Synthesis of ultra-long hierarchical ZnO whiskers in a hydrothermal system for dye-sensitised solar cells
Guo Gao*a,
Leping Yub,
Ajayan Vinuc,
Joseph G. Shapter *b,
Munkhbayar Batmunkhbd,
Cameron J. Shearer
*b,
Munkhbayar Batmunkhbd,
Cameron J. Shearer b,
Ting Yina,
Peng Huanga and
Daxiang Cui*a
b,
Ting Yina,
Peng Huanga and
Daxiang Cui*a
aInstitute of Nano Biomedicine and Engineering, Shanghai Engineering Research Center for Intelligent Diagnosis and Treatment Instrument, Department of Instrument Science and Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, P. R. China. E-mail: guogao@sjtu.edu.cn; dxcui@sjtu.edu.cn
bSchool of Chemical and Physical Sciences, Flinders University, Bedford Park, Adelaide 5042, Australia. E-mail: joe.shapter@flinders.edu.au
cFuture Industries Institute, University of South Australia, Mawson Lakes, Adelaide 5059, Australia
dSchool of Chemical Engineering, The University of Adelaide, Adelaide 5005, Australia
First published on 8th November 2016
Abstract
One-dimensional (1-D) ZnO structures are of great interest for many applications but the direct hydrothermal synthesis of ultra-long ZnO whiskers (>100 μm) remains a great challenge. Herein, we demonstrate the first synthesis of three kinds of ultra-long hierarchical ZnO whiskers, which are defined as ZnO-2 (>100 μm in length), ZnO-3 (>200 μm in length with relatively smooth surface) and ZnO-4 (>200 μm in length with relatively rough surface), via a one-pot hydrothermal process. The maximum length of hierarchical ZnO-4 whiskers can reach up to about 270 μm. The formation of oval-shaped quasi-hollow structural precursors plays a key role for the correct attachment of Zn2+-terminated and O2−-terminated active surfaces, producing well-ordered Zn2+⋯O2⋯Zn2+ bonds, and finally promoting the formation of ultra-long ZnO whiskers with hierarchical structures. When the synthesized ultra-long hierarchical ZnO-4 whiskers are mixed with commercial TiO2 for dye-sensitised solar cells (DSCs), the current density increases significantly from 13.68 mA cm−2 (commercial TiO2) to 16.81 mA cm−2 (TiO2–ZnO hybrid materials). The hybrid materials show a conversion efficiency of 7.95% which is higher as compared to that of commercial TiO2 (5.87%). This interesting performance of a hybrid material sheds light on the possibility of preparing ultra-long hierarchical ZnO whiskers (>100 μm) with tunable lengths through hydrothermal approaches and their application in DSCs.
Introduction
Recently, one-dimensional (1-D) ZnO whiskers have attracted considerable interest due to their unique physical and chemical properties which leads to potential application in electronics/optoelectronics, dye-sensitized solar cells, gas sensors and photocatalytic degradation.1–5 In particular, ultra-long (>100 μm) ZnO whiskers are highly desired for these device applications. As for the synthesis of ultra-long ZnO whiskers, physical vapor deposition, chemical vapor deposition, thermal oxidation methods and wet chemical approaches have been widely applied.4,5,32,34,35 Hu et al. reported a thermal evaporation method for the synthesis of ZnO whiskers (several hundred of micrometers in length) using ZnS and Fe(NO3)3 as reactants in an alumina tube at 1300 °C.6 Sun et al. suggested a simple two-step evaporation oxidation strategy for synthesis of highly-oriented ZnO whiskers (several hundred of micrometers in length) in a temperature range of 920–980 °C.7 Zhu et al. proposed heterogeneous substrates with catalysts for synthesis of ultra-long ZnO whiskers (130 μm in length) at 960 °C.8 All of these reaction processes need high temperature and hence are energy consuming and require specialist reaction equipment. Alternatively, low-cost hydrothermal methods (100–374 °C, 0.1–22 MPa) have been developed to prepare ultra-long 1-D ZnO whiskers which are attractive due to their potential for large-scale synthesis and commercial feasibility.ZnO is an amphoteric oxide with a direct band gap of 3.37 eV.1,9 It is known that the electron configuration of Zn is 1s22s22p63s23p63d104s2, O is 1s22s22p4 and valence electron of Zn is 4s2. Owing to the 3d10 electron configuration, Zn2+ can exist in many different intermediates. The c axis of growing ZnO precursors have high energy polar surfaces with alternating Zn2+-terminated and O2−-terminated active surfaces.10 The newly generated nuclei tend to absorb on the polar surface of the precursors in the hydrothermal system. After adsorption, the polar surface is converted into the opposite polar surface. With the repeated conversion of polar surfaces, the nuclei grow fast along the c axis, leading to the formation of 1-D ZnO whiskers. Although great efforts have been devoted to the hydrothermal synthesis of ultra-long ZnO whiskers,11–18 to the best of our knowledge, the maximum reported length of the ZnO whiskers is only 70–80 μm and the synthesis of ultra-long ZnO whiskers (>100 μm in length) has not been reported to date. The synthesis of ultra-long ZnO whiskers (>100 μm) in a hydrothermal system has long been considered difficult if not impossible due to the presence of other anions, cations and surfactant molecules in hydrothermal system which may occupy the active sites of polar surface, influencing the continuous ordered assembly on the polar surface along the c axis leading to the growth termination of 1-D ZnO whiskers. It has been reported that Na+ is attracted by the negative ions around the growing nanocrystal and produces a virtual capping layer which hinders the further growth of the nanocrystal.19 In order to successfully synthesise ultra-long ZnO whiskers (>100 μm) in a hydrothermal system, we must construct ideal precursors with desired structures that allow the oriented attachment of Zn2+-terminated and O2−-terminated active surfaces, forming well-ordered Zn2+⋯O2⋯Zn2+ bonds leading to the continuous growth of whiskers along c axis.
