability of MIL-88A. Unfortunately, whether through the first or the second method, MIL-88A ultimately exhibited a decline in OG removal, which was likely due to the loss of active catalytic sites of Fe(III) from MIL-88A. Therefore, it is important and necessary for further study to improve the stability of MIL-88A in PS solutions.Acknowledgements
This work was supported by National Natural Science Foundation of China (31570568, 31670585), State Key Laboratory of Pulp and Paper Engineering (201535), Guangdong High Level Talent Project (201339), Science and Technology Planning Project of Guangzhou City, China (201607010079, 201607020007), and Science and Technology Planning Project of Guangdong Province, China (2016A020221005).Notes and references
- Q. J. Yang, H. Choi, S. R. Al-Abed and D. D. Dionysiou, Iron cobalt mixed oxide nanocatalysts: heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications, Appl. Catal. B: Environ., 2009, 88, 462–469 CrossRef CAS
.
- H. Lin, H. Zhang and L. W. Hou, Degradation of C. I. acid orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process, J. Hazard. Mater., 2014, 276, 182–191 CrossRef CAS PubMed
.
- N. Stock and S. Biswas, Synthesis of Metal–Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites, Chem. Rev., 2011, 112, 933–969 CrossRef PubMed
.
- C. Janiak and J. K. Vieth, MOFs, MILs and more: concepts, properties and applications for porous coordination networks (PCNs), New J. Chem., 2010, 34, 2366–2388 RSC
.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, Metal–organic frameworks-prospective industrial applications, J. Mater. Chem., 2006, 16, 626–636 RSC
.
- J. R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H. K. Jeong, P. B. Balbuena and H. C. Zhou, Carbon dioxide capture-related gas adsorption and separation in metal–organic frameworks, Coord. Chem. Rev., 2011, 255, 1791–1823 CrossRef CAS
.
- J. W. Yoon, S. H. Jhung, Y. K. Hwang, S. M. Humphrey, P. T. Wood and J. S. Chang, Gas-Sorption Selectivity of CUK-1: A Porous Coordination Solid Made of Cobalt(II) and Pyridine-2,4-Dicarboxylic Acid, Adv. Mater., 2007, 19, 1830–1834 CrossRef CAS
.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Selective gas adsorption and separation in metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC
.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Metal–organic framework materials as catalysts, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC
.
- J. Gascon, A. Corma, F. Kapteijn and F. X. Llabrés i Xamena, Metal Organic Framework Catalysis: Quo vadis, ACS Catal., 2013, 361–378 Search PubMed
.
- A. Corma, H. García and F. X. Llabrés i Xamena, Engineering Metal Organic Frameworks for Heterogeneous Catalysis, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed
.
- L. G. Qiu, Z. Q. Li, Y. Wu, W. Wang, T. Xu and X. Jiang, Facile synthesis of nanocrystals of a microporous metal–organic framework by an ultrasonic method and selective sensing of organoamines, Chem. Commun., 2008, 3642–3644 RSC
.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur and R. Gref, Porous, metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed
.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Luminescent Functional Metal–Organic Frameworks, Chem. Rev., 2011, 112, 1126–1162 CrossRef PubMed
.
- M. Ranocchiari and J. A. V. Bokhoven, Catalysis by metal–organic frameworks: fundamentals and opportunities, Phys. Chem. Chem. Phys., 2011, 13, 6388–6396 RSC
.
- M. Zhao, S. Ou and C. D. Wu, Porous Metal–Organic Frameworks for Heterogeneous Biomimetic Catalysis, Acc. Chem. Res., 2014, 47, 1199–1207 CrossRef CAS PubMed
.
- Z. Hasan and S. H. Jhung, Removal of hazardous organics from water using metal–organic frameworks (MOFs): plausible mechanisms for selective adsorptions, J. Hazard. Mater., 2015, 283, 329–339 CrossRef CAS PubMed
.
