The preparation of sub-micron spherical Fe-Ph/Cl-20 by the spray-drying method and its combustion†
Nai-Meng
Song
,
Rui-Yi
Gan
,
Wen-Yuan
Zhao
,
Guo-Ying
Zhang
and
Li
Yang
 *
*
State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: yanglibit@bit.edu.cn; Fax: +86-10-6891-1682; Tel: +86-10-6891-1682
First published on 6th December 2016
Abstract
This study introduces the preparation of sub-micron composite particles of phloroglucinol-Fe and Cl-20 (Fe-Ph/Cl-20) with a spherical shape using spray drying technology. The influences of solution concentration and temperature on the morphology and particle size of the Fe-Ph/Cl-20 particles were studied using a scanning electron microscope (SEM) and a laser particle analyzer. The results show that when the concentration (ethyl acetate as solvent) of Fe-Ph/Cl-20 (0.3 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g) is 10.30 g/100 g and the temperature is 80 °C, the Fe-Ph/Cl-20 particles are well-defined spherical particles (D50 958.96 nm and D90 1647.63 nm). Thermal decomposition of Fe-Ph/Cl-20 is studied using a differential scanning calorimeter (DSC). The DSC illustrates that the apparent activation energy of Fe-Ph/Cl-20 is reduced by 41.9 kJ mol−1. The spray drying technology provides theoretical support and introduces a new direction for preparing the sub-micron composite particles of high explosives and combustion catalysts.
10 g) is 10.30 g/100 g and the temperature is 80 °C, the Fe-Ph/Cl-20 particles are well-defined spherical particles (D50 958.96 nm and D90 1647.63 nm). Thermal decomposition of Fe-Ph/Cl-20 is studied using a differential scanning calorimeter (DSC). The DSC illustrates that the apparent activation energy of Fe-Ph/Cl-20 is reduced by 41.9 kJ mol−1. The spray drying technology provides theoretical support and introduces a new direction for preparing the sub-micron composite particles of high explosives and combustion catalysts.
Introduction
HNIW (2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane), more commonly called Cl-20, is a novel high-density cyclic nitramine, synthesized as an energetic component in propellant formulations.1 Addition of a small amount of combustion catalyst to Cl-20 can not only accelerate its reaction rate, but also promote the energy released.2,3 Many studies have proven that a mixed mode is very important for improving the catalytic effects because it determines the dispensability or contact area between the reactant and catalyst,4–6 such as in core–shell nanocomposites ammonium perchlorate/Cu(II)–Cr(III)-oxides (AP/Cu–Cr–O) and mesoporous composites α-Fe2O3/AP.7,8 However, the process of this mixed mode is complex and the production rate is low.The spray drying method is a well-known, facile, inexpensive, and scalable technique that has been extensively used in the preparation of micro-sized spherical material.9,10 The micro–nano spheres (HMX/Viton, HMX/Estane and HMX/NC, etc.) were prepared using a B290 Spray Dryer. The impact sensitivity of the microspheres energetic composites is obviously lower than that of raw HMX.11–13 The super structures comprising nano MOFs assembled by spray drying explore new ways for fine-tuning their porosity and encapsulation of guest species.14 2,4-dihydroxybenzoates micro–nano spheres with controllable size effectively lower the decomposition temperature of ammonium perchlorate (AP).15 These applications make the spray drying technology one of the most attractive methods for micro/nano composites in various areas.16,17
Herein, we describe the preparation of a micro-sized composite of Cl-20/phloroglucinol iron (Fe-Ph/Cl-20) spheres using a facile and scalable spray drying method. The phase composition and morphology of the obtained microspheres were characterized by a nanometer laser particle analyzer, scanning electron microscopy (SEM), and EDX. Furthermore, the catalytic ability of the Fe-Ph/Cl-20 microspheres was tested by DSC.
Experimental
Materials and methods
All reagents and solvents (analytical grade) were purchased from Sinopharm Chemical Reagent Co, Ltd., Beijing, China. High purity nitrogen was supplied by Beijing Jinggao Gas Works (Beijing, China). The material was refined using a rotary evaporator (RE-52AA, Shanghai Yarong Biochemistry Instrument Factory, China). The particle size distribution was investigated by a laser granulometer (Winner801, Jinan Winner Particle Instruments Joint Stock Co. Ltd., China). Surface morphology and microstructure were determined by scanning electron microscopy (SEM, S4800, Hitachi, Japan; operating at 15.0 kV).Spray drying of the Fe-Ph/Cl-20 composite
Phloroglucinol and ferric chloride were reacted in water at 60 °C for an hour. Fe-Ph (phloroglucinol-ferric) powder was acquired after phloroglucinol ferric solution was refined using a rotary evaporator. The microspheres composite Fe-Ph/Cl-20 was spray dried from the composite precursor solution (CPS). The CPS, with a concentration of 0.5 wt%, was prepared by diluting the as-prepared Fe-Ph and Cl-20 (a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) with acetone.
100) with acetone.
First, the CPS was atomized into droplets by an ultrasonic atomizer. Second, the droplets were introduced into the spray drying equipment by a carrier gas (N2) and evaporated into microspheres. Finally, the particles were collected by a collector (Fig. 1). The detailed operation procedure is shown in the ESI.†
Results and discussion
Morphology and elemental composition of the Fe-Ph/Cl-20 microspheres
The morphology and composition of the Fe-Ph/Cl-20 microspheres were investigated by scanning electron microscopy (SEM) and EDS, as shown in Fig. 2. The SEM image show that the particles of Fe-Ph/Cl-20 composite prepared by spray drying have a spherical shape and rough surface.The EDS reveals that Fe-Ph/Cl-20 microspheres contain C, N, O, and Fe elements. The EDX data (Table 1) further shows that the content of Fe is 0.91%, close to the theoretical content (0.90%). The results show that the microspheric composite of Cl-20 and Fe-Ph can be successfully fabricated using the spray drying method.
| Element | C | N | O | Fe | Total |
|---|---|---|---|---|---|
| Weight (%) | 29.38 | 38.21 | 31.51 | 0.91 | 100 |
| Atom (%) | 34.17 | 38.10 | 27.51 | 0.23 | 100 |
Effect of CPS's concentration on the morphology of Fe-Ph/Cl-20 microspheres
The concentration of the CPS is crucial for controlling the particle size distribution (PSD) and final morphology of the products. Herein, the effect of the concentration of the CPS on the morphology and size of the Fe-Ph/Cl-20 particles was analyzed by SEM using the three values of (0.06 g/2 g)/100 mL, (0.18 g/6 g)/100 mL, and (0.30 g/10 g)/100 mL (Fig. 3). SEM images confirm that Fe-Ph/Cl-20 particle size obviously increased with the increasing concentration, and its particle shape became well-defined.As can be seen from Fig. 3, most of the particles have sizes below 1000 nm, up to the micro level. When the concentration is lower, particle size distribution is not uniform. However, with the increasing concentration, the shape of the sphere becomes more regular. When the size of the droplet is constant, the concentration is lower and the content of the solution in droplets is higher. At a certain temperature and at lower concentrations, a longer time is needed to reach a supersaturated state, resulting in smaller supersaturated droplets. However, in the process of mass transfer and heat transfer, the heat transfer rate is relatively slow, which leads to low particle sphericity and non-uniform distribution. Conversely, the situation is improved at high concentration, where mass transfer and heat transfer reach equilibrium. Therefore, the particles prepared at high concentration have relatively better sphericity and more uniform particle size distribution.
Effect of drying temperature
To explore the effect of temperature on the morphology of the sphere, the drying temperature was set to 80 °C (a), 110 °C (b), and 140 °C (c). According to the abovementioned results, the solution concentration was set to 0.30 g/10 g (c) of Fe-Ph/Cl-20 per 100 g of ethyl acetate. The flow rate of nitrogen gas was 0.25 L min−1. Scanning electron microscopy figures show the shape of Fe-Ph/Cl-20 powder after spray drying (Fig. 4). | ||
| Fig. 4 SEM images of the Fe-Ph/Cl-20 microspheres prepared at different inlet temperatures: (a) T = 80 °C, (b) T = 110 °C, and (c) T = 140 °C. | ||
Compared to the surface of Fe-Ph/Cl-20 microspheres prepared at 110 °C and 140 °C (Fig. 4(b) and (c)), the surface of Fe-Ph/Cl-20 microspheres prepared at 80 °C (Fig. 4(a)) is more smooth. This is because the drying temperature of the particles, as shown in Fig. 4(b) and (c), was higher than the boiling point of ethyl acetate. The heat transfer rate is higher than the mass transfer rate in the process of drying. When the heat transfer rate is faster than the mass transfer rate, the solute will accumulate in the outer layer of the droplet during the liquid–solid phase transition. At the same time, the gas formed by the evaporation of the solution in the inner layer breaks through the outer layer. This process leads to roughness or even cracks on the surface of the spherical particles (Fig. 4(b), (c) and 3). In Fig. 4(c), the reunion phenomenon occurs. This is because in the drying tower, droplets will unavoidably collide to form droplet clusters. At this state, the drying process would only be completed (to create spherical droplets) in cases where the droplet clusters have not been formed, due to the fact that the heat transfer rate is too fast. Therefore, at 140 °C, the reunion phenomenon of the product will exist. At 80 °C, the drying temperature is close to the boiling point of ethyl acetate, such that the heat transfer rate and mass transfer rate achieve a state of equilibrium. As a result, the sphere surface is smooth. The effect of mass transfer rate and heat transfer rate on the product morphology is shown in Fig. 5.
 | ||
| Fig. 5 Schematic of the drying process under different conditions (rh = heat transfer rate and rm = mass transfer rate). | ||
Nanometer laser particle size test
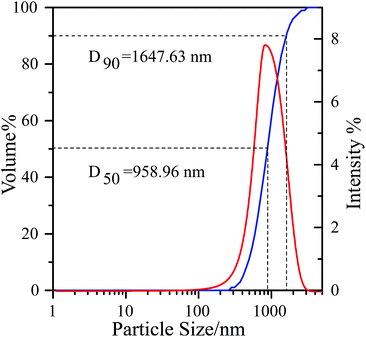
A small amount of solid powder was dispersed in deionized water. The sample was oscillated about 3 minutes in an ultrasonic cleaner before testing. The test result is shown in Fig. 6. D50 and D90 data is 958.96 nm and 1647.63 nm, respectively. We successfully obtained a micro–nano Fe-Ph/Cl-20 complex through the spray drying technology (Fig. 7).The catalytic effect of Fe-Ph on the decomposition of Cl-20
To investigate the difference between the spray mixing and mechanical blending, the DSC (DSC curves with the linear heating rate of 10 K min−1 were obtained under a nitrogen atmosphere) was used to test the thermal decomposition process of the mixture using two methods in this study.DSC data shows that compared with the decomposition peak temperatures of Cl-20, the mechanical doping lowers the required temperature by 9.4 °C, and spray mixing lowers it by 25.0 °C. This proves that the spray drying method can mix the components more fully to further strengthen the catalytic effect of Fe-Ph on the thermal decomposition of Cl-20.
Fig. 8 is a schematic of the catalytic effect. It can be seen that the effective contact area of the mixture of Fe-Ph and Cl-20 prepared by mechanical doping is much smaller than that of the Fe-Ph/Cl-20, resulting in the increased active sites of the Fe-Ph/Cl-20 particle. It is clear that the sub-micron spherical Fe-Ph/Cl-20 prepared by the spray drying method can greatly improve the effect of the catalyst. As a consequence, the sub-micron spherical Fe-Ph/Cl-20 particles prepared by the spray-drying method can greatly promote the thermal decomposition of Cl-20.
The thermal decomposition process of Cl-20 and Fe-Ph/Cl-20 were determined using the DSC method at 5, 10, 15, and 20 K min−1 heating rates, respectively. The Kissinger method and Ozawa–Doyle method were used to obtain the thermal decomposition kinetics parameters combined with the first exothermic peak temperatures of the DSC curves. Table 2 shows the thermodynamic parameters of Cl-20 and Fe-Ph/Cl-20 at different heating rates.
| β/(K min−1) | T p/K | E a/(kJ mol−1) | lg (A s−1) | R | ||||
|---|---|---|---|---|---|---|---|---|
| Kissinger | Ozawa–Doyle | Kissinger | Kissinger | Ozawa–Doyle | ||||
| Cl-20 | 5 | 519.85 | 163.0 | 163.4 | 14.16 | −0.9976 | −0.9978 | |
| 10 | 530.15 | |||||||
| 15 | 534.55 | |||||||
| 20 | 538.75 | |||||||
| Fe-Ph/Cl-20 (spray dried) | 5 | 496.65 | 121.1 | 123.2 | 10.46 | −0.9876 | −0.9892 | |
| 10 | 505.35 | |||||||
| 15 | 512.25 | |||||||
| 20 | 519.75 | |||||||
Table 2 shows that the kinetics parameters calculated by the Kissinger method and Ozawa–Doyle method are very close. The apparent activation energy of the first decomposition peak of Fe-Ph/Cl-20 is 121.1 kJ mol−1, which was measured by the Kissinger method. Corresponding to the initial thermal decomposition process, the Arrhenius equation is as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = 24.57 − 259.4 × 103/RT k = 24.57 − 259.4 × 103/RT | (1) |
By comparing the apparent activation energy of Cl-20 and Fe-Ph/Cl-20, it can be seen that the activation energy of Fe-Ph/Cl-20 is lower than that of Cl-20 by 41.9 kJ mol−1. Accordingly, the pre-exponential factor is reduced from 14.16 to 10.46.
Conclusions
In this study, the influences of the spray drying conditions on the Fe-Ph/Cl-20 particles are explored, obtaining the optimum process conditions. Under these conditions, the particles of Fe-Ph/Cl-20 prepared by the spray drying method have the advantages of small particle size and good sphericity. Clearly, the catalytic effect of compound Fe-Ph and Cl-20, doped by spray-drying, is superior to that of the Fe-Ph and Cl-20, which is prepared by simple mechanical blending. DSC data show that Fe-Ph and Cl-20 mixed by spray drying can lower the decomposition peak temperature of Cl-20 by 25.0 °C. The kinetic parameters illustrate that the microspheric composite Fe-Ph/Cl-20 reduces the activation energy of Cl-20 from 163.0 kJ mol−1 to 121.1 kJ mol−1. The microspheric composite Fe-Ph/Cl-20 prepared by the spray drying method exhibit excellent catalytic performance.Acknowledgements
We gratefully acknowledge the financial support received from the State Key Laboratory of Explosion Science and Technology (No. YB2016-17) and the National Natural Science Foundation of China (No. 11672040). We also thank the reviewers for their most valuable comments.References
- P. Karakaya, M. Sidhoum, C. Christodoulatos and S. Nicolich, Wendy Balas. Aqueous solubility and alkaline hydrolysis of the novel high explosive hexanitrohexaazaisowurtzitane (CL-20), J. Hazard. Mater., 2005, 120(1–3), 183–191 CrossRef CAS PubMed.
- W. Wei, X. Jiang, L. Lu, X. Yang and X. Wang, Study on the catalytic effect of NiO nanoparticles on the thermal decomposition of TEGDN/NC propellant, J. Hazard. Mater., 2009, 168(2–3), 838–842 CrossRef CAS PubMed.
- V. K. Balakrishnan, F. Monteil-Rivera, A. Halasz, A. Corbeanu and J. Hawari, Decomposition of the Polycyclic Nitramine Explosive, CL-20, by Fe0, Environ. Sci. Technol., 2004, 38(24), 6861–6866 CrossRef CAS PubMed.
- F. Liua, G. Feng, M. Lin, C. Wang, B. Hu and C. Qi, Superoleophilicnanoporous polymeric ionic liquids loaded with palladium acetate: reactants enrichment and efficient heterogeneous catalysts for Suzuki–Miyaura coupling reaction, J. Colloid Interface Sci., 2014, 435, 83–90 CrossRef PubMed.
- A. A. Lemonidou and M. Machli, Oxidative dehydrogenation of propane over V2O5-MgO/TiO2 catalyst Effect of reactants contact mode, Catal. Today, 2007, 127, 132–138 CrossRef CAS.
- T. Wei, Y. Zhang, K. Xu, Z. Ren, H. Gao and F. Zhao, Catalytic action of nano Bi2WO6 on thermal decompositions of AP, RDX, HMX and combustion of NG/NC propellant, RSC Adv., 2015, 5, 70323–70328 RSC.
- A. Eslami, N. M. Juibari and S. G. Hosseini, Fabrication of ammonium perchlorate/copper-chromium oxides core–shell nanocomposites for catalytic thermal decomposition of ammonium perchlorate, Mater. Chem. Phys., 2016, 181, 12–20 CrossRef CAS.
- S. G. Hosseini, R. Ahmadi, A. Ghavi and A. Kashi, Synthesis and characterization of α-Fe2O3 mesoporous using SBA-15 silica as template and investigation of its catalytic activity for thermal decomposition of ammonium perchlorate particles, Powder Technol., 2015, 278, 316–322 CrossRef CAS.
- L. Yu, Amorphous pharmaceutical solids: preparation, characterization and stabilization, Adv. Drug Delivery Rev., 2001, 48, 27–42 CrossRef CAS PubMed.
- V. Reinhard, Pharmaceutical particle engineering via spray drying, Pharm. Res., 2008, 25, 999–1022 CrossRef PubMed.
- X. Shi, J. Wang, X. Li and C. An, Preparation and Properties of HMX/Nitrocellulose Nanocomposites, J. Propul. Power, 2015, 31(2), 757–760 CrossRef.
- S. H. I. Xiaofeng, W. A. N. G. Jingyu, L. I. Xiaodong and A. N. Chongwei, Preparation and Characterization of HMX/Estane Nanocomposites, Cent. Eur. J. Energ. Mater., 2014, 11(3), 433–442 Search PubMed.
- S. H. I. Xiaofeng, W. A. N. G. Cailing, W. A. N. G. Jingyu, L. I. Xiaodong, A. N. Chongwei, W. A. N. G. Jiang and J. I. Wei, Process Optimization and Characterization of an HMX/Viton Nanocomposite, Cent. Eur. J. Energ. Mater., 2015, 12(3), 487–495 Search PubMed.
- A. Carne-Sanchez, I. Inhar, C.-S. Mary and M. Daniel, A spray-drying strategy for synthesis of nanoscale metal–organic frameworks and their assembly into hollow superstructures, Nat. Chem., 2013, 5(3), 203–211 CrossRef CAS PubMed.
- W.-Y. Zhao, T.-L. Zhang, L.-N. Zhang, L. Yang and Z.-N. Zhou, Large-scale production of (2,4-DHB)nM micro–nano spheres by spray drying and their application as catalysts for ammonium perchlorate, J. Ind. Eng. Chem., 2016, 38, 73–81 CrossRef CAS.
- A. A. Santana, D. M. Cano-Higuita, R. A. de Oliveira and V. R. N. Telis, Influence of different combinations of wall materials on the microencapsulation of jussara pulp (Euterpe edulis) by spray drying, Food Chem., 2016, 212, 1–9 CrossRef CAS PubMed.
- K. Du, X. Hongbin, H. Guorong, P. Zhongdong, C. Yanbing and Y. Fan, Enhancing the Thermal and Upper Voltage Performance of Ni-Rich Cathode Material by a Homogeneous and Facile Coating Method: Spray-Drying Coating with Nano-Al2O3, ACS Appl. Mater. Interfaces, 2016, 8(27), 17713–17720 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24343f |
| This journal is © The Royal Society of Chemistry 2016 |