In this contribution, a facile one-pot hydrothermal method has been developed for the first time to synthesis three kinds of ultra-long hierarchical ZnO whiskers (>100 μm) with different shapes. Whiskers with lengths up to about 270 μm have been produced. In addition, ZnO whiskers (∼50 μm in length) can also be achieved with a simple adjustment of the reaction parameters. When the synthesized ultra-long hierarchical ZnO whiskers have been mixed with commercial TiO2 paste for using them as photoanodes for dye-sensitized solar cells (DSCs), a measured maximum efficiency (η) of 7.95% has been achieved which is higher as compared with that of commercial TiO2 paste (5.87%).
Experimental section
Materials
ZnSO4·7H2O, Na2CO3, poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (F127) and cetyltrimethylammonium bromide (CTAB) are all analytical grade, and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).Synthesis of ultra-long hierarchical ZnO whiskers
The ZnO whiskers (>100 μm) were synthesized at 260 °C for 10 h in 100 mL Teflon-lined stainless steel autoclave by one-pot hydrothermal process (the temperature was increased at a rate of 4.3 °C min−1). In a typical procedure, 0.05 g poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (F127) was dissolved in 10 mL deionized water, then the F127 solution and another 10 mL cetyltrimethylammonium bromide (CTAB, 0.1 M) solution were simultaneously added into a certain concentration of ZnSO4·7H2O solution (20 mL). After stirring for 5–10 min, 25 mL of Na2CO3 solution was gradually added. The molar ratio between ZnSO4·7H2O solution and Na2CO3 solution was adjusted in the range of 0.4–0.8 according to the reaction processes. The synthesized ZnO whiskers (∼50 μm in length) are defined as ZnO-1, and other three kinds of ultra-long hierarchical ZnO whiskers are defined as ZnO-2 (>100 μm in length), ZnO-3 (>200 μm in length with relatively smooth surface) and ZnO-4 (>200 μm in length with relatively rough surface), respectively.Characterization of ultra-long hierarchical ZnO whiskers
The composition and structures of ZnO whiskers were characterized by X-ray powder diffractometer (XRD, Rigaku, Japan) with Cu Kα radiation (λ = 0.15418 nm), scanning electron microscopy (SEM, FEI-Sirion 200), transmission electron microscopy (TEM, JEM-2010), high-resolution transmission microscopy (HRTEM) and selected area electron diffraction (SAED). Raman spectra were recorded on a WITec alpha300R microscope using a 532 nm laser (NA 0.9) operating at constant power.Fabrication of the paste for DSCs
Fabrication of ZnO paste was carried out according the following procedure: 0.1 g ZnO whiskers, 0.02 g ethyl cellulose and 0.01 g terpinel was added into 5 mL of ethanol and dispersed mechanically at 175 °C until a soft uniform paste was obtained. Another 5 mL of ethanol was added into the paste to obtain a suitable viscosity for the coating preparation. The TiO2–ZnO hybrid paste was obtained by mixing the ZnO paste and TiO2 paste according to the weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97. The commercial TiO2 paste, and the obtained ZnO paste and TiO2–ZnO paste were used for DSCs.
97. The commercial TiO2 paste, and the obtained ZnO paste and TiO2–ZnO paste were used for DSCs.
Fabrication and evaluation of DSCs devices
The DSCs were fabricated by making photoanodes using the ZnO paste, commercial TiO2 paste and TiO2–ZnO hybrid paste by means of a screen printing method. The FTO was firstly cleaned by detergent (Pyroneg), and then washed by ethanol and deionized water under ultra-sonication for 10 min, respectively. Subsequently, the FTO was dried by a stream of nitrogen gas. The cleaned FTO (20 × 25 mm) was added into the TiCl4 solution (800 μL TiCl4 in 200 mL deionized water) and heated in oven at 70 °C for 35 min. Then, the paste was doctor-bladed on FTO with a thickness of about 15 μm. After that, the FTO was heated to 125 °C (6 min), 325 °C (7 min), 380 °C (6 min), 450 °C (16 min) and 500 °C (16 min). When the FTO was cooled to room temperature, the FTO was immersed in the TiCl4 solution again, and heated in an oven 70 °C for 35 min. The obtained FTO was then heated again according to the above procedure. When it was cooled to room temperature, FTO was immersed in N719 dye solution (0.3 mM) for 24 h. As for the fabrication of counter electrode, the clean FTO was firstly brushed with H2PtCl6 solution (2 mM), then heated at 450 °C for 20 min to give Pt-coated FTO. The active area of dye-sensitized TiO2 (ZnO, TiO2–ZnO) was about 0.25 cm2. The electrodes were sealed and filled with I3−/I− electrolyte by vacuum back-filling process. J–V curves of DSCs were measured by a solar simulator under AM1.5G. The power density of collimated xenon-arc light at the cell plane was calibrated to 100 mW cm−2 with standard test silicon cell (PV Measurements, NIST-traceable certification).Results and discussions
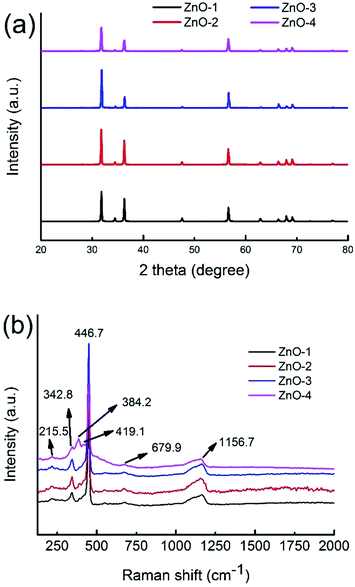
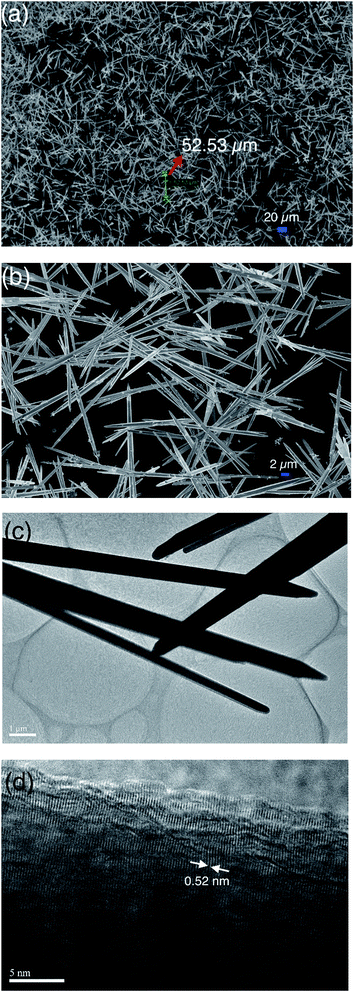
Fig. 1a shows the X-ray diffraction (XRD) patterns of the synthesized ZnO whiskers. All the diffraction peaks can be well indexed to the wurtzite ZnO structure (JCPDS No. 36-1451). No other impurities have been observed in the pattern. In order to study the vibrational properties of synthesized ZnO whiskers, Raman spectra were measured at room temperature, as shown in Fig. 1b. The Raman spectra of four ZnO samples with high intensity involve the E2(high)–E2(low) at about 342.8 cm−1, the E2(high) at about 449.8 cm−1 and the TO + LO at about 1164.7 cm−1.20 Compared with ZnO-1 whiskers (∼50 μm in length), the ultra-long hierarchical ZnO-4 whiskers (>200 μm in length with relatively rough surface) have an obvious strong peak in the A1(TO) region at about 384.2 cm−1. The peaks at 384.2 2 cm−1 are attributed to the intrinsic defects in ZnO whiskers such as zinc interstitials and oxygen vacancies,31 implying that the amount of zinc interstitials and oxygen vacancies in ultra-long hierarchical ZnO-4 whiskers is significantly higher than that of ZnO-1 whiskers (∼50 μm in length) which are beneficial to the promising device applications.When the molar ratio between ZnSO4·7H2O solution and Na2CO3 solution was in 0.4, ZnO whiskers with a length of about 50 μm can be obtained in large quantities. Fig. 2a shows the scanning electron microscopy (SEM) images of the ZnO-1 sample. The SEM images illustrate that the synthesized products are all whisker-like 1D structures. The majority of the ZnO whiskers are about 50 μm in length and usually 1.5–2.0 μm in diameter. Fig. 2b shows that the ZnO products have hexagonal whiskers with needle-like end planes. The surface of the synthesized ZnO whiskers was smooth and straight. The transmission electron microscopy (TEM) image of ZnO-1 sample is shown in Fig. 2c. It is observed that the surface of ZnO whiskers seems to be free of any particles or hierarchical structures, and the termination of the ZnO whiskers has a sharp tip. Fig. 2d shows the HRTEM image of as-prepared ZnO whiskers. The clear lattice fringes suggested that the as-prepared ZnO whiskers have high crystallisation. The lattice spacing is measured to be 0.52 nm, corresponding to the (0001) plane of ZnO. The (0001) plane is perpendicular to the c axis of the 1-D whiskers, suggesting that the growth direction of ZnO whiskers is along (0001) plane.
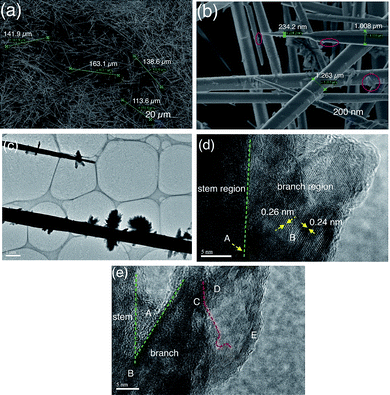
Interestingly, when the molar ratio between ZnSO4·7H2O solution and Na2CO3 solution was increased to 0.8, ultra-long hierarchical ZnO-2 whiskers (>100 μm) can be formed in large quantities, as shown in Fig. 3a. The length of four randomly selected whiskers is 113.6 μm, 138.6 μm, 141.9 μm and 163.1 μm, respectively. It can be seen that the length of majority ZnO-2 whiskers is over 100 μm. Fig. 3b shows that the ultra-long hierarchical ZnO-2 whiskers still exhibit hexagonal structures with needle-like tips and that the diameter of ZnO-2 whiskers decreases rapidly near the tip of whiskers, varying from 1.008 μm to 234.2 nm and then becomes even sharper. Compared with ZnO-1 whiskers, the surface of ultra-long ZnO-2 whiskers possesses hierarchical structure as shown in the red circular regions in Fig. 3b. The hierarchical structures of ultra-long ZnO-2 whiskers were further characterized by TEM, as shown in the image in Fig. 3c. The morphology of ultra-long ZnO-2 whiskers is similar to a tree. The TEM image shows that the hierarchical structures on the stem of ZnO-2 whiskers presented a flower-like shape. The stem of ZnO-2 whiskers is very dark whereas the branch of hierarchical structures is very light in color, suggesting the thickness of hierarchical structures is very thin. Fig. 3d shows the HRTEM image of ultra-long ZnO-2 whiskers. The continuous lattice fringes in the ‘A’ region across the stem and branch of ZnO-2 whiskers can be seen clearly suggesting that the flower-like hierarchical structures are epitaxial growth from the stem of ZnO-2 whiskers. The HRTEM image in the ‘B’ region shows the measured lattice spacing of nanocrystallites is 0.24 nm and 0.26 nm, respectively, which corresponds to the (101) and (002) planes of wurtzite ZnO structure. The inter-joint aggregation of nanocrystallites confirms the polycrystalline character of ZnO-2 whiskers. Fig. 3e shows further information regarding the flower-like hierarchical structures. The ‘A’ region in Fig. 3e is the junctional zone between the stem area and branch area, which has no evident lattice fringes suggesting an amorphous structure in this area. The amorphous structure in ‘A’ region shows that the chemical reactivity of this region is higher than other highly-crystalline regions, which facilitates the continuous growth of branch structures coming from the stem of the ZnO whiskers. The ‘B’ region is the foundation for the branch structures growth, which is completely embedded into the stem of ZnO whiskers. The ‘C’ region and ‘D’ regions are the secondary structures of the ZnO branches. The secondary structures are also built by a lot of ultra-thin ZnO sheets (the ‘E’ region). They fused and conjugated together, and finally assembled into 3-D flower-like architectures.
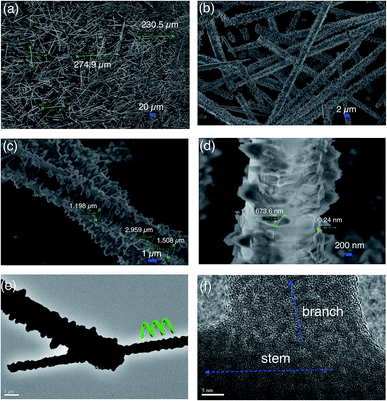
When the concentration both ZnSO4·7H2O solution and Na2CO3 solution was increased twofold but their molar ratio was still maintained at a value of 0.8, ultra-long hierarchical ZnO-3 whiskers (>200 μm) can be obtained in high yield. Fig. 4a shows that the length of randomly selected ZnO-3 whiskers is 222.1 μm, 229.5 μm and 230.9 μm, respectively. Fig. 4b shows that the diameter of ZnO-3 whiskers changes from 1.117 μm to 3.286 μm, and decreases sharply near the tip of whiskers with a diameter of ∼555.9 nm. Fig. 4c shows the TEM image of as-prepared ZnO-3 whiskers. It can be seen that the ultra-long ZnO-3 whiskers still present hierarchical structures. However, we observed that the branch region exhibit feather-like structures, unlike the flower-like structures observed for the ZnO-2 whiskers. Most of the feather-like branch structures were perpendicular to the stem of ZnO-3 whiskers. Fig. 4d shows the higher magnification of ZnO-3 whiskers. It is interesting to observe that the branch structures are connected with the stem of whiskers by the bamboo-like joint, which has the potential to endow the branch structures much more nucleation points for further growth. Fig. 4e shows the general morphology of the branch structures. It seems that the branch structures are composed of ultra-thin ZnO sheets. The HRTEM image in Fig. 4f shows that the ultra-thin ZnO sheets are an aggregation of numerous nanocrystallites. The selected area electron diffraction (SAED) patterns in Fig. 4g show the poly-crystalline character of the ultra-thin ZnO sheets.
