Approach to thiazole-containing tetrahydropyridines via Aza–Rauhut–Currier reaction and their potent fungicidal and insecticidal activity†
Yu-Jie Zhua,
Xiao-Feng Guoa,
Zhi-Jin Fan*ab,
Lai Chena,
Liu-Yong Maa,
Hai-Xia Wanga,
Yu Weic,
Xuan-Ming Xuc,
Jian-Ping Linc and
Vasiliy A. Bakulev*d
aState Key Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China. E-mail: fanzj@nankai.edu.cn; Fax: +86-22-23503620; Tel: +86-22-23499464
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China
cState Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300071, China
dThe Ural Federal University Named After the First President of Russia B. N. Yeltsin, Yeltsin UrFU 620002, Ekaterinburg, Russia. E-mail: v.a.bakulev@urfu.ru
First published on 7th November 2016
Abstract
A convenient and efficient synthesis of multi-substituted thiazole-containing tetrahydropyridine moieties was reported using the phosphine-catalyzed Aza–Rauhut–Currier reaction with excellent yields and diastereoselectivity. Thiazole-containing tetrahydropyridines were further transformed into the corresponding piperidine derivatives. The biological activity of the title compounds was explored; they exhibited moderate insecticidal activity against Aphis laburni Kaltenbach at 100 μg mL. A 3D QSAR model accurately predicted the insecticidal activity of the structurally diverse set of test compounds. Thiazole-containing tetrahydropyridines were active against normal fungi and also had good activity against resistant fungi mutations without cross resistance; thus, these compounds will be valuable for resistance management. The predicted potential fungicidal target of the title compounds is fumarate reductase.
Introduction
Heterocycles are important key pharmacophores in drug discovery.1 Specifically, nitrogen-containing heterocycles constitute a major class of natural products and bioactive ingredients.2 Piperidine is one N-heterocycle that is widely found in pharmaceutically active and natural compounds, therefore it has attracted the interest of chemists, biologists and pharmacists alike for its significant, diverse and promising biological activity.3 Numerous investigations have shown that sulfur-containing heterocycles can induce apoptosis in multiple cell lines and experimental animals.4 Sulfur- and nitrogen-containing heterocyclic compounds are recognized as important leads.5 In particular, thiazole presents an important opportunity to access a wide variety of natural and synthetic products with a broad spectrum of biological activities.6,7 A combination of these two active substructures into one target molecule is a simple and effective method for designing novel pesticides.8 Certain compounds that combine thiazole and piperidine substructures have been identified for their medicinal activity. The fungicide oxathiapiprolin, which has excellent efficacy against downy mildew, was discovered by DuPont via high throughput screening.9 Our group has concentrated on the agricultural activities of nitrogen- and sulfur-containing heterocycles for novel pesticide discovery.4 Thiazole-containing piperidine structures without chiral carbon atoms were constructed with certain types of difficulties; however, inclusion of a chiral carbon center generally resulted in unexpected biological activities.10 In our continued exploration of this field, the phosphine-catalyzed Aza–Rauhut–Currier reaction was a useful tool to combine nitrogen- and sulfur-heterocycles with chiral carbon atoms in a single molecule.11 Inspired by this reaction, herein we aimed to synthesize thiazole-containing piperidine derivatives for novel pesticide discovery via the method shown in Scheme 1. Using thiazole aldehydes as raw materials, an efficient and convenient Aza–Rauhut–Currier reaction was employed to obtain thiazole-containing tetrahydropyridine derivatives, which could then be easily transformed into the corresponding thiazole-containing piperidines. To our delight, thiazole N-sulfonyl-1-aza-1,3-dienes reacted smoothly with vinyl ketones with high diastereoselectivity to produce targets with moderate biological activities. Several target compounds were found to be valuable for fungicide resistance management and novel pesticide activities.Results and discussion
Chemistry
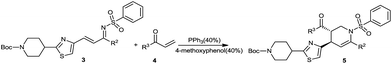
The results of the optimization study for the synthesis of benzenesulfonyl-substituted thiazole-containing tetrahydropyridine are summarized in Table 1. Observations at different temperatures were made in CH2Cl2 with 20% PPh3 and 20% 4-methoxyphenol for a 24 h reaction (Table 1, entries 1–3). The reaction did not proceed at room temperature, but was effectively advanced at 30 °C. The quantity of PPh3 and 4-methoxyphenol affected the yield of 5ea (Table 1, entries 4–8); when the reaction was conducted in CH2Cl2 with 40% PPh3 and 40% 4-methoxyphenol at 30 °C for 12 h, the yield of 5ea reached 90% (Table 1, entry 9). Further increases the reaction time and PPh3 and 4-methyoxyphenol loading did not improve the yield (Table 1, entries 7–8). However, under the other reaction conditions (Table 1, entries 1–6), reaction times were longer (24 h) and the yields were lower. Solvent screening (Table 1, entries 9–12) indicated that CH2Cl2 was the most effective solvent. Thus, the optimum reaction conditions were those illustrated in Table 1, entry 9.| Entry | PPh3 | Methoxyphenol | Solvent | Time, h | T, °C | Yielda, % |
|---|---|---|---|---|---|---|
| a Isolated yield. | ||||||
| 1 | 20% | 30% | CH2Cl2 | 24 | 25 | None |
| 2 | 20% | 30% | CH2Cl2 | 24 | 30 | 33 |
| 3 | 20% | 30% | CH2Cl2 | 24 | 35 | 32 |
| 4 | 10% | 30% | CH2Cl2 | 24 | 30 | None |
| 5 | 30% | 30% | CH2Cl2 | 24 | 30 | 51 |
| 6 | 40% | 30% | CH2Cl2 | 24 | 30 | 60 |
| 7 | 40% | 40% | CH2Cl2 | 24 | 30 | 90 |
| 8 | 50% | 50% | CH2Cl2 | 24 | 30 | 90 |
| 9 | 40% | 40% | CH2Cl2 | 12 | 30 | 90 |
| 10 | 40% | 40% | CHCl3 | 12 | 30 | 74 |
| 11 | 40% | 40% | Toluene | 12 | 30 | 41 |
| 12 | 40% | 40% | CH3CN | 12 | 30 | 57 |
The scope of the reaction was assessed using various substrates as 3 and 4. The group R1 was held constant as N-tert-butyl piperidine, whereas R2 and R3 groups were varied. Regardless of the identity of R3, when R2 was an electron-donating group such as 4-MeOC6H4, the reaction gave moderate yields with an excellent diastereoselectivity (Table 2, entries 1–6).
| Entry | 3 | 4 | R2 | R3 | 5 | d.r.,a yieldb% |
|---|---|---|---|---|---|---|
| a d.r. = trans/cis, determined by crude 1H NMR.b Yield of isolated trans isomer. | ||||||
| 1 | 3a | 4a | 4-MeOC6H4 | Et | 5aa | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 74 1, 74 |
| 2 | 3a | 4b | 4-MeOC6H4 | Phenyl | 5ab | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 83 1, 83 |
| 3 | 3a | 4c | 4-MeOC6H4 | 4-MeC6H4 | 5ac | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 70 1, 70 |
| 4 | 3a | 4d | 4-MeOC6H4 | 2-Thienyl | 5ad | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 79 1, 79 |
| 5 | 3a | 4e | 4-MeOC6H4 | 2-Furyl | 5ae | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 71 1, 71 |
| 6 | 3a | 4f | 4-MeOC6H4 | Cyclohexyl | 5af | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 68 1, 68 |
| 7 | 3b | 4a | Ph | Et | 5ba | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 88 1, 88 |
| 8 | 3b | 4b | Ph | Phenyl | 5bb | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 68 1, 68 |
| 9 | 3b | 4c | Ph | 4-MeC6H4 | 5bc | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 70 1, 70 |
| 10 | 3b | 4d | Ph | 2-Thienyl | 5bd | =12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 64 1, 64 |
| 11 | 3b | 4e | Ph | 2-Furyl | 5be | =12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 74 1, 74 |
| 12 | 3b | 4f | Ph | Cyclohexyl | 5bf | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 76 1, 76 |
| 13 | 3b | 4g | Ph | 4-CF3C6H4 | 5bg | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 72 1, 72 |
| 14 | 3c | 4h | 4-NO2C6H4 | Et | 5bh | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 80 1, 80 |
The reaction was also very tolerant to the substrates when the R2 group was changed into phenyl (Table 2, entries 7–13). Irrespective of the electronic characteristics of the substituent at the R3 position, good yields and excellent diastereomeric ratios (d.r.) were obtained (Table 2, entries 8–9, and entry 13). Even when the R3 group was ethyl and cyclohexyl, the protocol afforded good yields and excellent d.r. values (Table 2, entries 7 and 12). Furthermore, it was applicable to heteroaryl substituents such as 2-thiophenyl and 2-furyl with moderate yields and d.r. values (Table 2, entries 10–11).
When R2 remained unchanged, and R3 was an alkyl substituent such as ethyl and cyclohexyl, the reaction afforded excellent yields with low d.r. values (Table 3, entries 1 and 6). When R3 was changed to substituted phenyls with both electron-donating and electron-withdrawing groups, the reaction gave lower yields and d.r. values as compared with the equivalent reagent with a unsubstituted phenyl (Table 3, entries 2–3, 7). Substrate 4 with heteroaryl substituents such as 2-thiophenyl and 2-fury gave excellent yields and d.r. values (Table 3, entries 4–5).
The scope of the reaction was further explored using unsubstituted thiazole for substrate 3. As compared to the reactions of phenyl-substituted 3 and alkyl-substituted 4, ethyl-substituted 4 afforded excellent yield and d.r. value, whereas cyclohexyl-substituted 4 gave both lower yield and d.r. value (Table 4, entries 1 and 6). The reaction in which unsubstituted phenyl R3 gave lower yields than electron-donating substituent 4-methylphenyl, the d.r. values contrastingly were higher (Table 4, entries 2–3). Both substrates with 2-thiophenyl and 2-furyl at the R3 position gave excellent d.r. values (Table 4, entries 4–5), but only the 2-furyl substituted 4 provided excellent yields. If R2 (cyclopropyl) and R3 (ethyl) were both alkyl groups, lower d.r. value and moderate yield were obtained (Table 4, entry 7).
| Entry | 3 | 4 | R2 | R3 | 5 | d.r.,a yieldb% |
|---|---|---|---|---|---|---|
| a d.r. = trans/cis, determined by crude 1H NMR.b Yield of isolated trans isomer. | ||||||
| 1 | 3e | 4a | Ph | Et | 5ea | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 90 1, 90 |
| 2 | 3e | 4b | Ph | Phenyl | 5eb | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 66 1, 66 |
| 3 | 3e | 4c | Ph | 4-MeC6H4 | 5ec | =17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 93 1, 93 |
| 4 | 3e | 4d | Ph | 2-Thienyl | 5ed | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 65 1, 65 |
| 5 | 3e | 4e | Ph | 2-Furyl | 5ee | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 96 1, 96 |
| 6 | 3e | 4f | Ph | Cyclohexyl | 5ef | =8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60 1, 60 |
| 7 | 3f | 4i | Cyclopropyl | Et | 5ia | =8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 66 1, 66 |
Furthermore, the benzenesulfonyl substituted thiazole-containing tetrahydropyridine underwent smooth desulfonation under mild reaction conditions with TFA and thioanisole to give imine intermediate 6; the configuration of which was assigned by X-ray crystallography. This intermediate was easily reduced with sodium cyanoborohydride to obtain thiazole-containing piperidine 7 in moderate yield (Scheme 2).
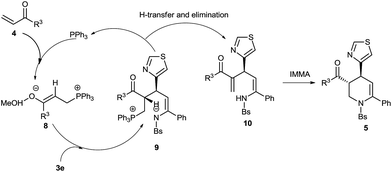
On the basis of the above experimental explorations and existing literature descriptions,12 a reaction mechanism was proposed as shown in Scheme 3. Triphenylphosphate played the role of catalyst and 4-methoxyphenol acted as an additive.
Biological activity
After obtaining the benzenesulfonyl substituted thiazole-containing tetrahydropyridines, biological screening was carried out. The insecticidal activity of all the title compounds against Aphis laburni Kaltenbach was evaluated. The results shown in Table 5 indicated that, to some extent, almost all of the compounds possessed activity against aphids at the tested concentration. Compounds 5bc, 5bd, 5df, 5ec, 5ee and 5ef presented good insecticidal activity at 100 μg mL−1 with over 50% mortality. Compounds 5bc, 5df, 5ee and 5ef were particularly active and presented insecticidal activities at 100 μg mL−1 with over 60% mortality.| No. | Compound | Mortality% (A) | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A A |
||
|---|---|---|---|---|---|
| Exp | Pred | Res | |||
| 1 | 5aa | 22.84 | −3.34 | −3.31 | 0.03 |
| 2 | 5ab | 45.78 | −2.92 | −2.91 | 0.01 |
| 3 | 5ad | 9.41 | −3.83 | −3.83 | 0.00 |
| 4 | 5af | 46.98 | −2.90 | −2.90 | 0.00 |
| 5 | 5ba | 14.83 | −3.55 | −3.56 | −0.01 |
| 6 | 5bc | 60.37 | −2.65 | −2.63 | 0.02 |
| 7 | 5bd | 55.20 | −2.74 | −2.79 | −0.05 |
| 8 | 5bg | 33.18 | −3.17 | −3.17 | −0.00 |
| 9 | 5bh | 35.86 | −3.08 | −3.09 | −0.01 |
| 10 | 5da | 34.81 | −2.93 | −2.92 | 0.01 |
| 11 | 5dc | 28.70 | −3.11 | −3.17 | −0.06 |
| 12 | 5de | 27.73 | −3.11 | −3.04 | 0.07 |
| 13 | 5dg | 24.63 | −3.24 | −3.26 | −0.02 |
| 14 | 5eb | 14.83 | −3.45 | −3.43 | 0.02 |
| 15 | 5ec | 58.92 | −2.54 | −2.51 | 0.03 |
| 16 | 5ed | 26.92 | −3.13 | −3.15 | −0.02 |
| 17 | 5ef | 70.28 | −2.32 | −2.34 | −0.02 |
| 18 | 5ia | 40.56 | −2.77 | −2.79 | −0.02 |
| 19 | 5ac | 48.78 | −2.87 | −2.95 | −0.08 |
| 20 | 5ae | 21.28 | −3.41 | −3.32 | 0.09 |
| 21 | 5bb | 45.37 | −2.91 | −2.76 | 0.15 |
| 22 | 5be | 27.27 | −3.25 | −3.30 | −0.05 |
| 23 | 5bf | 21.28 | −3.40 | −3.22 | 0.18 |
| 24 | 5db | 17.93 | −3.36 | −3.06 | 0.30 |
| 25 | 5dd | 48.70 | −2.73 | −2.84 | −0.09 |
| 26 | 5df | 68.70 | −2.36 | −2.90 | −0.54 |
| 27 | 5ea | 39.24 | −2.83 | −2.63 | 0.20 |
| 28 | 5ee | 64.73 | −2.41 | −2.76 | −0.35 |
The fungicidal screening results against Alternaria solani (AS), Botrytis cinerea (BC), Cercospora arachidicola (CA), Gibberella zeae (GZ), Phytophthora infestans (Mont) de Bary (PI), Physalospora piricola (PP), Pellicularia sasakii (Shirai) (PS), Sclerotinia sclerotiorum (SS), and Rhizoctonia cerealis (RC) are shown in Table 6. The majority of the target compounds showed good fungicidal activity against CA, SS, and BC. Compound 5bh exhibited over 50% activity against six of the fungi tested, whereas compound 5ia exhibited over 50% activity against seven of the tested fungi. Compound 5ba stood out as being particularly active, with growth inhibition of 93.85% against SS. Compound 5da showed 89.29% inhibition against BC. Compound 5df was also active against BC, with 91.07% inhibition.
| Compd | Growth inhibition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AS | CA | GZ | PP | SS | BC | PI | RC | PS | |
| a PC: positive control, azoxystrobin; AS: A. solani, BC: B. cinerea, CA: C. arachidicola, GZ: G. zeae, PI: P. infestans, PP: P. piricola, PS: P. sasakii (Shirai), SS: S. sclerotiorum, RC: R. cerealis. | |||||||||
| 5aa | 25.00 | 29.17 | 28.00 | 29.63 | 20.00 | 41.07 | 15.38 | 30.00 | 14.29 |
| 5ab | 40.00 | 58.33 | 38.67 | 29.63 | 56.92 | 39.29 | 34.62 | 27.50 | 19.05 |
| 5ac | 30.00 | 41.67 | 36.00 | 46.30 | 53.85 | 58.93 | 26.92 | 35.00 | 4.76 |
| 5ad | 35.00 | 37.50 | 20.00 | 59.26 | 50.77 | 30.36 | 15.38 | 37.50 | 19.05 |
| 5ae | 25.00 | 45.83 | 28.00 | 14.81 | 26.15 | 44.64 | 15.38 | 42.50 | 19.05 |
| 5af | 30.00 | 41.67 | 20.00 | 45.29 | 26.15 | 87.50 | 23.08 | 30.00 | 4.76 |
| 5ba | 25.00 | 37.50 | 52.00 | 51.85 | 93.85 | 62.50 | 19.23 | 7.50 | 4.76 |
| 5bb | 15.00 | 38.10 | 22.67 | 16.67 | 26.25 | 23.21 | 15.38 | 36.25 | 4.76 |
| 5bc | 30.00 | 37.50 | 28.00 | 48.15 | 26.15 | 41.07 | 15.38 | 37.50 | 4.76 |
| 5bd | 29.63 | 38.10 | 46.51 | 58.70 | 38.46 | 62.50 | 46.15 | 68.63 | 23.08 |
| 5be | 25.00 | 37.50 | 25.33 | 33.33 | 26.15 | 46.43 | 15.38 | 47.50 | 0 |
| 5bf | 30.00 | 37.50 | 36.00 | 42.59 | 35.38 | 53.57 | 23.08 | 50.00 | 19.05 |
| 5bg | 30.00 | 8.33 | 25.33 | 33.33 | 35.38 | 39.29 | 11.54 | 5.00 | 9.52 |
| 5bh | 67.23 | 47.88 | 68.35 | 73.89 | 56.45 | 80.30 | 60.23 | 69.66 | 58.87 |
| 5da | 26.53 | 28.57 | 50.00 | 39.53 | 31.82 | 89.29 | 22.58 | 46.43 | 50.00 |
| 5db | 20.00 | 45.83 | 33.33 | 38.89 | 35.38 | 35.71 | 23.08 | 42.50 | 19.05 |
| 5dc | 20.41 | 21.43 | 50.00 | 55.81 | 45.45 | 67.86 | 8.06 | 28.57 | 27.27 |
| 5dd | 30.00 | 37.50 | 36.00 | 42.59 | 35.38 | 53.57 | 23.08 | 50.00 | 19.05 |
| 5de | 45.00 | 54.71 | 49.33 | 51.89 | 50.77 | 57.14 | 34.62 | 67.50 | 23.81 |
| 5df | 40.00 | 50.00 | 44.00 | 35.19 | 44.62 | 91.07 | 34.62 | 60.00 | 33.33 |
| 5dg | 30.00 | 41.67 | 22.67 | 42.59 | 35.38 | 14.29 | 19.23 | 40.00 | 9.52 |
| 5ea | 8.16 | 21.43 | 42.86 | 20.93 | 68.18 | 78.57 | 22.58 | 25.00 | 22.73 |
| 5eb | 35.00 | 45.83 | 33.33 | 46.30 | 44.62 | 58.93 | 19.23 | 37.50 | 19.05 |
| 5ec | 29.63 | 47.62 | 46.15 | 32.61 | 38.46 | 56.25 | 19.23 | 54.90 | 0 |
| 5ed | 35.00 | 33.33 | 25.33 | 31.48 | 53.85 | 37.50 | 19.23 | 25.00 | 19.05 |
| 5ee | 44.44 | 23.81 | 19.23 | 47.83 | 7.69 | 50.00 | 19.23 | 68.63 | 19.23 |
| 5ef | 22.22 | 23.81 | 0 | 43.48 | 19.23 | 12.50 | 15.38 | 68.63 | 34.62 |
| 5ia | 60.47 | 67.56 | 50.83 | 78.69 | 60.12 | 68.99 | 64.32 | 60.78 | 68.91 |
| PC | 75.00 | 55.56 | 100 | 100 | 91.18 | 100 | 100 | 80.77 | 100 |
The detailed potency of precision toxicity was detected and the results were shown in Table 7. Compound 5ba exhibited excellent activity against SS with EC50 of 13.19 μg mL−1.
| Compound | Fungi | Regression equation | R2 | EC50/μg mL−1 |
|---|---|---|---|---|
| 5ba | S. sclerotiorum | y = 1.3860x + 3.4470 | 0.9610 | 13.19 |
| 5da | B. cinerea | y = 0.9390x + 2.4960 | 0.9310 | 463.35 |
| 5df | B. cinerea | y = 0.5820x + 3.7070 | 0.9560 | 166.62 |
BC resistance is a worldwide problem; in order to provide alternative measures for BC resistance management, four types of BC mutations were chosen for further screening using the compounds that possessed good activity against normal BC, and the results were shown in Table 8. Compound 5ba exhibited excellent activity, even higher than that of the positive control trifloxystrobin against all the tested mutations of BC, with almost 100% activity at 50 μg mL−1. This is the first report on diastereoselective thiazole piperdine compounds with biological activity against resistant fungi mutations.
| Compound | T3–10 | T1–12 | FJ1–10 | BC2 |
|---|---|---|---|---|
| a FJ1–10 of B. cinerea strains were mutations of 143th amino acid of azoxystrobin target point CYTB protein by GGT into GCT. T1–12 and T3–10 of B. cinerea strains were multiresistant to azoxystrobin and Boscalid. BC2 of B. cinerea strains were multiresistant to carbendazim, diethofencarb, procynidone, pyrimethanil and kresoxim-methyl. | ||||
| Trifloxystrobin | 66.00 | 87.30 | 59.26 | 80.33 |
| 5ba | 100 | 92.06 | 100 | 93.44 |
| 5da | 60.00 | 79.38 | 48.15 | 68.03 |
| 5df | 41.00 | 85.71 | 77.78 | 79.51 |
This provided the opportunity to find more potential novel leads with anti-resistant fungicide activity for resistance management.
Possible drug target prediction
Representative compound 5df was used to find putative targets based on the high fit score using Pharm Mapper Server. A total of 300 top potential target candidates were reserved. Among these target candidates, Escherichia coli fumarate reductase had a high fit score value of 3.75. The lowest fit score value was 3.48 for aldose reductase. Further sequence analysis studies on the fungicidal targets of this study were carried out to identify that the fumarate reductase in E. coli and B. cinerea possessed a low degree of sequence homology (about 18.6%). This suggested that benzenesulfonyl substituted thiazole-containing tetrahydropyridines might be inhibitors for fumarate reductase, a possibility deserving further experimental validation.3D-QSAR model analysis for insecticidal activity against Aphis laburni Kaltenbach
To obtain the relationship between structure and insecticidal activities against Aphis laburni Kaltenbach, 18 compounds, including 5aa, 5ab, 5ad, 5af, 5ba, 5bc, 5bd, 5bg, 5bh, 5da, 5dc, 5de, 5dg, 5eb, 5ec, 5ed, 5ef and 5ia, were chosen to perform 3D-QSAR analysis (Table 5, entries 1–18). The result showed an excellent statistical correlation between experimental (Exp) insecticidal activities and predicted (Pred) insecticidal activities (Fig. 1). The non-cross-validated correlation coefficient r2 was 0.993, which served as a measure of the quality of the model. The F-statistic and cross-validation q2 values were 472.65 and 0.593, respectively. The optimal number of components obtained from the cross-validated analysis was 4. In addition, the proportions of steric and electrostatic contributions were 83.1% and 16.9%, respectively. These results suggested that prediction using the 3D-QSAR model was credible for insecticidal activity of benzenesulfonyl substituted thiazole-containing tetrahydropyridine compounds. The 3D-QSAR analysis provided more information about the relationship between structure and insecticidal activities against Aphis laburni Kaltenbach.The CoMFA steric and electrostatic fields were plotted as 3D colored contour maps, which were helpful to identify important regions of change in the steric and electrostatic fields.
The compound 5ef, which exhibited the highest rate of insecticidal activity against Aphis laburni Kaltenbach at 100 mg mL−1, was used as a reference structure for the 3D-QSAR model. The steric and electrostatic contour maps from CoMFA analysis were presented in Fig. 2. The yellow regions represented areas where increasing the steric bulk would decrease the activity and the green regions illustrated areas where increasing the steric bulk might enhance the activity. Similarly, the red regions indicated areas where increasing electronegative substitution could enhance the activity, whereas the blue regions showed areas where increasing electropositive substitution might enhance the activity.
For comparison, the weakest compound 5ad and the most potent compound 5ef were overlaid on the map shown in Fig. 3. A small green contour around the cyclohexyl substituent of 5ef indicated that a bulky group was favored in this region. The least active compound 5ad tended to locate its piperidine moiety in a sterically unfavorable yellow contour. In general, extending the backbone into yellow areas would decrease the activity in steric fields, and green areas indicated that a large group at the R3 position would lead to increased activity. In addition, the influence of the electrostatic field was exerted mainly on the R2 position and the carbonyl oxygen of the R3 position.
 | ||
| Fig. 3 (A) The contour maps of compound 5ad for CoMFA model in steric fields. (B) The contour maps of compound 5ef for CoMFA model in steric fields. | ||
To test the utility of the model as a predictive tool, it was applied to predict the activity of a set of compounds not used in the model generation. The results were shown in entries 19–28 in Table 5. All of the 10 compounds in the test set were accurately predicted with relatively small residuals values except compound 5df. The predictive r2, which was calculated based on Patel's work13 from the CoMFA model, was found to be 0.707. The results showed that the 3D QSAR model could accurately predict the activity of the structurally diverse test set compounds and yield reliable information for further optimization of benzenesulfonyl substituted thiazole-containing tetrahydropyridine derivatives.
Experimental
Instruments
1H NMR and 13C NMR spectra were recorded at 400 MHz and 100 MHz in CDCl3, respectively. Chemical shifts were reported in ppm relative to TMS as an internal reference. FT-IR spectra were obtained on KBr plates and major peaks were reported in inverse centimeters. HRMS spectra were recorded on a spectrometer with an ESI source. X-ray single-crystal diffraction was performed on a CCD area detector.General procedure for synthesis of compounds 2 (2a as example)
Compound 2 was synthesized according to a revised reference procedure.14 A solution of 1a (200 mg, 0.67 mmol) and the corresponding vinyl ketone compound (100 mg, 0.81 mmol) in methanol (30 mL) was slowly dropped into 2.5% potassium hydroxide solution (110 mg, 2.01 mmol) in an ice bath. The obtained mixture was stirred for 30 min before removing the ice bath for another 2 h of stirring at room temperature. Methanol was evaporated under vacuum and the residue was extracted with ethyl acetate (3 × 10 mL). After condensation of the ethyl acetate phase, the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford the pure 2a as a yellow solid.
1) as the eluent to afford the pure 2a as a yellow solid.
General procedure for synthesis of compounds 3 (3a as example)
Compound 3 was synthesized according to a revision of reference procedures.11,15 To a solution of benzenesulfonamide (1140 mg, 7.22 mmol) and α,β-unsaturated carbonyl compound 2 (2400 mg, 6.0 mmol) in anhydrous DCM (40 mL) cooled to 0 °C, Et3N (1830 mg, 18.1 mmol) and TiCl4 (0.36 mL, 3.0 mmol), which was added slowly, were successively added under nitrogen atmosphere. The reaction mixture was then heated for 5 h at 50 °C. The solution was cooled to room temperature, and quenched with water (100 mL); the mixture was then extracted with DCM (2 × 30 mL). After condensation of the DCM phase, the residue was purified by column chromatography with petroleum ether/ethyl acetate (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford pure 3a as a yellow solid.
1) as the eluent to afford pure 3a as a yellow solid.
General procedure for the syntheses of N-sulfonyl-1-aza-1,3-diene compounds 5 (5aa as example)
Compound 5 was synthesized according to a revision of reference procedures.11,15 To a solution of compound 3a (100 mg, 0.18 mmol), PPh3 (8.9 mg, 0.07 mmol) and 4-methoxyphenol (9 mg, 0.07 mmol) in CH2Cl2, the corresponding vinyl ketone compound 4a was slowly added. The reaction was maintained for 12 hours at a constant temperature of 30 °C and the reaction mixture was directly condensed and purified by silica gel column chromatography with petroleum ether/ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford pure 5aa as a yellow solid.
1) as the eluent to afford pure 5aa as a yellow solid.
Synthesis procedure of 1-((3S,4R)-6-(4-methoxyphenyl)-4-(2-(piperidin-4-yl)thiazol-4-yl)-2.3,4,5-tetrahydropyridin-3-yl)propan-1-one (6)
To a solution of 5aa (760 mg, 1.68 mmol) in thioanisole (5 mL), TFA was slowly added in an ice bath, and stirred for another 3 h at room temperature. Finally, the pH was adjusted to 8–9 with a 1 mol L−1 sodium hydroxide solution, and the mixture was extracted with DCM (3 × 10 mL). After condensation of the DCM phase, the residue was purified by column chromatography with DCM/MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford pure 6 as a pale yellow solid.
1) as the eluent to afford pure 6 as a pale yellow solid.
Synthesis procedure of (1R)-1-((3S,4R)-6-(4-methoxyphenyl)-4-(2-(piperidin-4-yl) thiazol-4-yl)piperidin-3-yl)propan-1-ol (7)
Sodium cyanoborohydride (45.2 mg, 0.72 mmol) was added to a solution of compound 6 (100 mg, 0.24 mmol) in glacial acetic acid (5 mL) in an ice bath, followed by stirring for another 2 h at room temperature. The mixture was then adjusted to pH 9–10 with 1 mol L−1 sodium hydroxide solution, and extracted with EA (3 × 10 mL). After condensation of the EA phase, the residue was purified by column chromatography with DCM/MeOH (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford pure 7 as a pale yellow solid.
1) as the eluent to afford pure 7 as a pale yellow solid.
Insecticidal screening method for Aphis laburni Kaltenbach
The target compounds were evaluated for insecticidal activity against Aphis laburni.16 About 50 aphids were transferred to the shoot with 3 to 5 fresh leaves of horsebean. The shoot with aphids was cut and dipped into a solution of 100 μg mL−1 of the test compound for 2 seconds. After removing the extra solution on the leaf, the aphids were raised in the shoot at 27 °C ± 1 °C and 85% of relative humidity for 16 h by cutting it into the foam saturated with water, with a glass cylinder covering to avoid their escape. Each experiment for one compound was repeated for three times. The corrected death rate was calculated by Abbott's formula.Fungicide screening
Preliminary screening was conducted by fungal growth inhibition method.10 Fungi used in this study included Alternaria solani (AS), Botrytis cinerea (BC), Cercospora arachidicola (CA), Gibberella zeae (GZ), Phytophthora infestans (Mont) de Bary (PI), Physalospora piricola (PP), Pellicularia sasakii (Shirai) (PS), Sclerotinia sclerotiorum (SS), and Rhizoctonia cerealis (RC). Precision toxicity studies were conducted according to the above methods by determination of growth inhibition of specific fungi using the same compound in five to seven different of concentrations; the median effective concentration (EC50) was calculated by linear regression of the logarithm of the concentration with probability of the corresponding growth inhibition using Excel. The active compounds were evaluated against Botrytis cinerea resistant mutation fungi strains FJ1–10, SQ15, SJ4–8, T1–12 and T3–10 and multi-resistant mutation fungi strains Botrytis cinerea BC1 and BC2.Potential target prediction
PharmMapper server (http://59.78.96.61/pharmmapper/) was used to predict potential drug targets for benzenesulfonyl substituted thiazole-containing tetrahydropyridine compounds. The SDF format of the compound was submitted to PharmMapper server to retrieve targets with fit scores. Other settings were the default parameters.Molecular modelling and alignment for insecticidal activity
The 3D-QSAR analysis was carried out using comparative molecular field analysis (CoMFA) in SYBYL 8.0 (Tripos, St. Louis, MO, USA) molecular modeling software.17 Two-thirds of each packet was randomly selected to construct a training set, and the rest of the total compounds were used for the test set. Therefore, 18 compounds in entries 1–18 of Table 5 were chosen for CoMFA analysis, which showed insecticidal activities of the target compounds against aphids at 100 mg mL−1 and their activities were within 100%. The activity data was transformed and expressed in terms of A by the formula A = lg{a/[100 − a]Mw}, where a was the percentage of inhibition and Mw was the molecular weight of the tested compounds. Energy minimizations were carried out using the Tripos force field18 and Gasteiger-Hückel charges.19 The potent compounds in lines 1–18 were chosen as templates for molecular alignment. In this study, model validation was achieved using a standard Leave-One Out (LOO) cross-validation, and the optimal number of components was obtained. Genetic algorithm (GA) and setup stimulated annealing methods were used to modify the conformation with biggest residual values for optimizing the model.Conclusions
In summary, we reported a simple synthetic procedure to obtain substituted thiazole-containing tetrahydropyridines with high diastereoselectivity by a phosphine-catalyzed [4 + 2] annulation Aza–Rauhut–Currier reaction. Biological activity studies indicated that all the title compounds possessed moderate insecticidal activity against Aphis laburni Kaltenbach. The QSAR studies clearly elucidated the relationship between activity and structure characteristics. Some target compounds were effective against multi-resistant fungi mutations. Benzenesulfonyl substituted thiazole-containing tetrahydropyridines were predicted to be potential fumarate reductase inhibitors.Acknowledgements
Z.-J. Fan is grateful to the financial support from the National Natural Science Foundation of China (Grant No. 31571991, 21372132) and the International Science & Technology Cooperation Program of China (Grant No. 2014DFR41030), and the Tianjin Natural Science Foundation (No. 14JCYBJC20400). V. A. Bakulev thanks the Ministry of Education and Science of the Russian Federation (State task 4.1626.2014/K). Resistant mutation fungi strains FJ1–10, T1–12 and T3–10 were kindly provided by Professor Liu Xili at China Agricultural University, and resistant mutation fungi strains BC2 were kindly provided by Professor Zhou Mingguo and Duan Yabing at Nanjing Agricutural University with multi-resistance characteristics.Notes and references
- (a) A. M. Elwahy and M. R. Shaaban, RSC Adv., 2015, 5, 75659–75710 RSC; (b) P. Martins, J. Jesus, S. Santos, L. R. Raposo, C. Roma-Rodrigues, P. V. Baptista and A. R. Fernandes, Molecules, 2015, 20, 16852–16891 CrossRef CAS PubMed; (c) S. S. Rajput and S. S. Patole, World J. Pharm. Res., 2015, 4, 1566–1591 CAS; (d) T. M. Shaikh and F.-E. Hong, J. Org. Chem., 2016, 801, 139–156 CrossRef CAS; (e) A. M. Dar and Shamsuzzaman, Eur. Chem. Bull., 2015, 4, 249–259 CAS.
- (a) H. M. Aly, J. Iran. Chem. Soc., 2016, 13, 999–1009 CrossRef CAS; (b) A. L. Odom and T. J. McDaniel, Acc. Chem. Res., 2015, 48, 2822–2833 CrossRef CAS PubMed; (c) T. V. Beryozkina, I. V. Efimov, W. M. F. Fabian, N. A. Beliaev, P. A. Slepukhin, M. L. Isenov, W. Dehaen, G. Lubec, O. S. Eltsov, Z.-J. Fan, J. Thomas and V. A. Bakulev, Tetrahedron, 2015, 71(36), 6189–6195 CrossRef CAS; (d) F. Meyer, Chem. Commun., 2016, 52, 3077–3094 RSC; (e) M. A. Gouda, M. A. Sabah, W. K. Aljuhani, A. S. E. Gahani, S. A. E. Kare, E. Enazi, S. A. A. Enizi and M. M. A. Balawi, Eur. J. Chem., 2015, 6, 219–224 CrossRef CAS.
- (a) S. Kallstrom and R. Leino, Bioorg. Med. Chem., 2008, 16, 601–635 CrossRef PubMed; (b) S. Haider, Z. S. Saify, N. Begum, S. Ashraf, T. Zarreen and S. M. G. Saeed, World J. Pharm. Res., 2014, 3, 987–1024 CAS; (c) P. R. Girling, T. Kiyoi and A. Whiting, Org. Biomol. Chem., 2011, 9, 3105–3121 RSC.
- (a) X.-J. Wu, F. Kassie and V. M. Sundermann, Mutat. Res., 2005, 589, 81–102 CrossRef CAS PubMed; (b) T. Ma, Q. Zhao, J.-B. Wang, Z. Pan and J. Chen, Angew. Chem., Int. Ed., 2016, 55, 1–6 CrossRef; (c) V. L. S. Freitas and M. D. M. C. Ribeiro da Silva, J. Therm. Anal. Calorim., 2015, 121, 1059–1071 CrossRef CAS.
- (a) I. Efimov, V. Bakulev, N. Beliaev, T. Beryozkina, U. Knippschild, J. Leban, Z.-J. Fan, O. Eltsov, P. Slepukhin, M. Ezhikova and W. Dehaen, Eur. J. Org. Chem., 2014, 17, 3684–3689 CrossRef; (b) X. Zuo, N. Mi, Z. J. Fan, Q. X. Zheng, H. K. Zhang, H. Wang and Z. K. Yang, J. Agric. Food Chem., 2010, 58, 2755–2762 CrossRef CAS PubMed.
- (a) A. Ayati, S. Emami, A. Asadipour, A. Shafiee and A. Foroumadi, Eur. J. Med. Chem., 2015, 97, 699–718 CrossRef CAS PubMed; (b) C. B. Mishra, S. Kumari and M. Tiwari, Eur. J. Med. Chem., 2015, 92, 1–34 CrossRef CAS PubMed; (c) D. Mohanty, Curr. Pharm. Res., 2012, 3, 750–763 CAS; (d) F.-Y. Li, X.-F. Guo, Z.-J. Fan, Y.-Q. Zhang, G.-N. Zong, X.-L. Qian, L.-Y. Ma, L. Chen, Y.-J. Zhu, T. Kalinina, Y. Y. Morzherin and N. P. Belskaya, Chin. Chem. Lett., 2015, 26(10), 1315–1318 CrossRef CAS.
- (a) A. Rouf and C. Tanyeli, Eur. J. Med. Chem., 2015, 97, 911–927 CrossRef CAS PubMed; (b) N. Siddiqui, M. F. Arshad, W. Ahsan and M. S. Alam, Int. J. Pharm. Sci. Drug Res., 2009, 1, 136–143 CAS; (c) I. Hutchinson, S. A. Jennings, B. R. Vishnuvajjala, A. D. Westwell and M. F. G. Stevens, J. Med. Chem., 2002, 45, 744–747 CrossRef CAS PubMed; (d) K. D. Hargrave, F. K. Hess and J. T. Oliver, J. Med. Chem., 1983, 26, 1158–1163 CrossRef CAS PubMed; (e) D. Pansuriya, K. Menpara, N. Kachhadiya, J. Menpara and K. Ladva, Pharma Chem., 2015, 7, 328–333 CAS.
- (a) A. Guan, C.-L. Liu, X.-P. Yang and M. Dekeyser, Chem. Rev., 2014, 114, 7079–7107 CrossRef CAS PubMed; (b) S. S. Mosse, F. Cederbaum, C. Lamberth, G. Berthon, J. Umarye, V. Grasso, A. Schlereth, M. Blum and R. Waldmeier, Bioorg. Med. Chem., 2015, 23, 2129–2138 CrossRef PubMed.
- (a) J.-C. Yang, X.-F. Sun, F. Yang and C.-L. Liu, Agrochemicals., 2013, 52, 50–52 CAS; (b) G. Hung, J.-C. Yang, H.-C. Li, J. Zhang and C.-L. Liu, Agrochemicals., 2011, 50, 79–82 Search PubMed.
- F.-Y. Li, Y.-J. Zhu, Z.-J. Fan, J.-H. Xu, X.-F. Guo, G.-N. Zong, H.-B. Song, L. Chen, Y.-Q. Song, L.-Y. Ma and J.-R. Wang, Chin. J. Struct. Chem., 2015, 34, 659–666 CAS.
- (a) Z.-G. Shi, P.-Y. Yu, T. P. Loh and G.-F. Zhong, Angew. Chem., Int. Ed., 2012, 51, 7825–7829 CrossRef CAS PubMed; (b) Z.-G. Shi and T. P. Loh, Angew. Chem., Int. Ed., 2013, 52, 8584–8587 CrossRef CAS PubMed; (c) Z.-G. Shi, Q.-J. Tong, W. W. Y. Leong and G.-F. Zhong, Chem.–Eur. J., 2012, 18, 9802–9806 CrossRef CAS PubMed; (d) X.-N. Zhang, G.-Q. Chen, X. Dong, Y. Wei and M. Shi, Adv. Synth. Catal., 2013, 355, 3351–3357 CrossRef CAS.
- (a) X. Lu, Y. Du and C. Lu, Pure Appl. Chem., 2005, 77, 1985–1990 CrossRef CAS; (b) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102–3116 RSC; (c) Q. Zhang, L.-G. Meng, K. Wang and L. Wang, Org. Lett., 2015, 17, 872–875 CrossRef CAS PubMed; (d) L. Liang and Y. Huang, Org. Lett., 2016, 18, 2604–2607 CrossRef CAS PubMed; (e) E. Li, H.-X. Jin, P.-H. Jia, X.-L. Dong and Y. Huang, Angew. Chem., Int. Ed., 2016, 55, 11591–11594 CrossRef CAS PubMed; (f) C.-J. Yang, X.-Y. Chen, T. Tang and Z.-J. He, Org. Lett., 2016, 18, 1486–1489 CrossRef CAS PubMed.
- P. D. Patel, M. R. Patel, N. Kaushik-Basu and T. T. Talele, J. Chem. Inf. Model., 2008, 48, 42–55 CrossRef CAS PubMed.
- (a) G. Hilt and C. Hengst, J. Org. Chem., 2007, 72, 19 CrossRef PubMed; (b) M. A. Fakhfakh, A. Fournet, E. Prina, J. F. Mouscadet, X. Franck, R. Hocquemiller and B. Figadère, Bioorg. Med. Chem., 2003, 11, 5013–5023 CrossRef CAS PubMed; (c) C.-H. Tseng, C.-C. Tzeng, C.-Y. Hsu, C.-M. Cheng, C.-N. Yang and Y.-L. Chen, Eur. J. Med. Chem., 2015, 97, 306–319 CrossRef CAS PubMed; (d) F. D. Lewis, R. S. Kalgutkar and J. S. Yang, J. Am. Chem. Soc., 2001, 123, 3878–3884 CrossRef CAS PubMed.
- (a) E. Li, P.-H. Jia, L. Liang and Y. Huang, ACS Catal., 2014, 4, 600–603 CrossRef CAS; (b) L.-G. Meng, P.-J. Cai, Q.-X. Guo and S. Xue, J. Org. Chem., 2008, 73, 8491–8496 CrossRef CAS PubMed; (c) W.-D. Rao, Sally, M. J. Koh and P. W.-H. Chan, J. Org. Chem., 2013, 78, 3183–3195 CrossRef CAS PubMed; (d) C. Simal, T. Lebl, A. M. Z. Slawin and A. D. Smith, Angew. Chem., Int. Ed., 2012, 51, 3653–3657 CrossRef CAS PubMed.
- Y.-D. Li, W.-T. Mao, Z.-J. Fan, J.-J. Li, Z. Fang, X.-T. Ji, G. N. Zong and F.-Y. Li, Chem. Res. Chin. Univ., 2014, 30(3), 390–395 CrossRef CAS.
- D.-D. Guo, Z.-W. Wang, Z.-J. Fan, H. Zhao, W. Zhang, J.-G. Cheng, J.-Q. Yang, Q.-J. Wu, Y.-J. Zhang and Q. Fan, Chin. J. Chem., 2012, 30(10), 2522–2532 CrossRef CAS.
- M. Clark, R. D. Cramer and N. Van Opdenbosch, J. Comput. Chem., 1989, 10, 982–1012 CrossRef CAS.
- J. Gasteiger and M. Marsili, Tetrahedron, 1980, 36, 3219–3228 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1491770. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra24342h |
| This journal is © The Royal Society of Chemistry 2016 |