- N. A. Khan, Z. Hasan and S. H. Jhung, Adsorptive removal of hazardous materials using metal–organic frameworks (MOFs): a review, J. Hazard. Mater., 2013, 244–245, 444–456 CrossRef CAS PubMed
.
- F. Ke, L. G. Qiu, Y. P. Yuan, F. M. Peng, X. Jiang, A. J. Xie, Y. H. Shen and J. F. Zhu, Thiol-functionalization of metal–organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water, J. Hazard. Mater., 2011, 196, 36–43 CrossRef CAS PubMed
.
- X. Zhao, D. Liu, H. Huang, W. Zhang, Q. Yang and C. Zhong, The stability and defluoridation performance of MOFs in fluoride solutions, Microporous Mesoporous Mater., 2014, 185, 72–78 CrossRef CAS
.
- L. Li, J. C. Li, Z. Rao, G. W. Song and B. Hu, Metal Organic Framework [Cu3(BTC)2(H2O)3] for the adsorption of methylene blue from aqueous solution, Desalin. Water Treat., 2013, 1–7 CrossRef
.
- J. Q. Jiang, C. X. Yang and X. P. Yan, Zeolitic Imidazolate Framework-8 for Fast Adsorption and Removal of Benzotriazoles from Aqueous Solution, ACS Appl. Mater. Interfaces, 2013, 5, 9837–9842 CAS
.
- W. Guo, S. Su, C. Yi and Z. Ma, Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system, Environ. Prog. Sustainable Energy, 2013, 32, 193–197 CrossRef CAS
.
- E. R. Bandala, M. A. Peláez, D. D. Dionysiou, S. Gelover, J. Garcia and D. Macías, Degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) using cobalt peroxymonosulfate in Fenton-like process, J. Photochem. Photobiol., A, 2007, 186, 357–363 CrossRef CAS
.
- P. K. Malik and S. K. Saha, Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst, Sep. Purif. Technol., 2003, 31, 241–250 CrossRef CAS
.
- G. P. Anipsitakis and D. D. Dionysiou, Transition metal/UV based advanced oxidation technologies for water decontamination, Appl. Catal., B, 2004, 54, 155–163 CrossRef CAS
.
- J. J. Du, Y. P. Yuan, J. X. Sun, F. M. Peng, X. Jiang, L. G. Qiu, A. J. Xie, Y. H. Shen and J. F. Zhu, New photocatalysts based on MIL-53 metal–organic frameworks for the decolorization of methylene blue dye, J. Hazard. Mater., 2011, 190, 945–951 CrossRef CAS PubMed
.
- L. H. Ai, C. Zhang, L. Li and J. Jiang, Iron terephthalate metal–organic framework: revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation, Appl. Catal., B, 2014, 148–149, 191–200 CrossRef CAS
.
- H. C. Liu, X. H. Ren and L. G. Chen, Synthesis and characterization of magnetic metal-organic framework for the adsorptive removal of rhodamine B from aqueous solution, J. Ind. Eng. Chem., 2016, 34, 278–285 CrossRef CAS
.
- S. Bhattacharjee, J. S. Choi, S. T. Yang, S. B. Choi, J. Kim and W. S. Ahn, Solvothermal Synthesis of Fe-MOF-74 and Its Catalytic Properties in Phenol Hydroxylation, J. Nanosci. Nanotechnol., 2010, 10, 135–141 CrossRef CAS PubMed
.
- T. A. Vu, G. H. Le, C. D. Dao, L. Q. Dang, K. T. Nguyen, P. T. Dang, H. T. K. Tran, Q. T. Duong, T. V. Nguyen and G. D. Lee, Isomorphous substitution of Cr by Fe in MIL-101 framework and its application as a novel heterogeneous photo-Fenton catalyst for reactive dye degradation, RSC Adv., 2014, 4, 41185–41194 RSC
.
- K.-Y. Andrew Lin, H. A. Chang and C. J. Hsu, Iron-based metal organic framework, MIL-88A, as a heterogeneous persulfate catalyst for decolorization of rhodamine B in water, RSC Adv., 2015, 5, 32520–32530 RSC
.
- W. T. Xu, L. Ma, F. Ke, F. M. Peng, G. S. Xu, Y. H. Shen, J. F. Zhu, L. G. Qiu and Y. P. Yuan, Metal-organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye, Dalton Trans., 2014, 43, 3792–3798 RSC
.
- S. Gokulakrishnan, P. Parakh and H. Prakash, Photodegradation of methyl orange and photoinactivation of bacteria by visible light activation of persulphate using a tris(2,20-bipyridyl) ruthenium(II) complex, Photochem. Photobiol. Sci., 2013, 12, 456–466 Search PubMed
.
- M. G. Antoniou, A. A. de la Cruz and D. D. Dionysiou, Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms, Appl. Catal., B, 2010, 96, 290–298 CrossRef CAS
.
- S. Bougie and J. S. Dube, Oxidation of dichlorobenzene isomers with the help of thermally activated sodium persulfate, J. Environ. Eng. Sci., 2007, 6, 397–407 CrossRef CAS
.
- T. Chalat, P. Horcajada, R. Gref, P. Couvreur and C. Serre, Optimisation of the synthesis of MOF nanoparticles made of flexible porousiron fumarate MIL-88A, J. Mater. Chem., 2011, 21, 2220–2227 RSC
.
- C. Mellot-Draznieks, C. Serre, S. Surblé, N. Audebrand and G. Férey, Very Large Swelling in Hybrid Frameworks: A Combined Computational and Powder Diffraction Study, J. Am. Chem. Soc., 2005, 127, 16273–16278 CrossRef CAS PubMed
.
- X. H. Li, W. L. Guo, Z. H. Liu, R. Q. Wang and H. Liu, Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation, Appl. Surf. Sci., 2016, 369, 130–136 CrossRef CAS
.
- C. Y. Gao, Y. W. Li and G. Y. Liang, The new method to determine the contents of fumaric acid by ultraviolet spectrophotometry fumaric acid, Chin. J. Anal. Lab., 2007, 12, 281–283 Search PubMed
.
- H. Liu, T. A. Bruton, F. M. Doyle and D. L. Sedlak, In Situ Chemical Oxidation of Contaminated Groundwater by Persulfate: Decomposition by Fe(III)- and Mn(IV)-Containing Oxides and Aquifer Materials, Environ. Sci. Technol., 2014, 48, 10330–10336 CrossRef CAS PubMed
.
- X. Y. Yu, Z. C. Bao and J. R. Barker, Free Radical Reactions Involving Cl˙, Cl2–˙ and SO4−˙ in the 248 nm Photolysis of Aqueous Solutions Containing S2O82− and Cl, J. Phys. Chem. A, 2003, 108, 295–308 CrossRef
.
- C. Liang, C. P. Liang and C. C. Chen, pH dependence of persulfate activation by EDTA/Fe(III) for degradation of trichloroethylene, J. Contam. Hydrol., 2009, 106, 173–182 CrossRef CAS PubMed
.
- J. Sun, X. Li, J. Feng and X. Tian, Oxone/Co2+ oxidation as an advanced oxidation process: comparison with traditional Fenton oxidation for treatment of landfill leachate, Water Res., 2009, 43, 4363–4369 CrossRef CAS PubMed
.
- P. Horcajada, F. Salles, S. Wuttke, T. Devic, D. Heurtaux, G. Maurin, A. Vimont, M. Daturi, O. David, E. Magnier, N. Stock, Y. Filinchuk, D. Popov, C. Riekel, G. Ferey and C. Serre, How Linker's Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88, J. Am. Chem. Soc., 2011, 133, 17839–17847 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |