Surface modification of polymers with bis(arylcarbene)s from bis(aryldiazomethane)s: preparation, dyeing and characterization†
Abstract
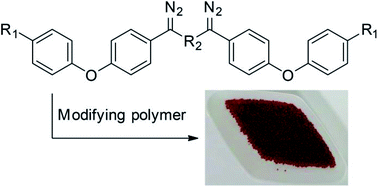
Modification of polymer beads by bis(arylcarbene) insertion provides access to materials with similar bulk properties, but different surface chemical characteristics, compared to the unmodified polymer. A subsequent dyeing process using different diazonium salts generates colored polymers with a variety of surface functional groups. XPS and solid state NMR spectra were used to characterize modified and dyed polymers, which showed this protocol was both successful and general. BET data showed that the surface area changed significantly after modification, while BJH data showed pore size distribution was unchanged. TG/DSC analysis and Elemental Analysis were also used to characterize modified polymers. This permitted calculation and comparison of the loadings of surface area and the modification effects with different chemical structure of bis(arylcarbene)s. This work shows that the bis(arylcarbene) system is as effective as mono(arylcarbene)s, but of significance since the starting bis(aryldiazomethane)s are more easily accessible and easier to handle than the mono(aryldiazomethane)s. All this data indicates that the surface property of polymers is modified.

 Please wait while we load your content...
Please wait while we load your content...