In-water facile synthesis of poly-substituted 6-arylamino pyridines and 2-pyrrolidone derivatives using tetragonal nano-ZrO2 as reusable catalyst†
Arijit Saha ,
Soumen Payra and
Subhash Banerjee
,
Soumen Payra and
Subhash Banerjee *
*
Department of Chemistry, Guru Ghasidas Vishwavidyalaya, Bilaspur-495009, Chhattisgarh, India. E-mail: ocsb2009@yahoo.com; Fax: +91 7752 260148; Tel: +91 7587 401979
First published on 14th October 2016
Abstract
Here, we report a facile synthetic protocol to access a series of ethyl 5-cyano-2-methyl-4-aryl-6-(arylamino)nicotinates and ethyl 4-hydroxy-2-(4-nitroaryl)-5-oxo-1-aryl-2,5-dihydro-1H-pyrrole-3-carboxylates using tetragonal nano-ZrO2 as reusable catalyst. It was observed that the ZrO2 nanoparticles were stable during reaction and apparently no leaching of Zr-content occurred when the catalyst was recycled eight times.
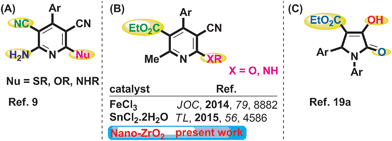
Poly-substituted pyridine moieties have emerged as one of the favoured medicinal scaffolds as they have found wide pharmaceutical applications and constitute a core entity in various natural products.1a–c These moieties also act as anti-prions,2a anti-hepatitis B virus,2b anti-bacterial,2c and anti-cancer2d agents. In addition, these pyridine scaffolds have revealed potential drug action against Parkinson's disease, hypoxia, asthma, kidney disease, epilepsy, cancer, and Creutzfeldt–Jakob disease.3 As a consequence, a number of methods have been developed for constructing functionalized pyridine derivatives.4,5 Among others, recently, one-pot multicomponent reactions (MCRs) have emerged as effective tools for the synthesis of these moieties from the perspective of green synthesis.4,5 This is because the MCRs offer rapid and direct access to a combinatorial library of highly convergent and functionalized bioactive organic scaffolds, e.g. pyridine derivatives, without producing unwanted by-products.6–8 Poly-substituted pyridines of general structure (A) (Fig. 1) were obtained via one-pot reactions of aldehyde, two molecules of malononitrile and nucleophile, NuH (Nu = S, O, NH) through a nucleophilic addition/intramolecular cyclization process.9 On the other hand, pyridines of diverse functionality of general structure (B) (Fig. 1) have recently been synthesized by the reactions of aldehyde, ethyl acetoacetate, malononitrile and a nucleophile (RXH; X = O, NH). Among these, the synthesis of pyridines (B) (Fig. 1) has rarely been reported and only two methods are present in the literature.10,11 He et al. have furnished a new method to access poly-substituted pyridine derivatives via nucleophilic addition/intermolecular cyclization catalysed by FeCl3 in ethanol medium.10 Reddy et al. have employed SnCl2·2H2O for the same reaction.11 Nevertheless, both the protocols were associated with long reaction time (6–10 h), and produced moderate to good yields (45–90%), and the catalysts were not reusable.
Functionalized 2-pyrrolidinone derivatives are of enormous significance as these moieties exhibit several important biological and pharmacological activities like anti-cancer,12 anti-tumour,13 HIV-1 integrase inhibitor,14 anti-microbial,15 anti-bacterial16 and anti-inflammatory.17 Over the years, several reports have been published for the synthesis of these important compounds.18 Recently, Ahankar et al. have reported a new protocol leading to 2-pyrrolidinones (Fig. 1C) via three-component condensation of aryl aldehyde, aryl amine and diethyl acetylenedicarboxylate catalysed by citric acid in ethanol under ultrasonic irradiation.19a As a part of our continuous interest in nano-catalysis,20 we have explored the excellent catalytic activity of ZrO2 nanoparticles (NPs) in MCRs.20g,i Here, a facile green protocol for the synthesis of poly-substituted pyridines (B) and 2-pyrrolidinone derivatives (C) by using reusable t-ZrO2 NPs in aqueous ethanol (Scheme 1) is reported.
 | ||
| Scheme 1 Tetragonal nano-ZrO2-catalysed synthesis of functionalized pyridine and 2-pyrrolidinone derivatives. | ||
Results & discussion
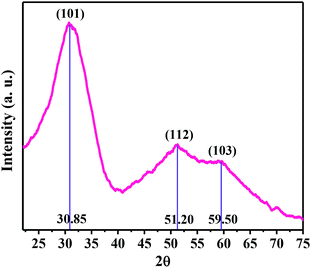
Primarily, tetragonal nano-ZrO2 was prepared via a sol–gel method involving the condensation of ZrO2Cl2·8H2O under basic conditions followed by the calcination of powder at 500 °C following our previously reported protocol20g (see ESI 1† for details). The formation of nano-ZrO2 with pure tetragonal phase was evident from the powder X-ray diffraction (XRD) and transmission electron microscopy (TEM) studies.The diffraction peaks at 2θ = 30.85, 51.20 and 59.50 in the powder XRD pattern (Fig. 2) of nano-ZrO2 are assigned to the {101}, {112} and {211} reflection planes and are in good agreement with pure tetragonal phase (JCPDS card no. 79-1771). The lattice fringes with lattice spacing 0.292 nm are associated with the {111} plane of t-ZrO2 (JCPDS card no. 17-0923), which also confirms the presence of a single tetragonal phase. The diffraction peaks, angles and the lattice spacing resemble previously reported data.20g,21 Peaks related to cubic or monoclinic phase are not detected in the powder XRD pattern. The broadening of diffraction peaks was due to the formation of small t-ZrO2 particles. The average particle size of the as-prepared NPs was evaluated to be 14 nm from the powder XRD pattern using the Scherrer formula.22
The TEM study revealed the formation of spherical ZrO2 NPs (Fig. 3a and b). The magnified high resolution TEM (HRTEM) image in Fig. 3c shows the lattice fringes with lattice spacing 0.292 nm associated with the {111} plane of t-ZrO2 (JCPDS card no. 17-0923), which also confirms the presence of single tetragonal phase.21b The selected electron diffraction (SAED) pattern (Fig. 3d) exposes the existence of t-ZrO2 NPs.21b Energy-dispersive X-ray analysis (EDX) through HRTEM shows that no impurity is present in the sample (Fig. 3e). The average size of the t-ZrO2 NPs was measured to be 13.6 nm from size distribution study (Fig. 3f) by HRTEM over 100 grains.
 | ||
| Fig. 3 (a) HRTEM image. (b) Magnified HRTEM image. (c) Showing lattice fringes with lattice spacing. (d) SAED pattern. (e) HRTEM-EDX. (f) Size distribution by HRTEM of t-ZrO2 NPs. | ||
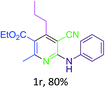
Next, the catalytic activity of as-prepared t-ZrO2 NPs was investigated in a four-component reaction for the synthesis of poly-substituted pyridines (B, Fig. 1). When a mixture of benzaldehyde (1 mmol), malononitrile (1 mmol), aniline (1 mmol), ethyl acetoacetate (1 mmol) and t-ZrO2 NPs (12 mg, 10 mol%) was heated at 80 °C in ethanol (4 ml), a moderate yield of ethyl 5-cyano-2-methyl-4-phenyl-6-(phenylamino)nicotinate (75%) was obtained after 2 h (entry 2, Table 1). To optimize the reaction conditions, we performed several reactions. The reaction did not run in absence of the catalyst (entry 1, Table 1). The rate of reaction was accelerated markedly in ethanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and produced an excellent yield (91%) of product (1a) in 2 h (entry 3, Table 1). However, at room temperature the same reaction produced only 40% desired product even after 4 h (entry 4, Table 1). The ZrO2 NPs were found to be less active in organic solvents like toluene, tetrahydrofuran (THF) and dimethyl formamide (DMF) (entries 5–7, Table 1). Next, investigating the effect of phase of ZrO2 on the reactivity, we observed that the t-ZrO2 NPs were superior to monoclinic (m-ZrO2) and cubic (c-ZrO2) ones (entries 8 and 9 Table 1). In addition, the t-ZrO2 NPs were able to produce better yields of product 1a (Table 2) than other oxide NPs such as Fe3O4, SiO2, CuO, NiO and ZnO (entries 10–14, Table 1). When only 5 mol% catalyst was used, the yield was decreased to 80% (entry 15, Table 1). Thus, the reaction using 10 mol% of t-ZrO2 NPs in ethanol–water (1
1) mixture and produced an excellent yield (91%) of product (1a) in 2 h (entry 3, Table 1). However, at room temperature the same reaction produced only 40% desired product even after 4 h (entry 4, Table 1). The ZrO2 NPs were found to be less active in organic solvents like toluene, tetrahydrofuran (THF) and dimethyl formamide (DMF) (entries 5–7, Table 1). Next, investigating the effect of phase of ZrO2 on the reactivity, we observed that the t-ZrO2 NPs were superior to monoclinic (m-ZrO2) and cubic (c-ZrO2) ones (entries 8 and 9 Table 1). In addition, the t-ZrO2 NPs were able to produce better yields of product 1a (Table 2) than other oxide NPs such as Fe3O4, SiO2, CuO, NiO and ZnO (entries 10–14, Table 1). When only 5 mol% catalyst was used, the yield was decreased to 80% (entry 15, Table 1). Thus, the reaction using 10 mol% of t-ZrO2 NPs in ethanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture at 80 °C was considered to have the optimized reaction conditions for the synthesis of poly-substituted pyridine derivatives.
1) mixture at 80 °C was considered to have the optimized reaction conditions for the synthesis of poly-substituted pyridine derivatives.
| Entry | Catalyst | Solvent/cond. | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), malononitrile (1 mmol), aniline (1 mmol), ethyl acetoacetate (1 mmol), catalyst (10 mol%), solvent (5 ml) with continuous stirring.b Isolated yield.c 5 mol% catalyst was used. RT means room temperature. | ||||
| 1 | No catalyst | EtOH/80 °C | 4 | — |
| 2 | t-ZrO2 NPs | EtOH/80 °C | 2 | 75 |
| 3 | t-ZrO2 NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 91 |
| 4 | t-ZrO2 NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/RT 1)/RT |
4 | 40 |
| 5 | t-ZrO2 NPs | Toluene/80 °C | 2 | 68 |
| 6 | t-ZrO2 NPs | THF/80 °C | 2 | 59 |
| 7 | t-ZrO2 NPs | DMF/80 °C | 2 | 60 |
| 8 | Bulk-ZrO2 | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 53 |
| 9 | m-ZrO2 NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 61 |
| 10 | Fe3O4 NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 79 |
| 11 | SiO2 NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 52 |
| 12 | CuO NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 71 |
| 13 | NiO NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 62 |
| 14 | ZnO NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 67 |
| 15 | t-ZrO2 NPs | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/80 °C 1)/80 °C |
2 | 80c |
a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), amine (1 mmol), ethyl acetoacetate (1 mmol), ZrO2 NPs (10 mol%), ethanol–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5 ml) with continuous stirring at 80 °C for 2 h. 1) (5 ml) with continuous stirring at 80 °C for 2 h. |
||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
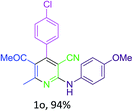
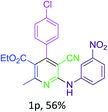
Next, utilizing these optimized conditions and the general experimental procedure (see ESI 2† for detail experimental procedure), we examined the scope of the methodology for the synthesis of pyridine derivatives. We observed that the t-ZrO2 NPs were able to furnish excellent yields (85–94%) of ethyl 5-cyano-2-methyl-4-aryl-6-(arylamino)nicotinates (1a–o, Table 2) by the condensation/cyclization of a variety of substituted aryl aldehydes and aryl amines with malononitrile and ethyl acetoacetate within a practical time period of 2 h. The results are summarized in Table 2. Aryl aldehydes containing both electron-donating (e.g. –OH, –OMe, –CH3, –Cl, –Br etc.) and electron-withdrawing (e.g. –NO2) groups participated smoothly in the reaction without any electronic effect, while in the case of aryl amines, the presence of electron-donating groups (e.g. –OMe, –CH3, –Cl, –Br) increased the product yield but an electron-withdrawing group (e.g. –NO2) in the aryl amine decreased the reaction rate as well as the product yield. The observations are listed in Table 2.
All the prepared pyridine derivatives are solid and were purified by recrystallization from hot ethanol. Thus, the present protocol does not require column chromatography. The compounds were identified by melting point determination followed by nuclear magnetic resonance (1H NMR and 13C NMR) spectroscopic studies. The NMR spectra are provided in ESI 5.†
To show the advantages of the present protocol, a comparison of present and previously reported methods is given in Table 3, which reveals the efficacy of the t-ZrO2 catalyst over the reported FeCl3 and SnCl2 catalysts in terms of yields, reaction times and recyclability.
The t-ZrO2 NPs showed better activity than others examined here. This is possibly due to the Lewis acid–base character and active surface of the material. The Lewis acid–base nature of ZrO2 is well known in the literature20g,23 and is due to the presence of active hydroxyl, oxide and Zr4+. In our previous reports, we have already proved the Lewis acid–base nature of the t-ZrO2 NPs.20g Here also, we studied the IR-based adsorption studies and we observed that both phenol and pyridine were adsorbed on the surface of the ZrO2 NPs, which clearly signifies the presence of acidic and basic sites (Fig. S2 and S3 in ESI-5†). It has also been reported that t-ZrO2 NPs contain more acidic and basic sites than c-ZrO2 and are more active.20g,23 In addition, we observed that in aqueous ethanol medium ZrO2 NPs showed basic character (pH = 8.1).
Next, we investigated the reusability of t-ZrO2 nano-catalyst for the synthesis of ethyl 5-cyano-2-methyl-4-phenyl-6-(phenylamino)nicotinate (1a, Table 2) as a model reaction. After reaction, t-ZrO2 NPs were separated from the reaction mixture by centrifugation, washed successively with ethanol and water, dried and reused for the subsequent run. We observed that tetragonal phase catalyst remained intact after the eighth cycle (confirmed by powder XRD; see Fig. S1, ESI-4†), with minimal loss (∼9%) in yield of isolated product at the eighth run (Fig. 4a). The TEM image of the reused t-ZrO2 NPs (Fig. 4b) indicates that the spherical morphology of particles remained intact even after the eighth cycle. The slight decrease in yield may possibly be due to loss of surface hydroxyl groups,20g decrease of oxygen vacancies20i or agglomeration of the nanoparticles.
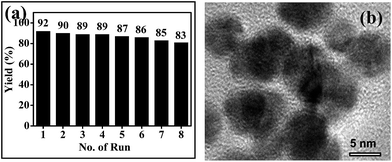 | ||
| Fig. 4 (a) Reusability of t-ZrO2 NPs for the synthesis of pyridine derivative (1a, Table 1) and (b) HRTEM image of t-ZrO2 NPs after the eighth reuse. | ||
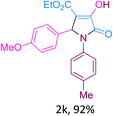
Inspired by the above synthetic results, next we extended the scope of t-ZrO2 NP-catalysed MCRs in the synthesis of bio-active 2-pyrrolidinone moieties by the reactions of aryl aldehyde, aryl amine and diethyl acetylenedicarboxylate. Thus, when a mixture of benzaldehyde (1 mmol), aniline (1 mmol) and diethyl acetylenedicarboxylate (1 mmol) was stirred at room temperature in the presence of 10 mol% t-ZrO2 in ethanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
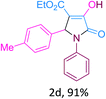
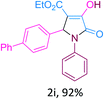
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by extraction and recrystallization, we obtained 92% yield of product, identified as ethyl 4-hydroxy-2-(4-nitrophenyl)-5-oxo-1-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate (2a, Table 4). In a simple experimental procedure using t-ZrO2 NPs, we have synthesized a variety of 2-pyrrolidone derivatives (2a-I, Table 4).
1), followed by extraction and recrystallization, we obtained 92% yield of product, identified as ethyl 4-hydroxy-2-(4-nitrophenyl)-5-oxo-1-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate (2a, Table 4). In a simple experimental procedure using t-ZrO2 NPs, we have synthesized a variety of 2-pyrrolidone derivatives (2a-I, Table 4).
a Reaction conditions: aryl aldehyde (1 mmol), aryl amine (1 mmol), diethyl acetylenedicarboxylate (1 mmol), ZrO2 NPs (10 mol%), ethanol–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5 ml), with continuous stirring at room temperature. 1) (5 ml), with continuous stirring at room temperature. |
||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
The detailed experimental procedure for the above syntheses is provided in ESI-3.† These reactions were very fast (5–12 min.) and high yielding (87–95%). Moreover, this protocol does not require any external energy source such as ultrasonic irradiation.
A comparative study for the synthesis of 2-pyrrolidinone moieties, presented in Table 5, clearly indicates that the present method using t-ZrO2 NPs is a better alternative to the existing protocols.19
Finally, a leaching study by hot filtration test was executed to examine the stability of t-ZrO2 in the synthesis of ethyl 5-cyano-2-methyl-4-phenyl-6-(phenylamino)nicotinate (1a, Table 2). The catalyst was removed from the reaction mixture after 45 minutes by ultracentrifugation under hot conditions and the remaining filtrate was further stirred for up to 2 h under similar conditions. It was observed that a negligible amount (16%) of desired product (1a, Table 1) was formed after removal of the catalyst at 45 minutes, without further improvement of yield. The results are depicted in Fig. 5, and prove that the t-ZrO2 NPs were highly stable during reaction and apparently no leaching of Zr-content occurred.
 | ||
| Fig. 5 Catalyst leaching study by hot filtration test performed with: (a) complete run (green line) and (b) catalyst removed after 45 min (red line). For the synthesis of pyridine derivative 1a (Table 2). | ||
Conclusions
In conclusion, we have demonstrated a simple and efficient method for the synthesis of ethyl 5-cyano-2-methyl-4-aryl-6-(arylamino)nicotinate and ethyl 4-hydroxy-2-(4-nitroaryl)-5-oxo-1-aryl-2,5-dihydro-1H-pyrrole-3-carboxylate using t-ZrO2 NPs. All the reactions listed in Tables 2 and 4 are very clean and high yielding (85–94%). Moreover, a significant acceleration of reaction rate (2 h) was observed for the synthesis of pyridines (1a–o, Table 2) compared with reported methods (6–10 h).10,11 The comparative results of present and previously reported methods for the synthesis of pyridines (B, Fig. 1) and 2-pyrrolidones (C, Fig. 1) presented in Tables 3 and 5 demonstrate a significant improvement in terms of reaction time, yields and greenness. Additionally, use of mild t-ZrO2 NPs as catalyst, ethanol–water mixture as solvent, simple isolation and purification of products without using column chromatography, and recycling of catalyst make the present synthetic methodology more convenient and environmentally friendly in nature.Acknowledgements
We are pleased to acknowledge the funding agency Department of Science and Technology, New Delhi, Govt of India (No.·SB/FT/CS-023/2012). AS and SP also thank Guru Ghasidas Vishwavidyalaya for their fellowship. Special thanks to Prof. B. C. Ranu and his group for their help in NMR studies.Notes and references
- (a) X.-Y. Xin, D.-P. Wang, X.-C. Li and B.-S. Wan, Tetrahedron, 2013, 69, 10245 CrossRef CAS; (b) D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC; (c) S. Lee, B. A. Rao and Y.-A. Son, Sens. Actuators, B, 2014, 196, 388 CrossRef CAS.
- (a) V. Perrier, A. C. Wallace, K. Kaneko, J. Safar, S. B. Prusiner and F. E. Cohen, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 6073 CrossRef CAS PubMed; (b) H. Chen, W. Zhang, R. Tam and A. K. Raney, PCT Int. Appl. WO 2005058315A120050630, 2005; (c) S. B. Levy, M. N. Alekshun, B. L. Podlogar, K. Ohemeng, A. K. Verma, T. Warchol, B. Bhatia, T. Bowser and M. Grier, US Pat. Appl. 2005124678A120050609, 2005; (d) D. R. Anderson, N. W. Stehle, S. A. Kolodziej and E. J. Reinhard, PCT Int. Appl. WO 2004055015A1 20040701, 2004.
- (a) T. R. K. Reddy, R. Mutter, W. Heal, K. Guo, V. J. Gillet, S. Pratt and B. J. Chen, Med. Chem., 2006, 49, 607 CrossRef CAS PubMed; (b) B. B. Fredholm, A. P. Ijzerman, K. A. Jacobson, K. N. Klotz and J. Linden, Pharmacol. Rev., 2001, 53, 527 CAS; (c) K. Guo, R. Mutter, W. Heal, T. R. K. Reddy, H. Cope, S. Pratt, M. J. Thompson and B. Chen, Eur. J. Med. Chem., 2008, 43, 93 CrossRef CAS PubMed.
- (a) A. E. J. Chichibabin, J. Russ. Phys.-Chem. Soc., 1906, 37, 1229 Search PubMed; (b) W. Zecher and F. Kröhnke, Chem. Ber., 1961, 94, 690 CrossRef CAS; (c) F. Kröhnke and W. Zecher, Angew. Chem., 1962, 74, 811 CrossRef; (d) D. L. Boger and J. S. J. Panek, J. Org. Chem., 1981, 46, 2179 CrossRef CAS; (e) A. Hantzsch, Justus Liebigs Ann. Chem., 1882, 215, 1 CrossRef; (f) T. R. Reddy, G. R. Reddy, L. S. Reddy, S. Jammula, Y. Lingappa, R. Kapavarapu, C. L. T. Meda, K. V. L. Parsa and M. Pal, Eur. J. Med. Chem., 2012, 48, 265 CrossRef PubMed; (g) M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, Bioorg. Med. Chem., 2007, 15, 1859 CrossRef CAS PubMed; (h) S. Banerjee and G. Sereda, Tetrahedron Lett., 2009, 50, 6959 CrossRef CAS.
- (a) W. J. W. Watson, Green Chem., 2012, 14, 251 RSC; (b) D. J. C. Constable, C. Jimenez-Gonzalez and R. K. Henderson, Org. Process Res. Dev., 2007, 11, 133 CrossRef CAS; (c) G. P. Taber, D. M. Pfistere and J. U. Colberg, Org. Process Res. Dev., 2004, 8, 385 CrossRef CAS; (d) C. Jimenez-Gonzalez, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Clean Technol. Environ. Policy, 2005, 7, 42 CrossRef CAS; (e) L. Weber, Curr. Opin. Chem. Biol., 2000, 4, 295 CrossRef CAS PubMed.
- (a) P. T. Anastas and J. C. Warner, Green chemistry: theory and practice, Oxford University Press, New York, 1998 Search PubMed; (b) R. S. Varma, in Green chemistry: challenging perspectives, ed. P. Tundo and P. T. Anastas, Oxford University Press, Oxford, 2000, p. 221 Search PubMed.
- A. M. Balu, B. Baruwati, E. Serrano, J. Cot, J. Garcia-Martinez, R. S. Varma and R. Luque, Green Chem., 2011, 13, 2750 RSC.
- (a) J. Ramón and Y. Miguel, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef PubMed; (b) M. B. Gawande, V. D. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC; (c) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC; (d) Y. Gu, Green Chem., 2012, 14, 2091 RSC; (e) M. B. Gawande, V. D. B. Bonifácio, R. S. Varma, I. D. Nogueira, N. Bundaleski, C. A. A. Ghumman, O. M. N. D. Teodoro and P. S. Branco, Green Chem., 2013, 15, 1226 RSC.
- (a) B. C. Ranu, R. Jana and S. Sowmiah, J. Org. Chem., 2007, 72, 3152 CrossRef CAS PubMed; (b) K. Guo, R. Mutter, W. Heal, T. R. K. Reddy, H. Cope, S. Pratt, M. J. Thompson and B. Chen, Eur. J. Med. Chem., 2008, 43, 93 CrossRef CAS PubMed; (c) M. Sridhar, B. C. Ramanaiah, C. Narsaiah, B. Mahesh, M. Kumaraswamy, K. K. R. Mallu, V. M. Ankathi and P. S. Rao, Tetrahedron Lett., 2009, 50, 3897 CrossRef CAS; (d) K. N. Singh and S. K. Singh, ARKIVOC, 2009, xiii, 153 Search PubMed; (e) R. Mamgain, R. Singh and D. S. Rawat, J. Heterocycl. Chem., 2009, 46, 69 CrossRef CAS; (f) P. V. Shinde, S. S. Sonar, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2010, 51, 1309 CrossRef CAS; (g) M. R. P. Heravi and F. Fakhr, Tetrahedron Lett., 2011, 52, 6779 CrossRef; (h) M. L. Kantam, K. Mahendar and S. Bhargava, J. Chem. Sci., 2010, 122, 63 CrossRef CAS; (i) M. Thimmaiah, P. Li, S. Regati, B. Chen and J. C.-G. Zhao, Tetrahedron Lett., 2012, 53, 4870 CrossRef CAS PubMed; (j) J. B. Gujar, M. A. Chaudhari, D. S. Kawade and M. S. Shingare, Tetrahedron Lett., 2014, 55, 6939 CrossRef CAS; (k) S. Maddila, S. N. Maddila, S. B. Jonnalagadda and P. Lavanya, J. Heterocycl. Chem., 2016, 53, 658 CrossRef CAS; (l) S.-J. Tu, T.-J. Li, S.-L. Zhu, X. Zou and Q. Wang, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2005, 61, o1883 CAS; (m) V. Raghukumar, D. Thirumalai, V. T. Ramakrishnan, V. Karunakarac and P. Ramamurthy, Tetrahedron, 2003, 59, 3761 CrossRef CAS; (n) S. Sarkar, D. K. Das and A. T. Khan, RSC Adv., 2014, 4, 53752 RSC; (o) J. Huang, J. Zhou, S. Song, H. Song, Z. Chen and W. Yi, Tetrahedron, 2015, 71, 8628 CrossRef CAS.
- X. He, Y. Shang, Z. Yu, M. Fang, Y. Zhou, G. Han and F. Wu, J. Org. Chem., 2014, 79, 8882 CrossRef CAS PubMed.
- D. N. K. Reddy, K. B. Chandrasekhar, Y. S. S. Ganesh, B. S. Kumar, R. Adepu and M. Pal, Tetrahedron Lett., 2015, 56, 4586 CrossRef CAS.
- (a) T. Michael, A. Michael, T. Andreas, H. Ulrich, B. Mirko and N. A. Johannes, Patent WO 2008055945 (A1), 2008; (b) V. O. Koz'minykh, N. M. Igidov, S. S. Zykova, V. E. Kolla, N. S. Shuklina and T. Odegova, Pharm. Chem. J., 2002, 36, 188 CrossRef.
- Y. Geng, X. Wang, L. Yang, H. Sun, Y. Wang, Y. Zhao, R. She, M.-X. Wang, D.-X. Wang and J. Tang, PLoS One, 2015, 10, 1 Search PubMed.
- (a) K. Ma, P. Wang, W. Fu, X. Wan, L. Zhou, Y. Chu and D. Ye, Bioorg. Med. Chem. Lett., 2011, 21, 6724 CrossRef CAS PubMed; (b) A. Pendri, T. L. Troyer, M. J. Sofia, M. A. Walker, B. N. Naidu, J. Banville, N. A. Meanwell, I. Dicker, Z. Lin, M. Krystal and S. W. Gerritz, J. Comb. Chem., 2010, 12, 84 CrossRef CAS PubMed.
- V. L. Gein, M. N. Armisheva, N. A. Rassudikhina, M. I. Vakhrin and E. V. Voronina, Pharm. Chem. J., 2011, 45, 162 CrossRef CAS.
- V. L. Gein, V. A. Mihalev, N. N. Kasimova, E. V. Voronina, M. I. Vakhrin and E. B. Babushkina, Pharm. Chem. J., 2007, 41, 208 CrossRef CAS.
- V. L. Gein, V. V. Yushkov, N. N. Kasimova, N. S. Shuklina, Y. M. Vasil'eva and M. V. Gubanova, Pharm. Chem. J., 2005, 39, 484 CrossRef CAS.
- (a) M. S. F. Franco, G. A. Casagrande, C. Raminelli, S. Moura, M. Rossatto, F. H. Quina, C. M. P. Pereira, A. F. C. Flores and L. Pizzuti, Synth. Commun., 2015, 45, 692 CrossRef CAS; (b) M. Astada and Sh.-I. Hashimoto, Tetrahedron Lett., 1998, 39, 79 CrossRef; (c) D.-R. Choi, K.-Y. Lee, Y.-S. Chung, J.-E. Joo, Y.-H. Kim, Ch.-Y. Oh, Y.-S. Lee and W.-H. Ham, Arch. Pharm. Res., 2005, 28, 151 CrossRef CAS PubMed; (d) L. E. Burgess and A. I. Meyers, J. Org. Chem., 1992, 57, 1656 CrossRef CAS; (e) L. E. Overman and T. P. Remarchuk, J. Am. Chem. Soc., 2002, 124, 12 CrossRef CAS PubMed; (f) V. Singh, R. Saxena and S. Batra, J. Org. Chem., 2005, 70, 353 CrossRef CAS PubMed; (g) R. Sarkar and C. Mukhopadhyay, Tetrahedron Lett., 2013, 54, 3706 CrossRef CAS; (h) J. Sun, Q. Wu, E.-Y. Xia and Ch.-G. Yan, Eur. J. Org. Chem., 2011, 2981 CrossRef CAS; (i) Q. Zhu, H. Jiang, J. Li, Sh. Liu, Ch. Xia and M. Zhang, J. Comb. Chem., 2009, 11, 685 CrossRef CAS PubMed; (j) Q. Zhu, L. Gao, Z. Chen, S. Zheng, H. Shu, J. Li, H. Jiang and S. Liu, Eur. J. Med. Chem., 2012, 54, 232 CrossRef CAS PubMed.
- (a) H. Ahankar, A. Ramazani, K. Ślepokura, T. Lis and S. W. Joo, Green Chem., 2016, 18, 3582 RSC; (b) J. Sun, Q. Wu, E.-Y. Xia and C.-G. Yan, Eur. J. Org. Chem., 2011, 2981 CrossRef CAS; (c) A. M. Zonouz, I. Eskandari and B. Notash, Synth. Commun., 2015, 45, 2115 CrossRef.
- (a) S. Banerjee and S. Santra, Tetrahedron Lett., 2009, 50, 2037 CrossRef CAS; (b) S. Banerjee, J. Das, R. Alverez and S. Santra, New J. Chem., 2010, 34, 302 RSC; (c) S. Banerjee, V. Balasanthiran, R. Koodali and G. Sereda, Org. Biomol. Chem., 2010, 8, 4316 RSC; (d) S. Banerjee, A. Horn, H. Khatri and G. Sereda, Tetrahedron Lett., 2011, 52, 1878 CrossRef CAS; (e) S. Banerjee and A. Saha, New J. Chem., 2013, 37, 4170 RSC; (f) S. Banerjee, S. Payra, A. Saha and G. Sereda, Tetrahedron Lett., 2014, 55, 5515 CrossRef CAS; (g) A. Saha, S. Payra and S. Banerjee, Green Chem., 2015, 17, 2859 RSC; (h) S. Banerjee, New J. Chem., 2015, 39, 5350 RSC; (i) A. Saha, S. Payra and S. Banerjee, RSC Adv., 2015, 5, 101664 RSC; (j) A. Saha, S. Payra, S. K. Verma, M. Mandal, S. Thareja and S. Banerjee, RSC Adv., 2015, 5, 100978 RSC; (k) S. Payra, A. Saha and S. Banerjee, RSC Adv., 2016, 6, 12402 RSC; (l) S. Payra, A. Saha, S. Guchhait and S. Banerjee, RSC Adv., 2016, 6, 33462 RSC; (m) S. Payra, A. Saha and S. Banerjee, RSC Adv., 2016, 6, 52495 RSC.
- (a) R. Malakooti, H. Mahmoudi, R. Hosseinabadi, S. Petrov and A. Migliori, RSC Adv., 2013, 3, 22353 RSC; (b) P. Zhang, Y. Su, F. Teng, Y. He, C. Zhao, G. Zhang and E. Xie, CrystEngComm, 2014, 16, 1378 RSC.
- (a) P. Scherrer, Nachr. Ges. Wiss. Goettingen, Math.-Phys. Kl., 1918, 2, 98 Search PubMed; (b) A. Patterson, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- Y. Zhao, W. Li, M. Zhang and K. Tao, Catal. Commun., 2002, 3, 239 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedure for preparation of catalysts and synthesis of poly functionalized pyridine and 2-pyrrolidinone derivatives, NMR copy of the representative compounds. See DOI: 10.1039/c6ra24367c |
| This journal is © The Royal Society of Chemistry 2016 |