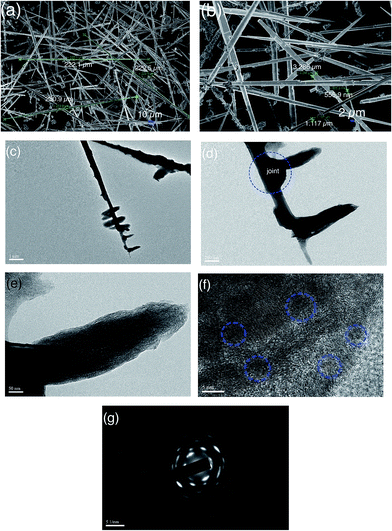 | ||
| Fig. 4 SEM image of as-prepared ZnO-3 sample (a and b), TEM image (c–e), HRTEM image (f) and SAED pattern (g). | ||
When using 10 mL of de-ionized water to replace the 10 mL CTAB (0.1 M) solution in the procedure used to make ZnO-3, ultra-long hierarchical ZnO-4 whiskers (>200 μm) can be prepared, as shown in Fig. 5a. The maximum length of the ZnO-4 whiskers observed was about 270 μm. Fig. 5b shows that the synthesized ultra-long ZnO-4 whiskers still exhibited a hierarchical structure. It can be seen that the surface of ZnO-4 whiskers is covered by numerous branch structures. The morphology and structure of ZnO-4 whiskers are different than those observed for the ZnO-3 whiskers. Fig. 5c shows that the diameter of ultra-long hierarchical ZnO-4 whiskers is about 2.959 μm, and the length of branch structures on the stem of ZnO-4 whiskers varies from 1.198 μm to 1.508 μm. Fig. 5d shows the width of branch structures is about 673.6 nm, and the thickness is about 90.24 nm. It is clearly seen that the amount of branch structures on the surface of ZnO-4 whiskers is significantly higher than that of ZnO-2 and Zn-3 whiskers. Fig. 5e shows that the ultra-long hierarchical ZnO-4 whiskers exhibit a special helix structure. HRTEM image of Fig. 5f shows that the branch structures are nearly perpendicular to the stem of ZnO-4 whiskers. The lattice fringes in the joint region of branch and stem are disordered inter-conjunction together, indicating the polycrystalline character of the synthesized ZnO-4 whiskers.
In order to reveal the formation mechanism of ultra-long hierarchical ZnO whiskers, the precursors at different temperatures from the hydrothermal reaction has been investigated. Fig. 6 shows the TEM images of the ZnO-4 precursors when the reaction temperature was at room temperature, 80 °C, 120 °C, 160 °C, 200 °C and 260 °C. Fig. 6a shows that the TEM image of precursors obtained at room temperature. It is seen the precursors are composed of numerous oval-shaped quasi-hollow structures. The HRTEM image of a selected oval-shaped quasi-hollow structure (OSQHS) in Fig. 6b suggested that the thickness of the OSQHS is thinner than that of the edge. We also observed that the tip of OSQHS exhibits an amorphous structure which suggests that the chemical reactivity of tip region is higher than other edge regions. When the temperature was elevated to 80 °C, the two neighbouring OSQHSs (A region and B region) are aggregated together (C region) via the active tip region, as show in Fig. 6c. With the increase of temperature (120 °C, Fig. 6d), the two neighbouring OSQHSs are continuously fused leading to formation of a 1-D dumb-bell shaped structure. The distance of the overlapping region of the two OSQHSs also has an evident decrease. With a further increase in temperature (160 °C), the other edge regions of OSQHS become active. The 1-D dumb-bell shaped structures assemble into 3-D architectures via the edge fusion of neighbouring dumb-bell shaped structures, as shown in Fig. 6e. When the temperature was elevated to 200 °C, short 1-D structures (∼100 nm) with hollow holes are observed, as shown in Fig. 6f. When the temperature reached 260 °C, long 1-D structures are formed (Fig. 6g). The higher resolution TEM image in Fig. 6h shows the surface of 1-D structures have many hollow holes. With increasing reaction time, the 1-D structures gradually grew longer and thicker. After reaction at 260 °C for 10 h, ultra-long hierarchical ZnO-4 whiskers are formed. We speculate that these hollow holes on the surface of 1-D precursor structures are devoted to the growth of the branch hierarchical structures.
