Graphene quantum dots: recent progress in preparation and fluorescence sensing applications
Shenghai Zhou
ad,
Hongbo Xuad,
Wei Gancd and
Qunhui Yuan
*bd
aCollege of Chemistry and Chemical Engineering, Hebei Normal University for Nationalities, Chengde 067000, China
bSchool of Materials Science and Engineering, Harbin Institute of Technology, Shenzhen 518055, China. E-mail: yuanqunhui@hitsz.edu.cn; Fax: +86-991-3838957; Tel: +86-991-3677875
cSchool of Natural Sciences and Humanities, Harbin Institute of Technology, Shenzhen 518055, China
dLaboratory of Environmental Science and Technology, The Xinjiang Technical Institute of Physics and Chemistry, Key Laboratory of Functional Materials and Devices for Special Environments, Chinese Academy of Sciences, Urumqi 830011, China
First published on 17th November 2016
Abstract
Fluorescent graphene quantum dots (GQDs) have attracted tremendous attention because of their unique 2D layered structure, large surface area, good water solubility, tunable fluorescence, high photostability, excellent biocompatibility and low toxicity, which make them promising candidates for applications in various fields. In this review, we summarize the latest progress in research on GQDs, focusing on their preparation via both top-down and bottom-up routes and application in fluorescence sensing of inorganic ions, organic molecules and biomaterials. This review provides insight into GQDs to inspire their further development, including their controllable preparation and use in a wider range of sensing applications, by the large community of researchers focusing on graphene.
1. Introduction
Graphene quantum dots (GQDs) consisting of two dimensional graphene sheets with lateral dimensions less than 100 nm in single, double or a few (3–10) layers have attracted tremendous attention.1,2 They are different from the spherical photoluminescent carbon nanodots within 10 nm in diameter, which are generally divided into carbon quantum dots (CQDs) with crystal lattices and carbon nanoparticles without crystalline structures.3 Compared with the above mentioned carbon nanodots, their cousin GQDs possess excellent physical and chemical properties, such as higher surface area, larger length-to-diameter ratio and better surface grafting via the π–π conjugated network because of their structural graphene layers. GQDs possess not only intriguing properties of the graphene,4–7 but also improved solubility and extended fluorescence compared with those of larger graphene sheets.2,8 Interestingly, as a new family member of the fluorescent materials, GQDs display many merits, such as lower toxicity, better resistance to photo bleaching and better biocompatibility, compared with commercial organic dyes and traditional semiconductor quantum dots (for example, CdS and CdTe).9 Therefore, fluorescent GQDs with unique structure and properties provide an unprecedented opportunity to improve the performance of organic light-emitting diodes, fuel cells, bioimaging and so on.8–11 Of particular interest is the recent finding that GQDs can be used as fluorescent sensing probes to detect inorganic ions, small organic molecules and large biomaterials.2,12 This is because (a) the high photostability of GQDs guarantees the stability of the fluorescence signal, which can ensure the accuracy of the detection results; (b) qualitative detection of an analyte can be easily achieved by simply monitoring the variation of the fluorescence intensity of GQDs without the need for expensive instruments, time-consuming operations and complicated sample pretreatment; and (c) their low toxicity and good biocompatibility make GQDs safe for humans and the environment. Therefore, GQD-based fluorescent probes are promising candidates for analyte detection, and many studies on this topic are emerging at present.Some noteworthy review articles focusing on the basic properties,1,8 fluorescence mechanism3 and applications of GQDs in energy, environmental and biological fields have already been published.1,2,8–10,12 In addition, reviews that mainly consider the preparation of GQDs emerged in 2012,8 2013,1,13 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 2015.2 However, the very rapid development of the GQD research field means that various new strategies for their preparation are continuously being published. Therefore, an updated review on the latest advances, especially those regarding the preparation of GQDs, is needed. Furthermore, with the development of various novel methods to prepare GQDs, they have also been used as fluorescent probes to detect numerous new targets. Based on these considerations, we mainly focus on recent progress in the field of GQDs in this review with an emphasis on the preparation of GQDs and their fluorescent sensing applications including simple detection of inorganic ions, small organic molecules and large biomaterials are briefly summarised. We hope this article will offer valuable insight to facilitate the investigation of GQDs and stimulate new ideas for further research.
9 and 2015.2 However, the very rapid development of the GQD research field means that various new strategies for their preparation are continuously being published. Therefore, an updated review on the latest advances, especially those regarding the preparation of GQDs, is needed. Furthermore, with the development of various novel methods to prepare GQDs, they have also been used as fluorescent probes to detect numerous new targets. Based on these considerations, we mainly focus on recent progress in the field of GQDs in this review with an emphasis on the preparation of GQDs and their fluorescent sensing applications including simple detection of inorganic ions, small organic molecules and large biomaterials are briefly summarised. We hope this article will offer valuable insight to facilitate the investigation of GQDs and stimulate new ideas for further research.
2. Synthesis of GQDs
Various novel methods to prepare fluorescent GQDs have been developed with the purpose of realising simple, effective, size-controllable or high-yield synthetic methods to obtain high-quality GQDs. Generally, the syntheses of GQDs can be divided into two main categories, top-down and bottom-up synthetic approaches, based on the relationship between the source and product.1,8 Top-down strategies involve directly cleaving bulk carbon materials into nanoscale GQDs via physical, chemical or electrochemical techniques.9,13 Conversely, bottom-up methods are growth strategies; namely, appropriate molecular precursors gradually grow into nanosized GQDs by applying external energy.2.1 Top-down routes
A variety of bulk carbon materials including graphite,14 graphene,15 carbon nanotubes (CNTs),16 coal,17 carbon nanofibers,18 carbon black,19 neem leaf derived carbon,20 carbon nano-onions,21 C60,22 activated carbon powder23 and, recently, rice husk-derived carbon,24 have been used as raw materials to prepare GQDs by top-down methods. Heteroatom-doped bulk carbon materials such as adenosine triphosphate-derived carbon containing N and P,25 metal–organic framework-derived carbon with N,26 porous polythiophene-derived carbon with S27 and fluorinated graphene oxide (FGO)28 have also been used to produce corresponding heteroatom-doped GQDs with excellent performance. These precursors are usually subjected to chemical/electrochemical oxidation, hydrothermal/solvothermal treatment, laser ablation method, microwave or ultrasound assisted treatment to obtain GQDs.1,8,29,30 Top-down strategies can achieve large-scale production of GQDs with numerous surface functional groups via relatively simple operations.8,9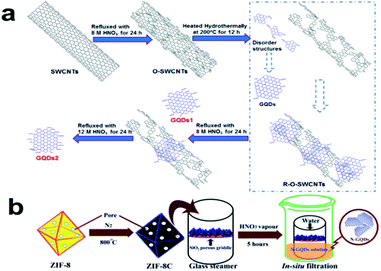 | ||
| Fig. 1 (a) Diagram outlining the preparation of two types of GQDs from SWCNTs. (Reprinted with permission from ref. 31. Copyright 2013 Elsevier.) (b) Schematic diagram of the preparation of N-GQDs. (Reprinted with permission from ref. 26. Copyright 2015 Royal Society of Chemistry.) | ||
Besides acidic oxidative reagents, powerful oxidants such as KMnO4 have been used to prepare GQDs. Kundu et al. chemically oxidized multi-walled carbon nanotubes (MWCNTs) in an acidic solution containing KMnO4 to prepare GQDs.16 The resultant GQDs were enriched with –COOH and –OH functional groups and possessed an average particle size of 12 nm within a size range of 10–15 nm. Although direct synthesis from bulk carbon materials using strong acids and powerful oxidants has advantages, a common problem is that it is difficult to thoroughly remove excess strong oxidant. In such a case, a complex purification process is needed, which can be inefficient. Thus, research on the preparation of GQDs using mild oxidants has recently appeared. For instance, Shin et al. heated several natural carbon resources (graphite, MWCNTs, carbon fiber (CF) and charcoal) at 200 °C in DMF using oxone as the oxidant, resulting in blue fluorescent GQDs.32 This novel acid-free approach does not require the neutralisation of strong acids, making it simple and eco-friendly.
Apparently, the current chemical oxidation methods used to prepare GQDs are chiefly based on chemical oxidation in the solution phase; namely, the oxidative reagent and bulk carbon material are usually in the same solution. Thus, these methods always need a large amount of oxidant and generally involve a tedious purification process. In this regard, we developed an efficient acid vapour cutting strategy to synthesize GQDs in which just 2 mL of concentrated HNO3 was used as “scissors” to cut metal–organic framework-derived carbon placed on a porous SiO2 grid without contact with HNO3 (see Fig. 1b).26 After the reaction, GQDs were easily and directly obtained by in situ filtration, while the unreacted residue was retained on the porous SiO2 grid. This method avoids complicated separation and purification processes and is low-cost, rapid and eco-friendly. Another group used the clean, mild oxidising agent H2O2 with the assistance of a tungsten oxide nanowire (W18O49) catalyst to oxidize and cleave graphene oxide (GO), leading to the formation of GQDs.33 The lateral size of the GQDs was controlled between 4 and 21 nm simply by changing the concentrations of W18O49 and H2O2. Furthermore, the reaction products were only H2O and GQDs, so the prepared GQD aqueous solution could be directly used for biological imaging without the need for further purification.
Regarding organic-phase synthesis of GQDs, Li and co-workers electrochemically cleaved a graphene film into N-doped GQDs using CV at a scan rate of 0.5 V s−1 in an organic electrolyte consisting of acetonitrile and nitrogen-rich tetrabutylammonium perchlorate.39 The as-prepared N-doped GQDs with a N/C atomic ratio of approximately 4.3% possessed diameters of around 2–5 nm and a typical topographic height of 1–2.5 nm (ca. 1–5 graphene layers). These GQDs remained homogeneous for a long period and exhibited electrocatalytic activity in the oxygen reduction reaction. Ananthanarayanan et al. prepared GQDs by electrooxidation of 3D porous graphene immersed in an organic electrolyte of 1-butyl-3-methylimidazolium hexafluorophosphate (BMIMPF6) and acetonitrile.37 The prepared GQDs possessed high crystallinity and uniform lateral size (∼3 nm) and thickness (mostly single-layered).
Interestingly, when adding chemicals with heteroatoms into the aqueous solutions, doped GQDs can be obtained by simple one-step hydrothermal treatment. Recently, Zhang and colleagues prepared a homogeneous solution containing GO, ammonia solution and powdered S.41 They transferred the mixture into an autoclave where it was heated at 180 °C for 12 h. After filtration and dialysis, N,S co-doped GQDs were obtained. By changing the amount of S added, the fluorescence intensity of the N,S-GQDs could be adjusted.
As an example of GQDs produced using a solvothermal route, Zhu et al. prepared GQDs with strong green fluorescence and high quantum yields of 11.4% by solvothermal treatment of GO (270 mg) in DMF (10 mL) at 200 °C for 5 h.42 The average diameter and height of the as-prepared GQDs were 5.3 and 1.2 nm, respectively, indicating that most of the GQDs were single- or bilayered.
 | ||
| Fig. 2 (a) Schematic representation of the preparation of blue and green GQDs. (Reprinted with permission from ref. 43. Copyright 2012 Wiley.) (b) Schematic illustration of a preparation strategy of GQDs. (Reprinted with permission from ref. 53. Copyright 2014 Wiley.) | ||
Recently, the microwave assisted method has also been used to prepare GQDs doped with elements such as F,28 B48 and N,F and S.47 For example, Sun et al. used FGO as a raw material to prepare fluorinated GQDs.28 In their approach, FGO was added to a solution of concentrated HNO3/H2SO4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then exposed to microwave irradiation for 6 h in a microwave oven operating at a power of 650 W. Kundu et al. reported a facile, rapid synthesis of GQDs co-doped with N,F and S by microwave-irradiation treatment of MWCNTs in the commercial ionic liquid (IL) (1-methyl-1-propylpiperidinium bis(trifluoromethylsulfonyl)imide).47 The researchers first dispersed MWCNTs in the IL by ultrasonic treatment for 1 h. The resulting mixture was heated in a 1100 W microwave for 15 min. After filtration and dialysis, N,F,S-GQDs with an average size of 2 nm and high quantum yield of 70% were obtained. The ultrafast preparation and heteroatom doping of GQDs were attributed to the use of a microwave assisted technique and an IL that contained heteroatoms.
1) and then exposed to microwave irradiation for 6 h in a microwave oven operating at a power of 650 W. Kundu et al. reported a facile, rapid synthesis of GQDs co-doped with N,F and S by microwave-irradiation treatment of MWCNTs in the commercial ionic liquid (IL) (1-methyl-1-propylpiperidinium bis(trifluoromethylsulfonyl)imide).47 The researchers first dispersed MWCNTs in the IL by ultrasonic treatment for 1 h. The resulting mixture was heated in a 1100 W microwave for 15 min. After filtration and dialysis, N,F,S-GQDs with an average size of 2 nm and high quantum yield of 70% were obtained. The ultrafast preparation and heteroatom doping of GQDs were attributed to the use of a microwave assisted technique and an IL that contained heteroatoms.
Recently, ultrasound assisted techniques have been used for the simple and mild synthesis of GQDs at room temperature. When a liquid is exposed to ultrasound, small vacuum bubbles constantly form and abruptly collapse, which results in high-speed liquid jets, deagglomeration and strong hydrodynamic shear forces that break down carbon materials.49 Zhuo and co-workers developed a direct, facile ultrasonic assisted method to prepare GQDs from graphene in aqueous solution in 2012.44 Since then, bulk carbon materials such as GO,50 carbon nanofibers,51 MWCNTs,52 and cheap graphite53 have been investigated as starting materials for the synthesis of GQDs using ultrasound in aqueous solution44,50,53 or organic solvents.51,54 Routh et al. reported a new method to synthesise GQDs from GO via a sono-Fenton reaction strategy; that is, the Fenton reaction under sonication conditions.50 In their strategy, GO was dissolved in deionised water, followed by the addition of H2O2 and FeCl3. After stirring, the mixture was sonicated for 4 h to form GQDs. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) of the resulting GQDs revealed their good dispersion, diameter of ∼5.6 ± 1.4 nm size and height of 0.2–5 nm. Song and colleagues prepared GQDs using graphite as a raw material with the aid of ultrasound (Fig. 2b).53 In their experiment, graphite and potassium sodium tartrate with a mass fraction ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were mixed and ground. The ground homogeneous mixture was heated in an autoclave at 250 °C for 24 h to prepare graphite intercalation compounds (GICs). After the formation of GICs, they were dispersed in water and sonicated for 1 h to prepare GQDs. The diameters of the GQDs were mainly distributed in the range of 1–5 nm and their heights were 0.5–1.5 nm. Interestingly, heteroatom-doped GQDs can also be fabricated by ultrasound-assisted methods. Zhao et al. prepared chlorine-doped GQDs (Cl-GQDs) by using chlorinated carbon fiber as a starting material in the organic solvent N-methylpyrrolidone with the help of ultrasound.51 CFs derived from degreasing cotton were chlorinated with HCl, resulting in the introduction of halogen and oxygen functional groups into the layered structure of the CFs. In this chlorination process, HCl acts not only as a Cl doping source but also exfoliates the CFs into small pieces. The chlorinated CFs were then added into N-methylpyrrolidone and sonicated for 10 h to prepare GQDs. The group found that the presence of Cl-GQDs substantially enhanced the performance of photovoltaic photodetectors.
10 were mixed and ground. The ground homogeneous mixture was heated in an autoclave at 250 °C for 24 h to prepare graphite intercalation compounds (GICs). After the formation of GICs, they were dispersed in water and sonicated for 1 h to prepare GQDs. The diameters of the GQDs were mainly distributed in the range of 1–5 nm and their heights were 0.5–1.5 nm. Interestingly, heteroatom-doped GQDs can also be fabricated by ultrasound-assisted methods. Zhao et al. prepared chlorine-doped GQDs (Cl-GQDs) by using chlorinated carbon fiber as a starting material in the organic solvent N-methylpyrrolidone with the help of ultrasound.51 CFs derived from degreasing cotton were chlorinated with HCl, resulting in the introduction of halogen and oxygen functional groups into the layered structure of the CFs. In this chlorination process, HCl acts not only as a Cl doping source but also exfoliates the CFs into small pieces. The chlorinated CFs were then added into N-methylpyrrolidone and sonicated for 10 h to prepare GQDs. The group found that the presence of Cl-GQDs substantially enhanced the performance of photovoltaic photodetectors.
As shown in Fig. 3, Zhu and co-workers have developed a novel, facile and eco-friendly magnetron sputtering method to prepare monodisperse GQDs directly from graphite.55 In their method, graphite and ZnO powder were first mixed uniformly and pressed into a composite target using a pressure of 30 MPa. The composite target was calcined at 1100 °C for 4 h in nitrogen to prepare a carbon source. Second, silicon plates were washed with acetone, methanol and water and then dried with a nitrogen stream to receive the GQDs. Third, magnetron sputtering was performed using a target-to-substrate distance of 100 mm, base pressure of 1 × 10−4 mTorr, working pressure of 0.55 mTorr, sputtering power of 40 W and deposition time of 30 min. Finally, the silicon plates with GQDs and ZnO were sonicated for 15 min in HCl to remove ZnO and obtain GQDs. The as-prepared GQDs possessed good dispersion and size uniformity with a diameter of 4–12 nm and thickness of 1–2 nm.
 | ||
| Fig. 3 Schematic diagram of one-step fabrication of a GQD/ZnO composite film via magnetron sputtering and subsequent isolation of GQDs through acid treatment and dialysis. (Reprinted with permission from ref. 55. Copyright 2015 Elsevier.) | ||
Xu et al. developed another approach to synthesise GQDs involving non-selective top-down cleavage of a bulk carbon precursor.56 Their approach of nanoreactor-confined synthesis and in situ filtration using a recyclable ordered mesoporous SiO2 (SBA-15) template produced GQDs that displayed yellow luminescence. An ordered mesoporous carbon replica of SiO2, namely C–SiO2, was first prepared by a nanocasting strategy in which SBA-15 was used as a template. Then, C–SiO2 powder was placed on a porous SiO2 grid and heated in an autoclave containing 2 mL of concentrated HNO3. After heating at 160 °C for 5 h, GQDs formed because the acid vapour cleaved the C–SiO2 composite in its confined mesopores. The large surface area and confined pores of the C–SiO2 nanocomposite resulted in the formation of GQDs with a narrow size distribution. Finally, the GQDs were obtained by in situ filtration. The as-prepared GQDs exhibited a relatively narrow size distribution ranging from 2.5 to 5.2 nm with an average diameter of ca. 3.6 nm and thickness of less than 2 nm.
2.2 Bottom-up routes
Recently, the number of bottom-up methods to prepare GQDs has been growing because these methods with good controllability can produce GQDs with well-defined sizes, shapes and properties. The main bottom-up methods include carbonisation of organic precursors,57–68 stepwise organic synthesis,69,70 chemical vapour deposition (CVD),71,72 and high-pressure and -temperature strategies.73–75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2
H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar) and keeping the same reaction time, they synthesised GQDs with different numbers of graphene and thickness-dependent visible photoluminescence.
Ar) and keeping the same reaction time, they synthesised GQDs with different numbers of graphene and thickness-dependent visible photoluminescence.Another group successfully prepared single-layered GQDs by hydrothermal treatment of citrate confined in 2D layered double hydroxides (LDHs).75 Citrate–intercalated Mg–Al-LDHs (Mg–Al–citrate-LDHs) were prepared by co-precipitation using Mg(NO3)2·6H2O, Al(NO3)3·9H2O, citrate and NaOH as raw materials. The solution of Mg–Al–citrate-LDHs and ammonia was reacted at 180 °C for 8 h in an autoclave, resulting in the formation of Mg–Al-GQD-LDHs. Finally, the GQDs were isolated by dissolving the Mg–Al-LDHs with HCl. The GQDs obtained by this strategy showed a small lateral size of 2.2 ± 0.2 nm and the average height of most GQDs (>90%) was 0.7 nm. Fig. 4 depicts the overall procedure to prepare GQDs using LDHs.
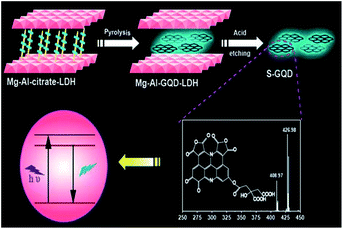 | ||
| Fig. 4 Schematic illustration of the formation of S-GQDs in the confined space of layered double hydroxides. (Reprinted with permission from ref. 75. Copyright 2015 Royal Society of Chemistry.) | ||
2.3 Quantum yield
Quantum yield (QY), an important factor for fluorescence sensing application, may vary with the synthesis method and the surface chemical environment of the GQDs, ranging from 2% to 86%.25,35,41,47,73,83,84 As shown in Table 1, the lowest QY of 2% is observed in GQDs prepared via stepwise organic synthesis while the highest QY of 86% for GQDs is obtained with a bottom-up process involving high pressure and temperature. Considering the rich structural oxygen-containing groups on the GQDs, the treatment containing reduction or surface passivation is generally to be applied to GQDs to deactivate those oxygen-containing groups in order to get higher QYs since the structural oxygen-containing groups are acting as the non-radiative electron–hole recombination centers.43,85 For example, Li et al.43 obtained greenish-yellow GQDs with QY of 11.7% by using microwave-assisted acidic oxidation strategy. Then the QY was further improved to 22.9% by a gentle reduction of GQDs with NaBH4. Tetsuka et al.85 synthesized amino-functionalized GQDs (af-GQDs) with high QY of 29% via hydrothermally cutting graphene sheets in ammonia solution. Subsequently, the af-GQDs were passivated by polyethylene glycol, which significantly enhanced the QY to 46%.| Methods | Sub classification | Quantum yield (QY) | Ref. |
|---|---|---|---|
| Top-down | Chemical oxidation | 27.5% | 25 |
| Top-down | Electrochemical exfoliation | 14% | 35 |
| Top-down | Hydrothermal/solvothermal treatment | 18.6% | 41 |
| Top-down | Microwave assisted method | 70% | 47 |
| Bottom-up | Carbonization of organic precursors | 78% | 83 |
| Bottom-up | Step organic synthesis of GQDs | 2% | 84 |
| Bottom-up | High pressure and high temperature | 86% | 73 |
3. Fluorescence sensing based on GQDs
The most important feature of GQDs is their extended optical performance compared with that of bulk graphene. Considerable effort has been devoted to developing the optical applications of GQDs, especially fluorescence-based applications. As novel fluorescent probes, GQDs are highly sensitive to minute perturbations, providing great potential for sensing applications. Meanwhile, the ultrasmall size, high photostability, low toxicity, good biocompatibility and excellent dispersion of GQDs result in improved detection sensitivity, stability, selectivity and security compared with traditional organic dyes and semiconductor quantum dots. To date, the detected analytes include inorganic ions,20,26,36,37,56,60,77,86–93 small organic molecules86,94–100 and large biomaterials101–106 based on either fluorescence turn-on or turn-off mechanisms. In this review, we focus on recent fluorescence sensing applications of GQDs.3.1 Detection of inorganic ions
GQDs have been widely used as fluorescence sensing probes to detect inorganic ions including metal cations20,26,36,37,56,60,77,86–92,95,107–111 and non-metallic anions.93,112,113Zhou et al. used pure GQDs exhibiting green fluorescence as a fluorescent probe for sensitive detection of Fe3+, achieving a lower detection limit of 5 × 10−9 M compared with those of reported Fe3+ probes, which include fluorescent nanographene, rhodamine 6G derivatives and 8-quinolinol (oxine) and quinazolinone derivatives.86 The GQDs also exhibited relatively high selectivity for Fe3+ with little interference from Cu2+, Fe2+ and Hg2+, which is caused by the specific coordination interaction between Fe3+ and the phenolic hydroxyl groups of GQDs. Our group developed a controllable top-down method to prepare novel GQDs displaying yellow fluorescence using mesoporous silica as a nanoreactor.56 The resulting GQDs with abundant oxygen-containing groups such as phenolic hydroxyl and carboxyl groups were used for highly selective determination of Fe3+ in tap water with satisfying recovery based on the fluorescence turn-off mechanism. The surface states of GQDs were modified with NaOH to investigate the quenching mechanism and high selectivity for Fe3+ of the GQDs. The NaOH-treated GQDs exhibited more noticeable quenching signals towards Cu2+, Co2+, Mn2+ and Ni2+ than other metal ions in neutral solution; in particular, a depressed response towards Fe3+ was observed. These results demonstrated that the high selectivity of the original GQDs for Fe3+ predominantly relied on the coordination between the phenolic hydroxyl groups of the GQDs and Fe3+.
Heteroatom doping can drastically alter the electronic characteristics of GQDs, thus leading to unusual fluorescent properties and novel applications. Li et al. electrochemically synthesised a S-GQD fluorescent probe with blue-green fluorescence that showed a more sensitive fluorescence response to Fe3+ than pure GQDs.36 This is because S doping tuned the electronic local density of GQDs, and thus promoted the coordination interaction between Fe3+ and phenolic hydroxyl groups on the surfaces of S-GQDs. This specific coordination interaction caused fluorescence quenching of the S-GQDs. The S-GQDs exhibited high selectivity towards Fe3+ with a low detection limit of 4.2 nM and linear range of 0–0.70 μM. Importantly, this novel fluorescent probe was successfully used for direct analysis of Fe3+ in human serum, indicating potential applications in clinical diagnosis. Fig. 5 illustrates the quenching mechanism of the S-GQDs and sensing performance of this Fe3+ sensor.
 | ||
| Fig. 5 (a) Fluorescence quenching mechanism of S-GQDs in the presence of Fe3+. (b) Electron transfer process from S-GQDs to Fe3+. (c) Fluorescence spectra of S-GQDs (5 μg mL−1) in the presence of different concentrations of Fe3+. The inset in (c) shows photographs of S-GQD aqueous solutions containing different concentrations of Fe3+ under UV irradiation (365 nm). (d) Fluorescence quenching values ΔF plotted against Fe3+ concentration. The inset in (d) is the linear calibration plot for Fe3+ detection. (Reprinted with permission from ref. 36. Copyright 2014 American Chemical Society.) | ||
Surface-functionalised GQDs have recently been used for the fluorescent determination of Fe3+. Ananthanarayanan et al. prepared GQDs functionalised with the IL BMIMPF6 that displayed blue fluorescence using an electrochemical cleavage method with the aid of BMIMPF6. The IL-functionalised GQDs were used for the optical detection of Fe3+ based on the quenching mechanism.37 The imidazole ring of BMIM+ both improved the dispersion of GQDs and endowed them with strong binding affinity for Fe3+. The constructed sensor exhibited good selectivity towards Fe3+ compared with Mg2+, Fe2+, Zn2+, Co2+, Ni2+, Cd2+ and K+, with a theoretical lower detection limit of ∼7.22 μM. More recently, Guo and co-workers used rhodamine B derivative (RBD)-functionalised GQDs (denoted RBD-GQDs) as an effective fluorescent probe for the sensitive detection of Fe3+ based on a rare fluorescent turn-on mechanism.87 The RBD-GQD probe displayed a detection limit as low as 0.02 μM in cancer stem cells. They found that the water solubility, sensitivity, photostability and biocompatibility of RBD were drastically improved when RBD was covalently linked to GQDs. The RBD-GQD-based fluorescent sensor also exhibited high selectivity for Fe3+ in comparison with Ca2+, Cd2+, Co2+, Cu2+, Hg2+, K+, Mg2+, Mn2+, Na+, NH4+, Ni2+, Pb2+, Zn2+ and Fe2+, with slight interference of Al3+.
Similar to the detection of Fe3+, pure GQDs,88,89 heteroatom-doped GQDs77 and surface-functionalized GQDs90,91 have been used to detect hazardous Hg2+. For example, Chakraborti et al. directly used pure GQDs as a fluorescent probe to develop a novel sensing platform for Hg2+ in aqueous solution based on the turn-off mechanism.88 High selectivity for Hg2+ was demonstrated by comparing the quenched fluorescence intensity of the GQD solution in the presence of only Hg2+ with that in the presence of other metal ions (Li+, Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cu2+, Zn2+, Al3+, Co2+, Ag+, Cr3+, Ni2+, Cd2+ and Pb2+) at the same concentration. The quenching mechanism was investigated using steady-state and time-resolved spectroscopy. The authors reported that the adsorption of Hg2+ onto the surface of the GQDs caused the electronic structure of the probe to change, which ultimately resulted in fluorescence quenching. The calculated detection limit for Hg2+ of this sensor was about 3.36 μM. Shi and colleagues used oxygen-rich nitrogen-doped GQDs (N-OGQDs) to develop an efficient fluorescent probe for the sensitive detection of Hg2+ in river water.77 The fluorescence quenching mechanism of the N-OGQDs was attributed to non-radiative electron transfer from the excited states of the N-OGQDs to the d orbital of Hg2+. This fluorescent sensor showed high sensitivity for Hg2+ with a low detection limit of 8.6 nM. It is worth noting that Pb2+, Cd2+, Cu2+, Ni2+ and Fe3+ all interfered with the detection of Hg2+ to a certain extent, so triethanolamine and sodium hexametaphosphate were added as chelating agents for Pb2+, Cd2+, Cu2+, Ni2+ and Fe3+. Recently, Tan's group achieved highly selective fluorescence detection of Hg2+ in HeLa cells based on the fluorescence quenching mechanism using a thymine-rich DNA-modified GQD (DNA-GQD) fluorescent probe.90 The developed Hg2+ sensor not only had an ultralow detection limit of 0.25 nM and relatively wide linear range of 1 nM to 10 μM, but also showed higher selectivity for Hg2+ than other metal ions, including Na+, K+, Li+, Ag+, Pb2+, Mg2+, Ni2+, Zn2+, Co2+, Cd2+, Cu2+, Mn2+, Ca2+ and Fe3+, at the same concentration. The excellent selectivity of the DNA-GQDs for Hg2+ was ascribed to the strong coordination of the spatially separated thymine bases with Hg2+. More importantly, they found that the unmodified GQDs displayed only a small fluorescence response towards Hg2+ compared with the DNA-GQD probe. This suggested that the fluorescent quenching mechanism involved the nonradiative electron transfer quenching of DNA-GQDs by Hg2+ binding to the thymine bases of DNA to form a T–T mismatch hairpin structure.
To detect Cu2+, Wang et al. utilised GQDs displaying blue fluorescence as a fluorescent probe.92 They achieved the successful detection of Cu2+ in water based on the quenching of GQD fluorescence by Cu2+. The sensor was very sensitive to Cu2+ compared with its sensitivity to other metal ions, including Fe3+, Al3+, Mn2+, Zn2+, Ca2+, Mg2+, Ag+, Ni2+, Co2+, Pb2+, Cd2+, Hg2+, Li+, Na+ and K+, and showed high selectivity. Furthermore, it also exhibited a linear range of 0–15 μM with a low detection limit of 0.226 μM. The authors also investigated the fluorescence quenching mechanism of this probe. They deemed that the quenching largely resulted from the formation of non-fluorescent complexes between Cu2+ and the oxygen-containing groups of the GQDs as electron donors. Qu's group reported an amino-functionalised GQD (afGQD) probe to sense Cu2+ based on the fluorescence turn-off mechanism.107 They found that the afGQDs displayed a larger fluorescence response towards Cu2+ than towards Al3+, Ag+, Co2+, Cd2+, Ni2+, Mg2+, Mn2+, Pb2+, Zn2+, Fe2+, Fe3+ and Hg2+. In contrast, pure GQDs had no selectivity for Cu2+ with non-negligible interference of Al3+, Co2+, Cd2+, Mn2+, Pb2+, Fe2+ and Fe3+. A possible reason for this may be that the introduction of amino groups increased the binding affinity of the afGQDS with N and O coexisting on their surface for Cu2+, increasing the chelation kinetics of Cu2+ compared with that of other metal ions. The constructed sensor showed a linear range of 0–100 nM with a detection limit of 6.9 nM and demonstrated fluorescence sensing of Cu2+ in living cells.
For the detection of Pb2+, Qian et al. developed a highly selective and sensitive fluorescent Pb2+ sensor based on aptamer-functionalized GQDs.109 In their study, the aptamer-functionalized GQDs fluorescent probe were firstly fabricated and then assembled on the surface of graphene oxide (GO) through electrostatic attraction and π–π stacking, resulting in a sequent fluorescence quenching due to a photoinduced electron transfer between GQDs and GO. The quantification of Pb2+ was achieved in the above aptamer/GQD/GO system through the liberation of aptamer–GQDs from GO triggered by the addition of Pb2+, leading to a recovery of fluorescence.
Similar to carbon quantum dots for highly selective detection of PPi,114 Lin et al. prepared N-GQDs with greenish-yellow fluorescence by hydrothermal treatment of lysine and GO (Fig. 6).112 An Eu3+-modulated N-GQD off–on fluorescent probe for PPi detection was constructed based on the following considerations: (a) Eu3+ can quench the fluorescence of N-GQDs by coordination of Eu3+ to the carboxyl and amido groups on the N-GQD surface; (b) PPi can recover the quenched fluorescence signal of N-GQDs by removing the Eu3+ because PPi possesses a higher affinity for Eu3+ than the carboxy groups of the N-GQDs. Other anions like F−, Cl−, Br−, I−, HCO3−, CO32−, Ac−, NO2−, S2− and PO43− did not recover the fluorescent signal, showing the good potential of this system for selective detection of PPi. The developed PPi sensor exhibited a linear range over 0.3–5 μM with a detection limit of 0.074 μM and successfully detected PPi in urine samples.
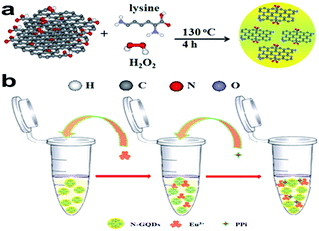 | ||
| Fig. 6 Schematic illustrations of (a) the preparation of N-GQDs and (b) the detection of PPi with Eu3+-modified N-GQDs. (Reprinted with permission from ref. 112. Copyright 2015 Royal Society of Chemistry.) | ||
3.2 Detection of small organic molecules using GQDs
Similar to carbon quantum dots for sensitive detection of organic molecules,78,80 monitoring small organic molecules with fluorescent GQDs has also become an interesting objective in recent years. Organic materials such as H2O2,86 ascorbic acid,94 bisphenol A,96 dihydroxybenzene,97 hydroquinone,98 2,4,6-trinitrotoluene,99 urea,100 melamine,115 glucose116 and 2,4,6-trinitrophenol (TNP)76 have been optically detected using fluorescent GQD probes based on a turn-on or turn-off fluorescence response.Inspired by their successful detection of metal ions, researchers have recently used GQDs as direct or indirect sensing probes to detect small organic molecules. As a direct sensing probe, the fluorescence of GQDs can be directly quenched or enhanced by specific organic molecules.76,116 For example, Lin et al. prepared N-GQDs displaying strong blue fluorescence that was directly quenched by TNP.76 Based on this discovery, the authors directly used N-GQDs as a probe to construct a TNP sensor that showed a linear range of 1–60 μM with a detection limit of 0.30 μM. The selectivity of the constructed TNP sensor was also investigated using structurally related aromatic materials (methylbenzene, phenol and nitrobenzene, 4-nitrotoluene, 2,4-dinitrotoluene, 2,4-dinitrophenol and 2,4,6-trinitrotoluene) and metal ions (Ca2+, Co2+, Ag+, Pb2+, Mn2+, Cd2+, Ba2+, K+, Na+, Al3+, Zn2+, Cu2+, Cr3+, Ni2+, Fe3+ and Hg2+) as interfering substances. The sensor displayed high selectivity towards TNP, with only small fluorescence responses towards Fe2+, Fe3+ and Hg2+, which were effectively eliminated by adding ethylenediaminetetraacetic acid. The researchers speculated that the fluorescence quenching of N-GQDs by TNP was caused by electron transfer and the formation of a non-fluorescent complex via the strong electrostatic interactions between N-GQDs and TNP.
Zhang and co-workers prepared B-GQDs with boronic acid groups on their surfaces.116 Interestingly, the fluorescence intensity of the B-GQDs increased linearly with glucose concentration in the range of 0.1–10 mM with a detection limit of 0.03 mM. As illustrated in Fig. 7, they postulated that the two cis-diol units in glucose can react with two boronic acid groups on the B-GQD surface to form structurally rigid B-GQD-glucose aggregates, restricting intramolecular rotation and thus resulting in enhanced fluorescence. The B-GQD sensor showed good selectivity for glucose compared with fructose, galactose and mannose. This is because fructose, galactose and mannose do not contain cis-diol units to enhance the fluorescence of B-GQDs by cross-linking-induced aggregation.
 | ||
| Fig. 7 (a) Schematic representation of boron-doped graphene quantum dots (BGQDs). (b) Proposed aggregation-induced photoluminescence (PL) mechanism of glucose sensing by BGQDs. (Reprinted with permission from ref. 116. Copyright 2014 American Chemical Society.) | ||
The fluorescence of GQDs can be quenched directly by the detected organic molecules with the aid of metal ions or oxidation agents.115 For instance, Zhu's group developed a rapid fluorescence sensing platform for melamine by direct fluorescence quenching of GQDs with predominantly aromatic sp2 domains in the presence of Hg2+.115 They speculated that after adding melamine into the GQD solution containing Hg2+, melamine first coordinated with Hg2+ through its nitrogen atoms (amine and triazine groups) and then Hg2+ interacted with the surface of GQDs through π–π stacking between the GQDs and melamine, thus leading to fluorescence quenching of the GQDs. The constructed melamine sensor exhibited a linear range of 0.15–20 μM with a detection limit of 0.12 μM and was successfully used for melamine detection in raw milk with satisfactory recovery. He and co-workers found that hydroquinone was oxidised to p-benzoquinone in the presence of dissolved oxygen with effective fluorescence quenching of GQDs.98 In their experiment, the GQDs served as a peroxidase-mimicking catalyst to oxidise hydroquinone to p-benzoquinone. The newly produced p-benzoquinone quenched the fluorescence of the GQD probe. The developed hydroquinone sensor had a good linear relationship in the range of 0.01–30 μM with a low detection of 5 nM. This novel sensor was successfully applied to hydroquinone detection in tap water, lake water and rain water samples. It should be pointed out catechol, dopamine and rutinum significantly interfered with hydroquinone detection.
As an indirect sensing probe, the fluorescence of GQDs can first be quenched by metal ions and then recovered by specific organic molecules.94,95 For example, Liu et al. developed a simple GQD-based fluorescence sensing system for rapid detection of ascorbic acid in the presence of Cu2+.94 Their system was based on the fluorescence quenching of GQDs by Cu2+ via efficient electron transfer between the GQDs and Cu2+. The addition of ascorbic acid allowed the fluorescence of the GQDs to be recovered by destroying the interaction between Cu2+ and the GQDs because ascorbic acid, as a strong reducing agent, could reduce Cu2+ to Cu+. The constructed sensor showed high selectivity for ascorbic acid compared with urine acid, citric acid, oxalic acid, dopamine, mannose, fructose, glucose, Na+, K+, Ca2+, Mg2+, Fe3+, Cl− and NO3−. Its linear range and detection limit were 0.3–10 μM and 94 nM, respectively. The sensor detected ascorbic acid in vitamin C samples with satisfactory results.
3.3 Detection of biomaterials using GQDs
The versatility of GQD-based fluorescent sensors is also evidenced by their ability to detect a range of biotargets including alkaline phosphatase,101 DNA,102–104 thrombin,105 protein kinase106 the mecA gene sequence,117 microRNAs,118 trypsin,119 phosphate-containing metabolites,120 heparin and chondroitin sulfate,121 acetylcholinesterase,122 cardiac marker antigen Troponin I (cTnI)123 and human immunoglobulin G.124 The most commonly used methods to detect biomaterials are based on specific fluorescence resonance energy transfer (FRET) between functional GQDs and a quencher. For example, Qian et al. developed an efficient fluorescence sensing platform for the selective and sensitive detection of DNA using GO as a quencher.103 As shown in Fig. 8a, they prepared fluorescent single-stranded DNA-functionalised GQDs (ssDNA-rGQDs) through a condensation reaction between connecting DNA (cDNA) and GQDs reduced by NaBH4. Fluorescence quenching of the ssDNA-rGQD probe occurred after the addition of GO quencher because of the adsorption of the ssDNA-rGQD probe on the GO surface through electrostatic attraction and π–π stacking interactions. The fluorescence of the probe was recovered by introducing target DNA (tDNA) into the testing solution containing ssDNA-rGQD and GO because the detachment of the dsDNA-rGQDs from GO, which were resulted from the hybridization between the ssDNA-rGQDs and tDNA. When tDNA was replaced with DNA with a single base mismatch, the recovered fluorescence intensity was much lower than that achieved using tDNA, indicating the high selectivity of the sensor for tDNA. The developed DNA sensor exhibited a wide linear range of 6.7–46.0 nM with a low detection limit of 75.0 pM. | ||
| Fig. 8 (a) Schematic illustration of a universal fluorescence sensing platform for the detection of DNA based on fluorescence resonance energy transfer (FRET) between graphene quantum dots (GQDs) and graphene oxide. (Reprinted with permission from ref. 103. Copyright 2014 Royal Society of Chemistry.) (b) Schematic illustration of a fluorescent biosensor for trypsin based on self-assembled GQDs. (Reprinted with permission from ref. 119. Copyright 2013 Royal Society of Chemistry.) | ||
More recently, Bhatnagar and co-workers used graphene as a quenching agent to construct a novel GQD-based fluorescent sensor to detect cardiac marker antigen cTnI in blood based on FRET.123 The detection of cTnl was accomplished by the following three steps: afGQDs were covalently conjugated with the antibody anti-cardiac Troponin I (anti-cTnI) to form a fluorescent anti-cTnl/afGQD nanoprobe. The anti-cTnl/afGQD probe was absorbed onto the surface of a graphene quencher through π–π stacking interactions, resulting in fluorescence quenching because of FRET between them. Finally, quantification of the target antigen cTnI was achieved by adding cTnI to the anti-cTnI/afGQD/graphene system to liberate anti-cTnI/afGQDs from graphene, leading to fluorescence recovery. Because of the strong interaction of cTnI with anti-cTnI, the constructed sensor showed high selectivity for cTnI compared with non-specific antigens. Detection of cTnI was realised over a wide range from 0.001 to 1000 ng mL−1 with a limit detection of 0.192 pg mL−1.
Besides graphene and GO, gold nanoparticles117 and CNTs102 have also been used as quenchers to detect biomaterials based on FRET. However, it should be noted that these GQDs required functionalization to prepare the sensors. Therefore, some novel label-free GQD-based fluorescent sensors for the detection of biomaterials have been developed. For example, Li et al. prepared a novel trypsin sensor based on the cytochrome c (Cyt c)-induced self-assembly of GQDs.119 Fig. 8b outlines the detection mechanism of the sensor. The fluorescence of the GQDs was first quenched by introducing an electron transfer protein (Cyt c) into the GQDs. The GQDs aggregated in the presence of Cyt c because of the electrostatic interaction between Cyt c and GQDs as well as the specific coordination interaction between Fe3+ in Cyt c and the phenolic hydroxyl groups of GQDs. The fluorescence of GQDs was then recovered by adding the target trypsin to the Cyt c/GQD system because the Cyt c quencher is cleaved by trypsin into smaller fragments (arginine and lysine residues) along with the reduction of Fe3+ and GQDs, leading to the restoration of fluorescence. The change of fluorescent intensity reflected the amount of trypsin added, providing a trypsin sensor with a low detection limit of 33 ng mL−1 and high selectivity for trypsin compared with bovine serum albumin, lysozyme, papain and pepsin. A similar GQD-based fluorescent sensor for heparin and chondroitin sulfate detection was also achieved by replacing Cyt c with dendrimer nanoparticles with multiple positive charges.121 Furthermore, with promising applications of CQDs in the detection of various enzymes such as acid phosphatase,125 alkaline phosphatase,126,127 tyrosinase,128 acetycholinesterase,129 hyaluronidase,130 human fetoprotein etc.,131 the detection of enzyme based on cousin GQDs may be expected in the future.
3.4 Fluorescence sensing mechanism
As previously mentioned, GQDs have been well applied as fluorescent probes for selective and sensitive detections of inorganic ions, small organic molecules and large biomaterials. Although there are various targets, the constructed fluorescence sensing platform was chiefly based on turn-off (fluorescence quenching) and turn-on (fluorescence recovering) fluorescence responses.92,94,103,107,120 As for the turn-off response, the fluorescence quenching of GQDs or functionalized GQDs by targets appears to be static quenching (complexation),92 dynamic quenching (collisional deactivation)120 or sometimes involving both static and dynamic quenching mechanisms,107 which usually can be analyzed based on the evaluation of fluorescence lifetime. For a turn-on fluorescence response, the mechanism for GQDs-based sensor is usually considered to contain two steps.94,103 Firstly, the fluorescence quenching of GQDs or functionalized GQD occurs because of the strong interaction between GQDs (or functionalized GQD) and quencher. Secondly, the pre-formed composite structure of GQDs and quencher is destroyed with the addition of targets, leading to the liberation of fluorescent GQDs into solution and the corresponding fluorescence recovery. However, the understanding on the fluorescence recovering process is still vague when GQD, quencher and target are in coexistence.4. Conclusion and outlook
In this review, we have summarised recent progress in fluorescent GQD research, focusing on their synthesis and fluorescence sensing applications for the detection of inorganic ions, small organic molecules and large biomaterials. Various approaches to prepare GQDs and construct fluorescent sensing platforms have been discussed in detail. Regarding the preparation of GQDs, both top-down and bottom-up methods have been developed that use hydrothermal/solvothermal, microwave, ultrasound, CVD and electrochemistry. Although numerous top-down and bottom-up methods have been developed, the exploration of the synthesis of high-quality GQDs with uniform lateral size and controllable layer number and surface composition is still ongoing. Nanoreactor-confined synthesis of GQDs based on either a top-down or bottom-up strategy provides GQDs with uniform lateral size and controllable layer number. Inspired by this, we expect that by using an appropriate nanoreactor and precursor, GQDs with a uniform structure may be obtained in the future. In particular, bottom-up methods rather than top-down approaches that involve nonselective cutting processes are anticipated to provide GQDs with desired structures. The unique planar structure, high surface area, tunable surface composition, high photostability, good biocompatibility and low toxicity of GQDs make them suitable for constructing a wide range of fluorescence sensing platforms for the sensitive detection of various targets based on turn-on and turn-off fluorescence responses. GQD-based fluorescent sensors show promise in detection of inorganic ions, small organic molecules and large biomaterials. The main issues facing GQD-based sensors are selectivity improvement and clear understanding of their fluorescence response mechanisms. Furthermore, GQDs with red-emitting fluorescence and high light efficiencies are hoped to be developed to produce GQDs for target detection, especially in biological system in future work. We hope this review stimulates continued interest and endeavour in the area of photoluminescent GQDs.Acknowledgements
The authors acknowledge financial support from the ‘1000 Talent Program’ (The Recruitment Program of Global Experts), the National Natural Science Foundation of China (21473247), the ‘Young Creative Sci-Tech Talents Cultivation Project’ (2013711012, 2013711016) and the ‘International Science and Technology Cooperation Project’ (20146003) of the Xinjiang Uyghur Autonomous Region.Notes and references
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J. J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- S. Benítez-Martínez and M. Valcárcel, TrAC, Trends Anal. Chem., 2015, 72, 93–113 CrossRef.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- J. Zhang, J. G. Yu, M. Jaroniec and J. R. Gong, Nano Lett., 2012, 12, 4584–4589 CrossRef CAS PubMed.
- G. C. Xie, K. Zhang, H. Fang, B. D. Guo, R. Z. Wang and H. Yan, Chem.–Asian J., 2013, 8, 2395–2400 CrossRef CAS PubMed.
- Q. Li, B. D. Guo, J. G. Yu, J. R. Ran, B. H. Zhang and H. J. Yan, J. Am. Chem. Soc., 2011, 133, 10878–10884 CrossRef CAS PubMed.
- P. P. Guo, F. Xiao, Q. Liu, H. F. Liu, Y. L. Guo and J. R. Gong, Sci. Rep., 2013, 3, 3499 Search PubMed.
- Z. Zhang, J. Zhang, N. Chen and L. Qu, Energy Environ. Sci., 2012, 5, 8869–8890 CAS.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS PubMed.
- Z. Wang, H. Zeng and L. Sun, J. Mater. Chem. C, 2015, 3, 1157–1165 RSC.
- Q. Liu, B. D. Guo, Z. Y. Rao, B. H. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- J. Ju and W. Chen, Curr. Org. Chem., 2015, 19, 1150–1162 CrossRef CAS.
- M. Bacon, S. J. Bradley and T. Nann, Part. Part. Syst. Charact., 2014, 31, 415–428 CrossRef CAS.
- W. Kwon, Y. H. Kim, C. L. Lee, M. Lee, H. C. Choi, T. W. Lee and S. W. Rhee, Nano Lett., 2014, 14, 1306–1311 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- S. Kundu, S. Ghosh, M. Fralaide, T. N. Narayanan, V. K. Pillai and S. Talapatra, Phys. Chem. Chem. Phys., 2015, 17, 24566–24569 RSC.
- R. Ye, C. Xiang, J. Lin, Z. Peng, K. Huang, Z. Yan, N. P. Cook, E. L. G. Samuel, C. C. Hwang, G. Ruan, G. Ceriotti, A. R. O. Raji, A. A. Martí and J. M. Tour, Nat. Commun., 2013, 4, 2943 Search PubMed.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemay, X. Zhang, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi and J. Zhu, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed.
- Z. Ding, Z. Hao, B. Meng, Z. Xie, J. Liu and L. Dai, Nano Energy, 2015, 15, 186–192 CrossRef CAS.
- A. Suryawanshi, M. Biswal, D. Mhamane, R. Gokhale, S. Patil, D. Guin and S. Ogale, Nanoscale, 2014, 6, 11664–11670 RSC.
- Y. Liu and D. Y. Kim, Chem. Commun., 2015, 51, 4176–4179 RSC.
- C. K. Chua, Z. Sofer, P. Šimek, O. Jankovský, K. Klímová, S. Bakardjieva, Š. H. Kučková and M. Pumera, ACS Nano, 2015, 9, 2548–2555 CrossRef CAS PubMed.
- T. L. Shao, G. D. Wang, X. T. An, S. J. Zhuo, Y. S. Xia and C. Q. Zhu, RSC Adv., 2014, 4, 47977–47981 RSC.
- Z. Wang, J. Yu, X. Zhang, N. Li, B. Liu, Y. Li, Y. Wang, W. Wang, Y. Li, L. Zhang, S. Dissanayake, S. L. Suib and L. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 1434–1439 CAS.
- A. Ananthanarayanan, Y. Wang, P. Routh, M. A. Sk, A. Than, M. Lin, J. Zhang, J. Chen, H. Sun and P. Chen, Nanoscale, 2015, 7, 8159–8165 RSC.
- H. Xu, S. Zhou, L. Xiao, H. Wang, S. Li and Q. Yuan, J. Mater. Chem. C, 2015, 3, 291–297 RSC.
- H. Xu, S. Zhou, L. Xiao, Q. Yuan and W. Gan, RSC Adv., 2016, 6, 36554–36560 RSC.
- H. Sun, H. Ji, E. Ju, Y. Guan, J. Ren and X. Qu, Chem.–Eur. J., 2015, 21, 3791–3797 CrossRef CAS PubMed.
- S. R. M. Santiago, T. N. Lin, C. T. Yuan, J. L. Shen, H. Y. Huang and C. A. J. Lin, Phys. Chem. Chem. Phys., 2016, 18, 22599–22605 RSC.
- P. Russo, R. Liang, E. Jabari, E. Marzbanrad, E. Toyserkani and Y. N. Zhou, Nanoscale, 2016, 8, 8863–8877 RSC.
- Y. Dong, H. Pang, S. Ren, C. Chen, Y. Chi and T. Yu, Carbon, 2013, 64, 245–251 CrossRef CAS.
- Y. Shin, J. Park, D. Hyun, J. Yang, J. H. Lee, J. H. Kim and H. Lee, Nanoscale, 2015, 7, 5633–5637 RSC.
- C. Zhu, S. Yang, G. Wang, R. Mo, P. He, J. Sun, Z. Di, Z. Kang, N. Yuan, J. Ding, G. Ding and X. Xie, J. Mater. Chem. B, 2015, 3, 6871–6876 RSC.
- D. B. Shinde and V. K. Pillai, Chem.–Eur. J., 2012, 18, 2522–2528 CrossRef PubMed.
- M. Zhang, L. Bai, W. Shang, W. Xie, H. Ma, Y. Fu, D. Fang, H. Sun, L. Fan, M. Han, C. Liu and S. Yang, J. Mater. Chem., 2012, 22, 7461–7467 RSC.
- S. Li, Y. Li, J. Cao, J. Zhu, L. Fan and X. Li, Anal. Chem., 2014, 86, 10201–10207 CrossRef CAS PubMed.
- A. Ananthanarayanan, X. Wang, P. Routh, B. Sana, S. Lim, D. H. Kim, K. H. Lim, J. Li and P. Chen, Adv. Funct. Mater., 2014, 24, 3021–3026 CrossRef CAS.
- X. Y. Tan, Y. C. Li, X. H. Li, S. X. Zhou, L. Z. Fan and S. H. Yang, Chem. Commun., 2015, 51, 2544–2546 RSC.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed.
- B. X. Zhang, H. Gao and X. L. Li, New J. Chem., 2014, 38, 4615–4621 RSC.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858–6860 RSC.
- L. L. Li, J. Ji, R. Fei, C. Z. Wang, Q. Lu, J. R. Zhang, L. P. Jiang and J. J. Zhu, Adv. Funct. Mater., 2012, 22, 2971–2979 CrossRef CAS.
- S. Zhuo, M. Shao and S. T. Lee, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- Y. Shin, J. Lee, J. Yang, J. Park, K. Lee, S. Kim, Y. Park and Y. Lee, Small, 2014, 10, 866–870 CrossRef CAS PubMed.
- C. C. Wang and S. Y. Lu, Nanoscale, 2015, 7, 1209–1215 RSC.
- S. Kundu, R. M. Yadav, T. N. Narayanan, M. V. Shelke, R. Vajtai, P. M. Ajayan and V. K. Pillai, Nanoscale, 2015, 7, 11515–11519 RSC.
- X. Hai, Q. X. Mao, W. J. Wang, X. F. Wang, X. W. Chen and J. H. Wang, J. Mater. Chem. B, 2015, 3, 9109–9114 RSC.
- H. Li, X. He, Y. Liu, H. Huang, S. Lian, S. T. Lee and Z. Kang, Carbon, 2011, 49, 605–609 CrossRef CAS.
- P. Routh, S. Das, A. Shit, P. Bairi, P. Das and A. K. Nandi, ACS Appl. Mater. Interfaces, 2013, 5, 12672–12680 CAS.
- J. Zhao, L. Tang, J. Xiang, R. Ji, Y. Hu, J. Yuan, J. Zhao, Y. Tai and Y. Cai, RSC Adv., 2015, 5, 29222–29229 RSC.
- L. Lin and S. Zhang, Chem. Commun., 2012, 48, 10177–10179 RSC.
- S. H. Song, M. H. Jang, J. Chung, S. H. Jin, B. H. Kim, S. H. Hur, S. Yoo, Y. H. Cho and S. Jeon, Adv. Opt. Mater., 2014, 2, 1016–1023 CrossRef CAS.
- J. Ali, G. U. D. Siddiqui, Y. J. Yang, K. T. Lee, K. Um and K. H. Choi, RSC Adv., 2016, 6, 5068–5078 RSC.
- H. Zhu, A. Liu, Y. Xu, F. Shan, A. Li, J. Wang, W. Yang, C. Barrow and J. Liu, Carbon, 2015, 88, 225–232 CrossRef CAS.
- H. Xu, S. Zhou, L. Xiao, S. Li, T. Song, Y. Wang and Q. Yuan, Carbon, 2015, 87, 215–225 CrossRef CAS.
- W. Zhu, H. Song, L. Zhang, Y. Weng, Y. Su and Y. Lv, RSC Adv., 2015, 5, 60085–60089 RSC.
- X. Li, S. P. Lau, L. Tang, R. Ji and P. Yang, Nanoscale, 2014, 6, 5323–5328 RSC.
- X. Li, S. P. Lau, L. Tang, R. Ji and P. Yang, J. Mater. Chem. C, 2013, 1, 7308–7313 RSC.
- T. Yang, F. Cai, X. Zhang and Y. Huang, RSC Adv., 2015, 5, 107340–107347 RSC.
- D. Qu, Z. Sun, M. Zheng, J. Li, Y. Zhang, G. Zhang, H. Zhao, X. Liu and Z. Xie, Adv. Opt. Mater., 2015, 3, 360–367 CrossRef CAS.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. N. Chen, Carbon, 2012, 50, 4738–4743 CrossRef CAS.
- X. Wu, F. Tian, W. Wang, J. Chen, M. Wu and J. X. Zhao, J. Mater. Chem. C, 2013, 1, 4676–4684 RSC.
- S. Umrao, M. H. Jang, J. H. Oh, G. Kim, S. Sahoo, Y. H. Cho, A. Srivastva and I. K. Oh, Carbon, 2015, 81, 514–524 CrossRef CAS.
- W. Shi, H. Fan, S. Ai and L. Zhu, New J. Chem., 2015, 39, 7054–7059 RSC.
- Y. Yin, Q. Liu, D. Jiang, X. Du, J. Qian, H. Mao and K. Wang, Carbon, 2016, 96, 1157–1165 CrossRef CAS.
- L. Tang, R. Ji, X. Li, K. S. Teng and S. P. Lau, J. Mater. Chem. C, 2013, 1, 4908–4915 RSC.
- X. Yan, X. Cui and L. S. Li, J. Am. Chem. Soc., 2010, 132, 5944–5945 CrossRef CAS PubMed.
- Q. Li, S. Zhang, L. Dai and L. S. Li, J. Am. Chem. Soc., 2012, 134, 18932–18935 CrossRef CAS PubMed.
- L. Fan, M. Zhu, X. Lee, R. Zhang, K. Wang, J. Wei, M. Zhong, D. Wu and H. Zhu, Part. Part. Syst. Charact., 2013, 30, 764–769 CrossRef CAS.
- X. Ding, J. Mater. Chem. C, 2014, 2, 3717–3722 RSC.
- C. Zhu, S. Yang, G. Wang, R. Mo, P. He, J. Sun, Z. Di, N. Yuan, J. Ding, G. Ding and X. Xie, J. Mater. Chem. C, 2015, 3, 8810–8816 RSC.
- R. Li, Y. Liu, Z. Li, J. Shen, Y. Yang, X. Cui and G. Yang, Chem.–Eur. J., 2016, 22, 272–278 CrossRef CAS PubMed.
- L. Song, J. Shi, J. Lu and C. Lu, Chem. Sci., 2015, 6, 4846–4850 RSC.
- L. Lin, M. Rong, S. Lu, X. Song, Y. Zhong, J. Yan, Y. Wang and X. Chen, Nanoscale, 2015, 7, 1872–1878 RSC.
- B. Shi, L. Zhang, C. Lan, J. Zhao, Y. Su and S. Zhao, Talanta, 2015, 142, 131–139 CrossRef CAS PubMed.
- X. Y. Shan, L. J. Chai, J. J. Ma, Z. S. Qian, J. R. Chen and H. Feng, Analyst, 2014, 139, 2322–2325 RSC.
- J. Zhou, X. Y. Shan, J. J. Ma, Y. M. Gu, Z. S. Qian and J. R. Chen, RSC Adv., 2014, 4, 5465–5468 RSC.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. J. Ma, J. R. Chen and H. Feng, ACS Appl. Mater. Interfaces, 2014, 6, 6797–6805 CAS.
- H. Chakraborti, S. Sinha, S. Ghosh and S. K. Pal, Mater. Lett., 2013, 97, 78–80 CrossRef CAS.
- S. L. Hu, A. Trinchi, P. Atkin and I. Cole, Angew. Chem., Int. Ed., 2015, 54, 2970–2974 CrossRef CAS PubMed.
- D. Qu, M. Zheng, P. Du, Y. Zhou, L. G. Zhang and D. Li, Nanoscale, 2013, 5, 12272–12277 RSC.
- M. L. Mueller, X. Yan, J. A. McGuire and L. S. Li, Nano Lett., 2010, 10, 2679–2682 CrossRef CAS PubMed.
- H. Tetsuka, R. Asahi, A. Nagoya, K. Okamoto, I. Tajima, R. Ohta and A. Okamoto, Adv. Mater., 2012, 24, 5333–5338 CrossRef CAS PubMed.
- L. Zhou, J. Geng and B. Liu, Part. Part. Syst. Charact., 2013, 30, 1086–1092 CrossRef CAS.
- R. Guo, S. Zhou, Y. Li, X. Li, L. Fan and N. H. Voelcker, ACS Appl. Mater. Interfaces, 2015, 7, 23958–23966 CAS.
- H. Chakraborti, S. Sinha, S. Ghosh and S. K. Pal, Mater. Lett., 2013, 97, 78–80 CrossRef CAS.
- B. Wang, S. Zhuo, L. Chen and Y. Zhang, Spectrochim. Acta, Part A, 2014, 131, 384–387 CrossRef CAS PubMed.
- X. Zhao, J. Gao, X. He, L. Cong, H. Zhao, X. Li and F. Tan, RSC Adv., 2015, 5, 39587–39591 RSC.
- T. Van Tran, S. H. Hong and W. M. Choi, RSC Adv., 2015, 5, 97598–97603 RSC.
- F. Wang, Z. Gu, W. Lei, W. Wang, X. Xia and Q. Hao, Sens. Actuators, B, 2014, 190, 516–522 CrossRef CAS.
- N. Yu, H. Peng, H. Xiong, X. Wu, X. Wang, Y. Li and L. Chen, Microchim. Acta, 2015, 182, 2139–2146 CrossRef CAS.
- J. J. Liu, Z. T. Chen, D. S. Tang, Y. B. Wang, L. T. Kang and J. N. Yao, Sens. Actuators, B, 2015, 212, 214–219 CrossRef CAS.
- S. Huang, H. Qiu, F. Zhu, S. Lu and Q. Xiao, Microchim. Acta, 2015, 182, 1723–1731 CrossRef CAS.
- H. Huang, Z. Feng, Y. Li, Z. Liu, L. Zhang, Y. Ma and J. Tong, Anal. Methods, 2015, 7, 2928–2935 RSC.
- Y. Li, H. Huang, Y. Ma and J. Tong, Sens. Actuators, B, 2014, 205, 227–233 CrossRef CAS.
- Y. He, J. Sun, D. Feng, H. Chen, F. Gao and L. Wang, Biosens. Bioelectron., 2015, 74, 418–422 CrossRef CAS PubMed.
- L. Fan, Y. Hu, X. Wang, L. Zhang, F. Li, D. Han, Z. Li, Q. Zhang, Z. Wang and L. Niu, Talanta, 2012, 101, 192–197 CrossRef CAS PubMed.
- T. Shao, P. Zhang, L. Tang, S. Zhuo and C. Zhu, Microchim. Acta, 2015, 182, 1431–1437 CrossRef CAS.
- Y. Zhu, G. Wang, H. Jiang, L. Chen and X. Zhang, Chem. Commun., 2015, 51, 948–951 RSC.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. J. Ma, J. R. Chen and H. Feng, Biosens. Bioelectron., 2014, 60, 64–70 CrossRef CAS PubMed.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. J. Ma, J. R. Chen and H. Feng, Nanoscale, 2014, 6, 5671–5674 RSC.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. R. Chen and H. Feng, Chem.–Eur. J., 2014, 20, 16065–16069 CrossRef CAS PubMed.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. R. Chen and H. Feng, Nanotechnology, 2014, 25, 415501 CrossRef PubMed.
- Y. Wang, L. Zhang, R. P. Liang, J. M. Bai and J. D. Qiu, Anal. Chem., 2013, 85, 9148–9155 CrossRef CAS PubMed.
- H. Sun, N. Gao, L. Wu, J. Ren, W. Wei and X. Qu, Chem.–Eur. J., 2013, 19, 13362–13368 CrossRef CAS PubMed.
- L. Zhang, D. Peng, R. P. Liang and J. D. Qiu, Anal. Chem., 2015, 87, 10894–10901 CrossRef CAS PubMed.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. R. Chen and H. Peng, Biosens. Bioelectron., 2015, 68, 225–231 CrossRef CAS PubMed.
- Y. X. Qi, M. Zhang, Q. Q. Fu, R. Liu and G. Y. Shi, Chem. Commun., 2013, 49, 10599–10601 RSC.
- H. Huang, L. Liao, X. Xu, M. Zou, F. Liu and N. Li, Talanta, 2013, 117, 152–157 CrossRef CAS PubMed.
- L. Lin, X. Song, Y. Chen, M. Rong, T. Zhao, Y. Jiang, Y. Wang and X. Chen, Nanoscale, 2015, 7, 15427–15433 RSC.
- S. Chen, Y. Song, Y. Li, Y. Liu, X. Su and Q. Ma, New J. Chem., 2015, 39, 8114–8120 RSC.
- L. J. Chai, J. Zhou, H. Feng, J. J. Lin and Z. S. Qian, Sens. Actuators, B, 2015, 220, 138–145 CrossRef CAS.
- L. Li, G. Wu, T. Hong, Z. Yin, D. Sun, E. S. Abdel-Halim and J. J. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 2858–2864 CAS.
- L. Zhang, Z. Y. Zhang, R. P. Liang, Y. H. Li and J. D. Qiu, Anal. Chem., 2014, 86, 4423–4430 CrossRef CAS PubMed.
- J. Shi, C. Chan, Y. Pang, W. Ye, F. Tian, J. Lyu, Y. Zhang and M. Yang, Biosens. Bioelectron., 2015, 67, 595–600 CrossRef CAS PubMed.
- H. Zhang, Y. Wang, D. Zhao, D. Zeng, J. Xia, A. Aldalbahi, C. Wang, L. San, C. Fan, X. Zuo and X. Mi, ACS Appl. Mater. Interfaces, 2015, 7, 16152–16156 CAS.
- X. Li, S. Zhu, B. Xu, K. Ma, J. Zhang, B. Yang and W. Tian, Nanoscale, 2013, 5, 7776–7779 RSC.
- J. J. Liu, X. L. Zhang, Z. X. Cong, Z. T. Chen, H. H. Yang and G. N. Chen, Nanoscale, 2013, 5, 1810–1815 RSC.
- Y. Li, H. Sun, F. Shi, N. Cai, L. Lu and X. Su, Biosens. Bioelectron., 2015, 74, 284–290 CrossRef CAS PubMed.
- N. Li, X. Wang, J. Chen, L. Sun and P. Chen, 2D Mater., 2015, 2, 034018 CrossRef.
- D. Bhatnagar, V. Kumar, A. Kumar and I. Kaur, Biosens. Bioelectron., 2016, 79, 495–499 CrossRef CAS PubMed.
- H. Zhao, Y. Chang, M. Liu, S. Gao, H. Yu and X. Quan, Chem. Commun., 2013, 49, 234–236 RSC.
- Z. S. Qian, L. J. Chai, Q. Zhou, Y. Y. Huang, C. Tang, J. R. Chen and H. Feng, Anal. Chem., 2015, 87, 7332–7339 CrossRef CAS PubMed.
- Z. S. Qian, L. J. Chai, C. Tang, Y. Y. Huang, J. R. Chen and H. Feng, Anal. Chem., 2015, 87, 2966–2973 CrossRef CAS PubMed.
- Z. S. Qian, L. J. Chai, Y. Y. Huang, C. Tang, J. J. Shen, J. R. Chen and H. Feng, Biosens. Bioelectron., 2015, 68, 675–680 CrossRef CAS PubMed.
- L. J. Chai, J. Zhou, H. Feng, C. Tang, Y. Y. Huang and Z. S. Qian, ACS Appl. Mater. Interfaces, 2015, 7, 23564–23574 CAS.
- Z. S. Qian, L. J. Chai, C. Tang, Y. Y. Huang, J. R. Chen and H. Feng, Sens. Actuators, B, 2016, 222, 879–886 CrossRef CAS.
- S. Y. Liu, N. Zhao, Z. Cheng and H. G. Liu, Nanoscale, 2015, 7, 6836–6842 RSC.
- Y. Y. Wu, P. Wei, S. Pengpumkiat, E. A. Schumacher and V. T. Remcho, Anal. Chem., 2015, 87, 8510–8516 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |