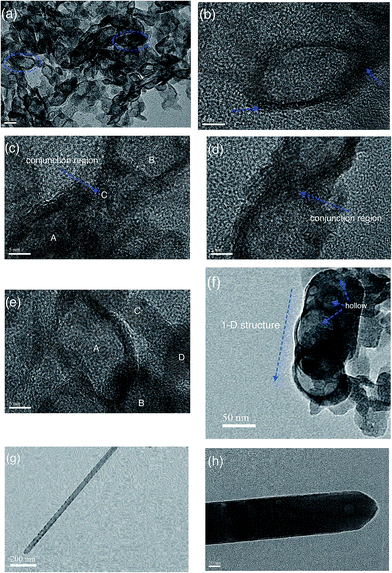 | ||
| Fig. 6 TEM and HRTEM images of the obtained precursors at room temperature (a and b), 80 °C (c), 120 °C (d), 160 °C (e), 200 °C (f) and 260 °C (g–h). | ||
The chemical reaction between Zn2+ and CO32− in alkaline solution is:
| 5Zn2+ + 2CO32− + 6OH− → Zn5(CO3)2(OH)6 |
| Zn5(CO3)2(OH)6 → 5ZnO + 2CO2↑ + 3H2O↑ |
When the ZnSO4·7H2O solution and Na2CO3 solution were mixed at room temperature, numerous Zn5(CO3)2(OH)6 precursors with oval-shaped quasi-hollow structures are formed in the presence of the surfactant. At the elevated temperatures, the precursors gradually decompose and assemble into 1-D structures with the assistance of the surfactant. It is known that the surfactant plays a key role for the formation of micro/nano-shaped architectures with desired sizes and shapes.27,28 We believe that the formation of oval-shaped quasi-hollow structural precursors contribute to the formation of ultra-long hierarchical ZnO whiskers in the hydrothermal system. In our experiments, control of the precursor's morphology is critical for the correct oriented attachment of Zn2+-terminated and O2−-terminated active surfaces, producing well-ordered Zn2+⋯O2⋯Zn2+ bonds, and resulting in the continuous growth of 1-D whiskers along c axis. These nuclei grow their own preferred growth direction along c axis during the hydrothermal process leading to formation of ultra-long 1-D structures.33
In order to explore a potential application of the synthesized ultra-long hierarchical ZnO whiskers for DSCs, the performance of photovoltaic cells with photoanodes made using the synthesized ultra-long hierarchical ZnO whiskers and TiO2–ZnO hybrid materials was compared against the commercial TiO2 materials (Table 1). Fig. 7a shows the photovoltaic performance of cells using the ultra-long hierarchical ZnO whiskers only for the photoanodes. For ZnO-2 sample (>100 μm in length), the device shows a current density (Isc) of 13.59 mA cm−2, an open-circuit voltage (Voc) of 0.30 V and an overall conversion efficiency of 1.29% under 1 Sun (AM 1.5G condition). For ZnO-3 sample (>200 μm in length with relatively smooth surface), no evident change on the current density is observed whereas the voltage increases from 0.30 V (ZnO-2) to 0.41 V (ZnO-3) and the fill factor has increased by 0.1. The increase in voltage and fill factor lead to an overall increase of conversion efficiency to 2.32%. As for ZnO-4 sample (>200 μm in length with relatively rough surface), the voltage of ZnO-4 changes slightly compared with ZnO-3 whereas a significant increase in the current density from 13.49 mA cm−2 to 17.43 mA cm−2 is observed, and an overall conversion efficiency of 2.77% is obtained. Fig. 7b shows the photovoltaic performance of the commercial TiO2 and TiO2–ZnO hybrid materials. It is clear that the open circuit voltage changes very little whereas the current density increases from 13.68 mA cm−2 (commercial TiO2) to 16.81 mA cm−2 (TiO2–ZnO hybrid materials) which was responsible for the 2.08% increase in conversion efficiency. The hierarchical ZnO-4 whiskers in the TiO2–ZnO hybrid materials plays a dual function in DSCs with a high dye loading and a high light scattering capability, offering a maximum conversion efficiency of 7.95% in comparison to that of commercial TiO2 (5.87%).
| Device | Current density Jsc (mA cm−2) | Voc | Fill factor (%) | Efficiency (%) |
|---|---|---|---|---|
| ZnO-2 whiskers | 13.59 | 0.30 | 0.32 | 1.29 |
| ZnO-3 whiskers | 13.49 | 0.41 | 0.42 | 2.32 |
| ZnO-4 whiskers | 17.43 | 0.39 | 0.40 | 2.77 |
| Commercial TiO2 | 13.68 | 0.67 | 0.64 | 5.87 |
| TiO2–ZnO-4 hybrid material | 16.81 | 0.67 | 0.70 | 7.95 |
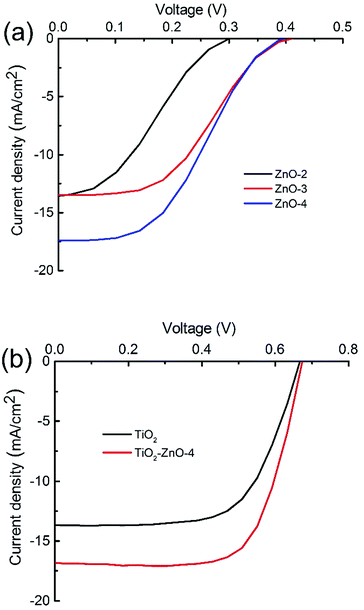 | ||
| Fig. 7 J–V curves of DSCs fabricated using ZnO whiskers (a), commercial TiO2 and TiO2–ZnO hybrid materials (b). | ||
As for pure TiO2 nanomaterials, a further increase in conversion efficiency has been limited by energy loss due to the recombination between electrons and either the oxidized dye molecules or electron-accepting species in the electrolyte during the charge transport.20 The introduction of 1-D wide-band-gap ZnO whiskers (Eg = 3.37 eV) are favourable for electron transport and light scattering and reduction of the recombination losses when used in DSCs.21,22 Under visible light, DSCs use the dye's capacity to inject photo-excited electrons into the conduction band of n-type semiconductor TiO2/ZnO structures. These carriers are subsequently transported toward and collected by a back-contact electrode. The synthesized ultra-long ZnO whiskers exhibit three-dimensional hierarchical structures which are favourable for the light scattering and harvesting. The hierarchical ZnO whiskers have larger surface areas which are favourable for the adsorption of a large amount of dyes that will generate electrons by absorbing visible solar light. Furthermore, 1-D hierarchical ZnO whiskers will provide large surface areas for charge separation and also provide direct conducting channels for the charge transfer. It has been demonstrated that the hybrid TiO2/ZnO hierarchical structures could greatly enhance the DCS's electrochemical performance.23–26 Our results also confirmed that the ultra-long hierarchical ZnO whiskers can enhance the conversion efficiency of TiO2 for DSCs. The improved photovoltaic performance can be attributed to the rational design and construction that make full utilisation of the advantages of hierarchical ZnO whiskers and TiO2 materials.
Conclusions
We have reported the first one-pot hydrothermal process for synthesis of three kinds of ultra-long hierarchical ZnO-2 whiskers (>100 μm in length), ZnO-3 whiskers (>200 μm in length with relatively smooth surface) and ZnO-4 whiskers (>200 μm in length with relatively rough surface) thus demonstrating the possibility of synthesising ultra-long ZnO whiskers (>100 μm) in a hydrothermal system. Our strategy for successfully controlling the precursors' morphology with an oval-shaped quasi-hollow structure contributes to the formation of ultra-long hierarchical ZnO whiskers, and promisingly inspires a novel way to design and synthesize other ultra-long metal oxides whiskers using a hydrothermal approach. Photoanodes based on the ultra-long hierarchical ZnO whiskers exhibited a superior conversion efficiency of 7.95% in comparison with that of commercial TiO2 (5.87%) which makes a huge breakthrough in the field of DSCs.Acknowledgements
We thank the funding support from 863 High-Tech project of China (2014AA020701), National Natural Science Foundation of China (No. 81671737) and 973 Project (2015CB931802). The support of the Australian Research Council Discovery Program (DP130101714) is gratefully acknowledged. Munkhbayar Batmunkh acknowledges International Postgraduate Research Scholarship (IPRS) and Australian Postgraduate Award (APA) for their financial support during his study in Australia. We acknowledge the use of South Australian nodes of the Australian Microscopy & Microanalysis Research Facility (AMMRF) and the Australian National Fabrication Facility (ANFF) at Flinders University.Notes and references
- Z. W. Pan, Z. R. Dai and Z. L. Wang, Science, 2001, 291, 1947 CrossRef CAS PubMed.
- J. Xu, Z. Chen, J. A. Zapien, C.-S. Lee and W. Zhang, Adv. Mater, 2014, 26, 5337 CrossRef CAS PubMed.
- S. Wang, P. Wang, C. Xiao, B. Yao, R. Zhao and M. Zhang, CrystEngComm, 2013, 15, 9170 RSC.
- J. Shi, S. Grutzik and X. Wang, ACS Nano, 2009, 3, 1594 CrossRef CAS PubMed.
- D. K. Dubey, D. N. Singh, S. Kumar, C. Nayak, P. Kumbhakar, S. N. Jha, D. Bhattacharya, A. K. Ghosh and S. Chatterjee, RSC Adv., 2016, 6, 22852 RSC.
- J. Q. Hu, X. L. Ma, Z. Y. Xie, N. B. Wong, C. S. Lee and S. T. Lee, Chem. Phys. Lett., 2001, 344, 97 CrossRef CAS.
- X. Sun, Z. Deng and Y. Li, Mater. Chem. Phys., 2003, 80, 366 CrossRef CAS.
- G. Zhu, Y. Zhou, S. Wang, R. Yang, Y. Ding, X. Wang, Y. Bando and Z. Wang, Nanotechnology, 2012, 23, 055604 CrossRef PubMed.
- K. Omichi, K. Kaiya, N. Takahashi, T. Nakamura, S. Okamoto and H. Yamamoto, J. Mater. Chem., 2001, 11, 262 RSC.
- R. A. Laudise and A. A. Ballman, J. Phys. Chem., 1960, 64, 688 CrossRef CAS.
- C. Lu, L. Qi, J. Yang, L. Tang, D. Zhang and J. Ma, Chem. Commun., 2006, 33, 3551 RSC.
- Q. D. Truong, T. H. Le, T. Kimura, S. Yin, T. Satob and Y. C. Ling, RSC Adv., 2013, 3, 19154 RSC.
- G. S. Wang, Y. Y. Wu, X. J. Zhang, Y. Li, L. Guo and M. S. Cao, J. Mater. Chem. A, 2014, 2, 8644 CAS.
- H. S. Qian, S. H. Yu, J. Y. Gong, L. B. Luo and L. L. Wen, Cryst. Growth Des., 2005, 5, 935 CAS.
- J. Wang, X. Li, Y. Xia, S. Komarneni, H. Chen, J. Xu, L. Xiang and D. Xie, ACS Appl. Mater. Interfaces, 2016, 8, 8600 CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2003, 125, 4430 CrossRef CAS PubMed.
- J. Wang, L. Zhang, S. Hou, J. Chen and L. Xiang, CrystEngComm, 2014, 16, 7115 RSC.
- G. C. Park, S. M. Hwang, S. M. Lee, J. H. Choi, K. M. Song, H. Y. Kim, H. S. Kim, S. J. Eum, S. B. Jung, J. H. Lim and J. Joo, Sci. Rep., 2015, 5, 10410 CrossRef CAS PubMed.
- R. Viswanatha, H. Amenitsch and D. D. Sarma, J. Am. Chem. Soc., 2007, 129, 4470 CrossRef CAS PubMed.
- L. M. Peter, J. Phys. Chem. C, 2007, 111, 6601 CAS.
- Q. Zhang, C. S. Dandeneau, X. Zhou and G. Cao, Adv. Mater., 2009, 21, 4087 CrossRef CAS.
- V.-M. Guérin, J. Rathousky and T. Pauporté, Sol. Energy Mater. Sol. Cells, 2012, 102, 8 CrossRef.
- K. E. Kim, S. R. Jang, J. Park, R. Vittal and K. J. Kim, Sol. Energy Mater. Sol. Cells, 2007, 91, 366 CrossRef CAS.
- T. Guo, L. Chen, L. Liu, Y. Cheng, X. Zhang, Q. Li, M. Wei and B. Ma, J. Power Sources, 2012, 201, 408 CrossRef CAS.
- A. K. Chandiran, M. Abdi-Jalebi, M. K. Nazeeruddin and M. Grätzel, ACS Nano, 2014, 8, 2261 CrossRef CAS PubMed.
- S. Alimirsalari, F. Tajabadi, S. M. Salehkoutahi, R. Ghahary and N. Taghavinia, RSC Adv., 2014, 4, 45174 RSC.
- T. Kawawaki, H. Wang, T. Kubo, K. Saito, J. Nakazaki, H. Segawa and T. Tatsum, ACS Nano, 2015, 9, 4165 CrossRef CAS PubMed.
- Z. R. Tian, J. A. Voigt, J. Liu, B. Mckenzie, M. J. Mcdermott, M. A. Rodriguez, H. Konishi and H. Xu, Science, 2003, 2, 821 CAS.
- M. Y. Nassar, M. M. Moustafa and M. M. Taha, RSC Adv., 2016, 6, 42180 RSC.
- Y. Qiu, W. Chen and S. Yang, J. Mater. Chem., 2010, 20, 1001 RSC.
- X. Peng, Nanowires-Recent Advances, InTech, New York, 2012 Search PubMed.
- M. H. Huang, Y. Wu, H. Feick, N. Tran, E. Weber and P. Yang, Adv. Mater., 2001, 13, 113 CrossRef CAS.
- S. Chakraborty, C. S. Tiwary and P. Kumbhakar, J. Phys. Chem. Solids, 2015, 78, 84 CrossRef CAS.
- S. Chakraborty and P. Kumbhakar, Mater. Lett., 2013, 100, 40 CrossRef CAS.
- W. I. Park, G. C. Yi, M. Kim and S. J. Pennycook, Adv. Mater., 2002, 14, 1481 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |