Study on the synthesis of MFI and FER in the presence of n-butylamine and the property of n-butylamine in a confined region of zeolites
Shaoqing Zhang,
Xin Liu,
Yifu Zhang ,
Tianming Lv,
Jiqi Zheng,
Wenyan Gao,
Xiaoyu Liu,
Miao Cui* and
Changgong Meng*
,
Tianming Lv,
Jiqi Zheng,
Wenyan Gao,
Xiaoyu Liu,
Miao Cui* and
Changgong Meng*
School of Chemistry, Dalian University of Technology, Dalian 116024, China. E-mail: mcui@dlut.edu.cn; cgmeng@dlut.edu.cn; Fax: +86-411-84708545; Tel: +86-411-84708545
First published on 6th December 2016
Abstract
ZSM-5 and ferrierite have been successfully hydrothermally crystallized in a gel system of sodium aluminosilicate using n-butylamine (n-BA) as structure directing agent. The zeolite crystals obtained were characterized by X-ray diffraction (XRD), infrared spectra (IR), Raman spectra, thermal analysis (TG/DTA), elemental analysis (EA), inductively coupled plasma emission spectrometry (ICP) and 13C NMR spectroscopy. The location and charge of the n-BA present within the solid materials were found to be significant, and it is possible to identity the nature of the organic species occluded in the channel system of the as-synthesized zeolites. n-BA is occluded in its protonated form together with dibutylamine in ZSM-5, while incorporated intact in its protonated form in ferrierite. Furthermore, the oxidation of n-BA restricted in zeolites by H2O2 has been studied. It is found that the restricted organic species in zeolites were oxidized by H2O2, and the oxidation products are different from the bulk reaction. The adsorption tests illustrated that the as-synthesized zeolites and their oxidized products have a high adsorption capacity for Ni(II). This work not only opens new opportunities for further research of zeolites and inorganic hybrids, but also provides a new idea for the application study of the SDAs present in molecular sieves.
1. Introduction
It is well established that the addition of organic structure-directing agents (SDAs) to an amorphous aluminosilicate or silicate gel can affect the framework structure and/or composition of the zeolite formed.1,2 The presence of organic molecules would result in the organization of tetrahedral TO4 units around them to form the building blocks for the nucleation and growth of a desired phase.3,4 In addition, they usually end up entrapped inside the void spaces of the crystallized product. However, the precise mechanism of the structure direction by organic additives such as amines and alkylammonium ions in the synthesis of zeolites and related materials is still poorly understood. Thus, detailed knowledge of the nature and extent of interactions between the occluded organic molecules and the zeolite framework is of fundamental importance in understanding the exact role of the organic SDAs during the zeolite crystallization process.2 In exploratory research aimed at the synthesis of new zeolite phases, it is often important to know how the organic species are being incorporated and whether a specie has been occluded intact5–10 or has undergone some degradation or other reaction.3,11–14 Specific template agents usually direct different structure types of zeolites,15,16 and their states in the synthesis process are not always in the same.2,17–20According to the previously reports, ZSM-5 and ferrierite could both be synthesized in the presence of n-BA.21,22 ZSM-5 with MFI structure has crystallographically distinct 12 T-sites and consists of parallel and straight 10-membered ring (MR) channels intersected by sinusoidal 10-MR channels.23 Ferrierite zeolite, with the FER topology, contains a two-dimensional (2D) pore system consisting of 10-MR channels intersected by 8-MR channels.24 Due to the different structure types of ZSM-5 and ferrierite, the conformation of the n-BA molecules and the relative mobilities of the different atoms in the organic cations are different. Hence, the molecular property of n-BA should produce a different result.
To the best of our knowledge, there has been no report on the investigation of the variation of properties of n-BA in a restricted region. In the present work, ZSM-5 and ferrierite have been synthesized using n-BA as structure directing agent. Our current work is aimed at examining the state of n-BA occluded during the course of synthesis of different zeolites, ZSM-5 and ferrierite. Furthermore, since primary amines can be partly oxidized to oximes by H2O2,25–27 the oxidation of n-BA within the zeolites has been studied by adding H2O2. Due to the results of our study, the oxidized products of n-BA in ZSM-5 and ferrierite by H2O2 are different with the organics in bulk reaction. The adsorption of Ni(II) by the as-synthesized samples and the oxidized products were also studied. This work promotes the study of the roles of SDAs in the synthesis of zeolites. What's more, the oxidation transformation of n-BA in confined regions of zeolites opens new opportunities for further research of molecular reactions on the inorganic hybrids and provides a new idea for the application study of the SDAs present in the molecular sieves.
2. Experimental
2.1 Synthesis procedure of ZSM-5 and ferrierite
The hydrogels were obtained by adding the source of silica (99.8% silica fume, Aldrich) to a previously prepared homogeneous solution consisting of n-butylamine C4H11N (Prolabo 99.7%), sodium hydroxide (Carlo Erba), sodium aluminate (45% Al2O3, 55% Na2O, Aldrich) and distilled water.In the synthesis of ZSM-5, the molar composition of the resultant mixture was 91SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Al2O3
1Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6Na2O
6Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.5NaCl
15.5NaCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.6n-BA
13.6n-BA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718.2H2O. The gel was sealed in 50 mL stainless steel Teflon lined autoclaves and hydrothermal treatment began after 2 h of stirring and carried out at 180 °C for 2 d. In the synthesis of ferrierite, it is necessary to add ammonium fluoride NH4F (96.0%, Aldrich) and the synthesis gel to obtain ferrierite has the following molar composition: 13.58SiO2
718.2H2O. The gel was sealed in 50 mL stainless steel Teflon lined autoclaves and hydrothermal treatment began after 2 h of stirring and carried out at 180 °C for 2 d. In the synthesis of ferrierite, it is necessary to add ammonium fluoride NH4F (96.0%, Aldrich) and the synthesis gel to obtain ferrierite has the following molar composition: 13.58SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Al2O3
1Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30NH4F
30NH4F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31n-BA
31n-BA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57.36H2O. The gel was sealed in 50 mL stainless steel Teflon lined autoclaves and hydrothermal treatment began after 2 h of stirring and carried out at 200 °C for 10 d. The as-synthesized samples were filtered, washed with distilled water, and dried at 100 °C overnight. The organic species were removed by calcining the as-synthesized samples in air at 550 °C for 6 h.
57.36H2O. The gel was sealed in 50 mL stainless steel Teflon lined autoclaves and hydrothermal treatment began after 2 h of stirring and carried out at 200 °C for 10 d. The as-synthesized samples were filtered, washed with distilled water, and dried at 100 °C overnight. The organic species were removed by calcining the as-synthesized samples in air at 550 °C for 6 h.
2.2 Oxidation of as-synthesized ZSM-5 and ferrierite by H2O2
0.5 mL H2O2 (30 wt% solution in water) was added to a suspension of as-synthesized ZSM-5 or ferrierite (0.07 g) in deionized water (50 mL). The mixture was stirred for 24 h. The product was subsequently isolated by filtration under suction and dried in a vacuum oven at 100 °C overnight.2.3 Characterization
X-ray diffraction (XRD) patterns were measured by a PANalytical X'Pert Powder diffractometer (equipped with a graphite monochromator), using Cu Kα radiation. The range of scanning was 3–50° at a rate of 8° min−1. IR transmission spectra were recorded with a Nicolet 6700 FTIR spectrometer over the spectral region from 400 to 4000 cm−1 with a resolution of 4 cm−1 using the KBr disc technique. Raman spectra were measured by a Thermo Scientific Raman spectrometer, with a 532 nm excitation line. The content of Si, Al, Na and Si/Al ratio were recorded by inductively coupled plasma emission spectrometer (ICP, Optima2000DV, PerkinElmer). The thermogravimetric analysis was performed on a TA Instrument, Q50. The measurement was conducted from 25 to 800 °C at a heating rate of 10 °C min−1 under nitrogen flow. ElementarvarioEL III elemental analyzer was used to determine the content of carbon and nitrogen in the synthesized zeolites. The Brunauer–Emmet–Teller (BET) surface areas of the samples were determined by a Micromeritics ASAP-2020 porosity analyzer after being degassed at 150 °C for 10 h. The 13C MAS NMR spectra were recorded on a Bruker AVANCE III 600 spectrometer at a resonance frequency of 150.9 MHz. Magicangle spin rates of 8 kHz and a 4 mm MAS probe was used for the 13C MAS measurement. The chemical shifts of 13C were externally referenced to TMS.2.4 Theoretical methods and models
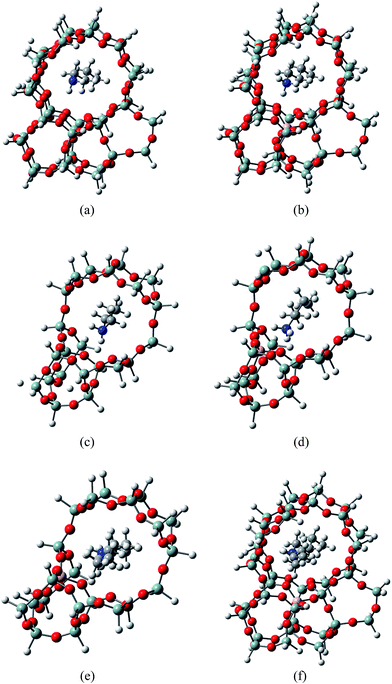
Density functional theory based calculations were performed to investigate the existence form of n-BA and dibutylamine (d-BA) within the framework of ZSM-5 and ferrierite. A 37 T cluster model truncated from the single crystalstructure was used to reproduce the micropore structures of ZSM-5 zeolite (Fig. 1a).28 The Si atom at the T12 site was replaced with an Al atom, to simulate the interaction of n-BA with the Bronsted acid site in the framework of ZSM-5.29 The pore structure of ferrierite was simulated with a 22 T cluster truncated from the bulk lattice and T4 site was considered as the most preferential Al site (Fig. 1b).30 n-BA and d-BA adsorption within the zeolite channels without Bronsted acid sites was also investigated for comparison. During the calculations, the n-BA and d-BA molecules and the Si and O atoms around the Bronsted acid site were full relaxed while the remaining atoms were constrained at their crystal position. The C, H, and N atoms were all treated with 6-311++g(2d,p) basis sets while the Si and O atoms were treated with 6-31g(d,p) basis sets,31 and the calculations were performed with B3LYP functional32,33 as implemented in Gaussian 09 package.34 | ||
| Fig. 1 The 37 T model of ZSM-5 (a) and the 22 T model of ferrieite (b). (O: red; Si: gray; Al: pink; H: white). | ||
2.5 Batch adsorption study
All the experiments were carried out by using batch technique under ambient conditions and the materials included the as-synthesized ZSM-5 and ferrierite, ZSM-5 and ferrierite calcined at 550 °C for 6 h, as-synthesized ZSM-5 and ferrierite oxidized by H2O2. Metal ion solutions were prepared by dissolving analytical NiCl2·6H2O in deionized water. Batch experiments were conducted in 50 mL Erlenmeyer flasks containing 100 mg L−1 Ni(II) concentrations. The flasks were sealed and agitated in a vibrator at 150 rpm at ambient temperature of 25 °C for 24 h. The solution was filtered through a 0.45 μm nylon syringe filter and the nickel content was determined by atomic absorption spectroscopy. In these experiments, the data were the averages of duplicate determinations. The relative errors of the data were controlled about 5%.The adsorption capacity of the adsorbent at equilibrium was also calculated using the following equation:35,36
 | (1) |
3. Results and discussion
3.1 Characterization of as-synthesized ZSM-5 and ferrierite
Fig. 2 shows the XRD diagram of the as-synthesized ZSM-5 (Fig. 2b) and ferrierite (Fig. 2d) in the n-BA–H2O system, respectively. Fig. 2a and c are stick pattern indicators for ZSM-5 and ferrierite, respectively. As observed, the XRD diagram exhibited no peaks other than standard diagram and those corresponding to the crystalline ZSM-5 and ferrierite the reported in the literature,9,37,38 indicating that pure phase ZSM-5 and ferrierite are obtained.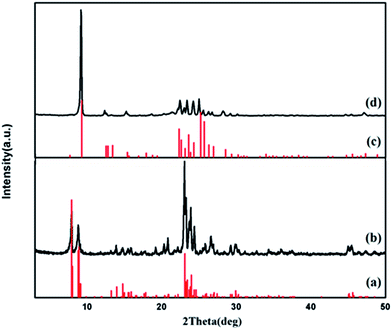 | ||
| Fig. 2 XRD patterens of standard ZSM-5 (a), as-synthesized ZSM-5 (b), standard ferrierite (c) and as-synthesized ferrierite (d). | ||
The texture properties of the calcined ZSM-5 and ferrierite were characterized by nitrogen adsorption analysis. As shown in Fig. 3, both of the samples exhibit a typical type I adsorption isotherms which reveal an essentially microporous nature of these materials.3 Table 1 lists the detailed textural properties of the calcined samples. The nitrogen BET area of ZSM-5 and ferrierite are 348 and 225 m2 g−1, respectively. The microporous volume of ZSM-5 and ferrierite are 0.11 and 0.10 cm3 g−1, respectively.
| Sample | SBET (m2 g−1) | Vmicro (cm3 g−1) |
|---|---|---|
| Calcined ZSM-5 | 348 | 0.11 |
| Calcined ferrierite | 225 | 0.10 |
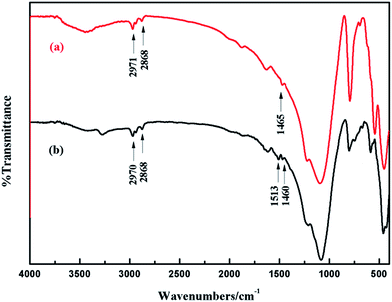
FT-IR spectra of n-BA, as-synthesized ZSM-5 and ferrierite are shown in Fig. 4. The broad band at 3413 cm−1 and the sharp peak at 1635 cm−1 are attributed to the structural hydroxyl groups and bending mode of physically adsorbed water, respectively. A characteristic band around 1221 cm−1 is represented T–O (T = Si or Al) symmetric stretching.39 The bands associated with the asymmetric and symmetric stretching vibrations of T–O–T bridges are at 1097 cm−1 and 796 cm−1 in ZSM-5 while at 1086 cm−1 and 791 cm−1 in ferrierite. The band at 545 cm−1 was assigned to the presence of distorted double five member rings (DDR-5) in ZSM-5 and ferrierite framework.40,41 A characteristic peak at 451 cm−1 is due to the T–O–T bending vibrations. The bands at 2965 cm−1 and 2873 cm−1 empirically attributed to the C–H stretching vibration of n-BA. The FT-IR spectrum of ferrierite (Fig. 4c) exhibits similar overall characteristics of that of ZSM-5. The remaining band at 1476 cm−1 in Fig. 4a and 1514 cm−1 and 1467 cm−1 in Fig. 4c could be ascribed to –CH2 deformation vibration.42 It is found that almost all adsorption bands observed for the n-BA/ZSM-5 and n-BA/ferrierite samples shown in Fig. 4 are different from those of n-BA (Fig. 4e), indicating that n-BA interacts strongly with the zeolites framework.43,44 After calcined at 550 °C for 6 h, the peaks at 1476 cm−1, 1514 cm−1 and 1467 cm−1 are absent, indicating that the organic species are removed (see Fig. 4b and d).
 | ||
| Fig. 4 IR spectra of samples: (a) the as-synthesized ZSM-5; (b) ZSM-5 calcined at 550 °C for 6 h; (c) the as-synthesized ferrierite; (d) ferrierite calcined at 550 °C for 6 h and (e) n-BA. | ||
Fig. 5A shows the Raman spectra of the calcined ZSM-5 (Fig. 5A(a)) and ferrierite (Fig. 5A(b)). The bands at 466 cm−1 in ZSM-5 and 424 cm−1 in ferrierite are assigned to the presence of 5-membered rings, respectively.45,46 Fig. 5B shows the Raman spectra of the as-synthesized products. The broad and weak bands at 1601 cm−1 in Fig. 5B(a) and 1608 cm−1 in Fig. 5B(b) are assigned to the –NH2 scissoring vibration. The bands at 2940 cm−1 and 1443 cm−1 in Fig. 5B(a) and 2935 cm−1 and 1445 cm−1 in Fig. 5B(b) could be ascribed to –CH3 and –CH2 deformation vibration, respectively.47,48 Because of the occluded of n-BA molecules in the two zeolites, the presence of 5-membered rings in ZSM-5 framework leads to a shift of the prominent band to 463 cm−1, while ferrierite exhibit the band at 426 cm−l.
 | ||
| Fig. 5 (A) Raman spectra of the calcined samples at 550 °C for 6 h: (a) ZSM-5 and (b) ferrierite; (B) Raman spectra of the as-synthesized ZSM-5 (a) and ferrierite (b). | ||
The TG/DTA curves of ZSM-5 and ferrierite are shown in Fig. 6. The first weight loss between 25 and 100 °C in Fig. 6a and below 300 °C in Fig. 6b correspond to desorption of physisorbed water. The peak in Fig. 6a between 100 and 250 °C is due to the removal of adsorbed n-BA. The third weight loss in Fig. 6a illustrates that ZSM-5 using n-BA as structure directing agent give rise to a sharp exothermic peak in the range from 250 to 460 °C which is due to the n-BA decomposition. The last weight loss from 460 to 700 °C should be attributed to the removal of dibutylamine which was formed during the synthetic process. This speculate was later confirmed by elemental analysis and 13C NMR spectroscopy (see below). In Fig. 6b, the weight loss between 300 and 700 °C is due to the removal of n-BA. The percentage composition of organic species in ZSM-5 is 5.84% (by weight) and in ferrierite presented to 8.19% (by weight).
The compositions of as-synthesized ZSM-5 and ferrierite are determined according to the ICP, elemental and TG analyses (Table 2). The Si/Al ratio of ZSM-5 is 28.63 while the ferrierite is 9.48. The n-BA/Al ratios of ZSM-5 and ferrierite are 1.71 and 1.04, respectively. The calculated cell compositions for the as-synthesized products of ZSM-5 and ferrierite are |Na0.9(C4H11N)3.2(H2O)6.6|[(Si91.9Al4.1O192)] and |(C4H11N)3.2(H2O)2.8|[(Si32.8Al3.2O72)], respectively. The ratio of C/N obtained for the sample ZSM-5 (C/N = 4.27) is slightly higher than that corresponding to the n-BA (C/N = 4), suggesting that some chemical fragments resulting from the polymerization of the n-BA in the synthesis gel must have been incorporated within the cavities during crystal growth. However, the ratio obtained for the ferrierite (C/N = 3.37) is lower, probably because of the addition of NH4F and the ratio of n-BA and NH4F is about 5.35. These results were later confirmed by 13C NMR spectroscopy (see below). The content of the organic molecules determined by CHN analysis is in agreement with the results measured by TG analysis.
| Samples | Content (%) | Si/Al | n-BA/Al | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | Na | Ha | C/Na | organica | Sic | Alc | Ratioc | Ratiod | |
| a Measured by CHN analysis.b Measured by TG analysis.c Measured by ICP analysis.d Measured by CHN and ICP analysis. | |||||||||
| n-BA/ZSM-5 | 3.96 | 1.08 | 1.31 | 4.27 | 6.02 | 33.43 | 1.13 | 28.63 | 1.71 |
| n-BA/ferrierite | 6.01 | 2.08 | 1.45 | 3.37 | 9.14 | 32.04 | 3.26 | 9.48 | 1.04 |
| n-BA/ZSM-5 + H2O2 | 3.83 | 0.81 | 0.65 | 5.57 | 5.32b | 34.37 | 1.35 | 24.55 | — |
| n-BA/ferrierite + H2O2 | 13.07 | 1.30 | 0.84 | 11.73 | 29.24b | 23.36 | 1.83 | 12.29 | — |
13C NMR can be used to investigate changes of the chemical environment around the structure-directing agent of the materials. The 13C NMR spectra of n-BA in solution and the as-synthesized samples are reported in Fig. 7, and their chemical shifts are given in Table 3. In solution (Fig. 7a), the resonance at 41.4 ppm indicates methylene groups attached to N, namely –CH2– of NH2–CH2–, while the peaks at 35.7 ppm and 19.5 ppm corresponds to methylene groups of –CH2–CH2–, the last resonance at 13.1 ppm is ascribed to methyl.21 The peaks of as-synthesized ZSM-5 (Fig. 7b) and ferrierite (Fig. 7c) appear broader and shifted when compared with n-BA, supporting that the organic cations are indeed incorporated into the zeolite pores. These data clearly show that n-BA not only remains intact (only a small portion of n-BA in ZSM-5 is exception) upon its occlusion into the pores of zeolites prepared here, but also changes in the chemical environment which is distinctly different in each of these two materials. According to the 13C NMR spectra of as-synthesized ferrierite and n-BA, we can conclude that n-BA molecules were occluded in ferrierite in their protonation states.49 A significant resonance around 49.5 ppm in the 13C NMR spectrum of ZSM-5 is assigned to an impurity of protonated dibutylamine of which compound three resonances coincide with the n-BAH+ resonances.21,50 This interpretation is supported by elemental analysis of as-synthesized ZSM-5 which showed a C/N ratio > 4. However, the fact that the resonance appearing around 49.5 ppm is much weaker than the resonances from n-BA clearly shows that most of organic SDAs used for ZSM-5 synthesis have not polymerized under the crystallization conditions. In fact, the molar ratio of n-BA and dibutylamine is about 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which can be calculated by CHN analysis data.
1, which can be calculated by CHN analysis data.
 | ||
| Fig. 7 13C MAS NMR spectra of n-BA (a), as-synthesized ZSM-5 (b) and ferrierite (c) (the star at 49.5 ppm is due to dibutylamine). | ||
| Compound | Chemical shift (ppm) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| CH3CH2CH2CH2NH2 | 13.1 | 19.5 | 35.7 | 41.4 | ||
| CH3CH2CH2CH2NH3+ | 14.2 | 20.2 | 30.0 | 40.5 | ||
| n-BA/ZSM-5 | 13.3 | 20.9 | 29.0 | 42.3 | 49.5 | |
| n-BA/ferrierite | 13.3 | 20.7 | 29.7 | 42.6 | ||
| n-BA/ZSM-5 + H2O2 | 12.3 | 20.5 | 29.1 | 41.8 | 48.9 | 51.5 |
| n-BA/ferrierite + H2O2 | 13.3 | 20.6 | 29.6, 35.3 | 42.6 | ||
Due to the notable differences in the pore structure of zeolites prepared with n-BA, on the other hand, the flexible n-BA molecules ended up encapsulated within the void spaces of the crystallized products should adopt a particular narrow range of conformations that match spatially with the structural aspects of each zeolite host. This implies that the conformations of n-BA molecules in the two zeolites are different and hence the resulting host–guest interactions in these zeolites may be considerably different from one another.51
Density functional theory based calculations were performed to investigate the existence form of n-BA and dibutylamine (d-BA) within the framework of ZSM-5 and ferrierite. The most plausible adsorption structure of n-BA in the channel of ZSM-5 were shown in Fig. 8a and b. The free energy change associated with n-BA adsorption inside the ZSM-5 channel without the introduction of Bronsted acid site at T12 (Si-ZSM-5, Fig. 8a) is −218.06 kJ mol−1. The large negative value of the free energy change proves that, apart from cationized at the Bronsted acid site, the diffusion of n-BA into the channel of ZSM-5 and serving as pore fillers is also thermodynamically preferred. When the Bronsted acid is introduced (Al-ZSM-5, Fig. 8b), the free energy change is further enlarged to −339.65 kJ mol−1. In this structure, the shortest O–H distance is only 1.80 Å and the N atoms is positively charged, showing the enhanced n-BA adsorption is contributed by both the electrostatic interaction among the cationized n-BA and the negatively charged O atoms, and the formed hydrogen bonds.
The case for n-BA adsorption in ferrierite is quite similar to that in ZSM-5 and the most plausible adsorption structures are shown as Fig. 8c and d. Due to the lack of electrostatic interaction and less efficient hydrogen bonding with the zeolite framework, the free energy change of n-BA adsorption in ferrierite without Bronsted acid site (Si-FER, Fig. 8c) is only −143.33 kJ mol−1, and is smaller by 122.73 kJ mol−1 with respect to n-BA adsorption on the Bronsted acid site (Al-FER, Fig. 8d). In this sense, no matter whether the Bronsted acid site exists, n-BA will diffuse into the channels of FER and act as pore fillers.
We also examined the adsorption of d-BA in ferrierite and ZSM-5 (Fig. 8e and f). Due to the basicity difference as compared with n-BA and the confinement effect of 10-member ring channel of ferrierite, the free energy change for d-BA adsorption at Bronsted acid sites in channels of ferrierite (Fig. 8e) is −323.75 kJ mol−1 and is 56.69 kJ mol−1 more significant as compared with that of n-BA (−266.06 kJ mol−1). For the same reasons, the free energy change for d-BA adsorption in Si-FER is also enhanced to −204.47 kJ mol−1. Taking the n-BA adsorption in Al-FER as a reference, the formation of d-BA and a NH3 adsorbed at Al-FER (free energy change as −88.53 kJ mol−1) is a free energy increase process and the free energy change is 113.12 kJ mol−1, showing that the formation of d-BA in FER is not plausible. Similar to the case in ferrierite, the d-BA adsorption in Al-ZSM-5 is as strong as −541.09 kJ mol−1. However, the strong adsorption of d-BA in Al-ZSM-5 turns the formation of a d-BA (Fig. 8f) and a NH3 adsorption in Al-ZSM-5 (free energy change as −94.17 kJ mol−1) a thermodynamically plausible process. These theoretical findings are in good agreement with the 13C NMR results that no peaks associated to d-BA were observed in the synthesized ferrierite samples, while the 13C NMR peaks with a chemical shift of 49.5 ppm can be explained as the d-BA formed in ZSM-5 samples.
3.2 Oxidation of as-synthesized ZSM-5 and ferrierite by H2O2
The oxidation of as-synthesized ZSM-5 and ferrierite were carried out by adding H2O2 to the suspension of ZSM-5 and ferrierite in deionized water.The XRD diagram exhibited no peaks other than those corresponding to the as-synthesized ZSM-5 and ferrierite (not shown).
Fig. 9 shows the FT-IR spectra of the oxidized ZSM-5 and ferrierite. Compared with the as-synthesized ZSM-5 and ferrierite in Fig. 4, there is no distinct difference in the oxidized products.
Fig. 10 displays the Raman spectra of the n-BA/ZSM-5 + H2O2 and n-BA/ferrierite + H2O2. Compared with the as-synthesized ZSM-5 and ferrierite in Fig. 5B, the spectra of the oxidized products show some differences. The bands at 1601 cm−1 in Fig. 5B(a) and 1608 cm−1 in Fig. 5B(b) which are assigned to the –NH2 scissoring vibration disappeared or weaker in Fig. 10a and b, respectively. This means that the –NH2 in n-BA reduced visually after the oxidation process. What's more, the new weak peak appears at 692 cm−1 in Fig. 10a and the obvious peak at 732 cm−1 in Fig. 10b determine the appearance of new organic substances.52,53
Fig. 11 shows the TG/DTA curves of the oxidized ZSM-5 and ferrierite. The total weight loss occurs in three and two steps in the oxidized ZSM-5 and ferrierite, respectively. The first weight loss below 250 °C in Fig. 11a and below 380 °C in Fig. 11b correspond to the removal of water. The second weight loss between 250 and 500 °C is due to the n-BA and dibutylamine decomposition. The third weight loss between 500 and 700 °C in Fig. 11a is due to the partly oxidized products during the oxidation process by H2O2. In Fig. 11b, the weight loss between 380 and 600 °C should be due to the removal of n-BA and its partly oxidized products. The percentage composition of organic in the oxidized ZSM-5 and oxidized ferrierite are both different compared with the as-synthesized samples. This is due to the formation of different organic species during the oxidation process.
The compositions of n-BA/ZSM-5 + H2O2 and n-BA/ferrierite + H2O2 are given in Table 2. The experimental C/N molar ratio obtained for the oxidized ZSM-5 (C/N = 5.57) is higher than that corresponding to the as-synthesized ZSM-5 (C/N = 4.27), suggesting that some chemical fragments formed during the oxidation process. However, the ratio obtained for the oxidized ferrierite (C/N = 12.73) is significantly higher than the as-synthesized one (C/N = 3.37), probably because of the elimination of NH4F and the formation of other organics. Compared with the as-synthesized products, the organic content of the oxidized ZSM-5 has a slight decline, while the oxidized ferrierite increased distinctly. However, the changing trend of the organic component are agree with TG analysis.
Fig. 12 shows the 13C NMR spectra of the as-synthesized ZSM-5 and oxidized ZSM-5, their chemical shifts are given in Table 3. In Fig. 12, oxidation treatment with H2O2 results in a slightly shift in the first four resonances while the last resonance at 49.5 ppm splitted into two signals (48.9 and 51.5 ppm in Fig. 12c), this splitting should be ascribed to the oxidation of NH in dibutylamine to hydroxylamine.54
The 13C NMR spectra of the as-synthesized ferrierite and its oxidized product are shown in Fig. 13. Compared to the as-synthesized ferrierite in Fig. 13b, a weak additional resonance at 35.5 ppm was observed in the n-BA/ferrierite + H2O2 (Fig. 13c). This new resonance in the oxidized product is similar with the resonance at 35.7 ppm in the 13C NMR spectrum of bulk n-BA.
 | ||
| Fig. 13 13C MAS NMR spectra of n-BA (a), as-synthesized ferrierite (b) and n-BA/ferrierite + H2O2 (c). | ||
In conclusion, a combination of FT-IR, Raman spectra, TG/DTA, elemental analysis and 13C NMR measurements has shown that n-BA changed a lot during the oxidation process by H2O2 in the two different zeolites prepared here. The oxidation products in the ZSM-5 and ferrierite are distinctly different.
3.3 Adsorption experiments
In the batch sorption studies, 0.07 g of the as-synthesized ZSM-5 and ferrierite, ZSM-5 and ferrierite calcined at 550 °C for 6 h, ZSM-5 and ferrierite oxidized by H2O2 was added to 50 mL 100 mg L−1 Ni(II) solution, respectively. The flasks were sealed and agitated in a vibrator at 150 rpm at ambient temperature of 25 °C for 24 h. For contrast, n-BA and the oxidation product of n-BA by H2O2 were also added as sorbent. As shown in Table 4, both of n-BA and the oxidation product of n-BA by H2O2 have a high adsorption capacity for Ni(II) in aqueous solutions. There is a promotional adsorption capacity from the calcined ZSM-5 to as-synthesized ZSM-5 and n-BA/ZSM-5 + H2O2, and so as the calcined ferrierite, as-synthesized ferrierite and its oxidized product. By computation, the capacity of the organic in the as-synthesized ZSM-5 and n-BA/ZSM-5 + H2O2 for the removal of Ni(II) are close to the n-BA and n-BA + H2O2 in bulk reactions. However, the capacities of the organic in the as-synthesized ferrierite and n-BA/ferrierite + H2O2 are much lower on the contrary. These results can be an evidence for the different changes during the synthesis and oxidation process in the two different zeolites.| Samples | mg of removed Ni(II) per g | mg of removeda Ni(II) per g |
|---|---|---|
| a The capacity of the organic species in the zeolite for the removal of Ni(II). | ||
| n-BA | 156.48 | — |
| n-BA + H2O2 | 141.46 | — |
| Calcined ZSM-5 | 1.17 | — |
| n-BA/ZSM-5 | 8.23 | 120.89 |
| n-BA/ZSM-5 + H2O2 | 8.51 | 137.97 |
| Calcined ferrierite | 2.37 | — |
| n-BA/ferrierite | 3.38 | 12.33 |
| n-BA/ferrierite + H2O2 | 5.67 | 11.29 |
4. Conclusions
Two kinds of zeolites have been prepared by direct synthesis using n-BA as structure directing agent. Analysis of FT-IR, Raman spectra, TG/DTA, EA and 13C MAS NMR show that n-BA is occluded in its protonated form together with dibutylamine in ZSM-5, while incorporated intact in its protonated form in ferrierite. Density functional theory based calculations were also performed to investigate the existence form of n-BA and dibutylamine within the framework of ZSM-5 and ferrierite.In the case of H2O2 as oxidant, it has been found that the restricted organic species were partly oxidized, and the oxidation products are different from the bulk reaction. The oxidation product of organic trapped in ZSM-5 was hydroxylamine, whereas unchanged in ferrierite. This was due to the different structure types of the two zeolites. The adsorption tests illustrated that the as-synthesized ZSM-5, ferrierite and their oxidized products have higher adsorption capacity for Ni(II) than the calcined samples. Thus, these materials can be applied in the adsorption of Ni(II) in aqueous solution and the application reduces the pollution caused by the calcining of SDAs.
This work opens new opportunities for further research of zeolites on the inorganic hybrids, and provides a new idea for the application study of the SDAs present in the molecular sieves.
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (Grant No. 21271037, 21573034 and 21373036), the Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase, No. NSFC2015_65 and NSFC2015_66), the Fundamental Research Funds for the Central Universities (DUT15LK18) and Science research project of Liaoning Province Education Department (L2015123).References
- B. M. Lok, T. R. Cannan and C. A. Messina, Zeolites, 1983, 3, 282–291 CrossRef CAS.
- W. C. Paik, C.-H. Shin, J. M. Lee, B. J. Ahn and S. B. Hong, J. Phys. Chem. B, 2001, 105, 9994–10000 CrossRef CAS.
- S. Candamano, P. Frontera, T. I. Koranyi, A. Macario, F. Crea and J. B. Nagy, Microporous Mesoporous Mater., 2010, 127, 9–16 CrossRef CAS.
- A. V. Goretsky, L. W. Beck, S. I. Zones and M. E. Davis, Microporous Mesoporous Mater., 1999, 28, 387–393 CrossRef CAS.
- R. A. Hearmon and A. Stewart, Zeolites, 1990, 10, 608–611 CrossRef CAS.
- M. B. Park, N. H. Ahn, R. W. Broach, C. P. Nicholas, G. J. Lewis and S. B. Hong, Chem. Mater., 2015, 27, 1574–1582 CrossRef CAS.
- L. Zhang, D. Chen, H.-Y. Nie and Y. Huang, Microporous Mesoporous Mater., 2013, 175, 147–156 CrossRef CAS.
- M. A. Miller, S. R. Miller, R. W. Broach, M. M. Galey, S. Prabhakar, B. Lyons, C. L. Nicholas and C. P. Nicholas, Microporous Mesoporous Mater., 2015, 202, 250–258 CrossRef CAS.
- G.-q. Guo, Y.-c. Long and Y.-j. Sun, Chem. Commun., 2000, 1893–1894, 10.1039/b005453o.
- B. Han, C.-H. Shin, P. A. Cox and S. B. Hong, J. Phys. Chem. B, 2006, 110, 8188–8193 CrossRef CAS PubMed.
- C. Pellegrino, R. Aiello, F. Testa, F. Crea, A. Tuel and J. B. Nagy, Microporous Mesoporous Mater., 2001, 47, 85–96 CrossRef CAS.
- C. Marichal, J. M. Chézeau, M. Roux, J. Patarin, J. L. Jordá, L. B. McCusker, C. Baerlocher and P. Pattison, Microporous Mesoporous Mater., 2006, 90, 5–15 CrossRef CAS.
- H. Suk Bong, M. Hyung-Ki, S. Chae-Ho, P. A. Cox, S. J. Warrender and P. A. Wright, J. Am. Chem. Soc., 2007, 129, 10870–10885 CrossRef PubMed.
- A. B. Pinar, L. Gomez-Hortiguela and J. Perez-Pariente, Chem. Mater., 2007, 19, 5617–5626 CrossRef.
- C. A. Fyfe, R. J. Darton, H. Mowatt and Z. S. Lin, Microporous Mesoporous Mater., 2011, 144, 57–66 CrossRef CAS.
- M. Arranz, J. Pérez-Pariente, P. A. Wright, A. M. Z. Slawin, T. Blasco, L. Gómez-Hortigüela and F. Corà, Chem. Mater., 2005, 17, 4374–4385 CrossRef CAS.
- N. R. Forbes and L. V. C. Rees, Zeolites, 1995, 15, 444–451 CrossRef.
- R. H. Jarman and M. T. Melchior, J. Chem. Soc., Chem. Commun., 1984, 7, 414–416 RSC.
- S. Hayashi, K. Suzuki, S. Shin, K. Hayamizu and O. Yamamoto, Chem. Phys. Lett., 1985, 113, 368–371 CrossRef CAS.
- S.-H. Lee, C.-H. Shin, D.-K. Yang, S.-D. Ahn, I.-S. Nam and S. B. Hong, Microporous Mesoporous Mater., 2004, 68, 97–104 CrossRef CAS.
- G. Boxhoorn, R. A. Santen van, W. A. Erp Van, G. R. Hays, N. Alma, R. Huis and A. Clague, Proceedings of the Sixth International Congress on Catalysis, 1983, pp. 694–703 Search PubMed.
- W. Fan, L. Gao and J. Dong, Mater. Lett., 2006, 60, 386–388 CrossRef CAS.
- T. Yokoi, H. Mochizuki, S. Namba, J. N. Kondo and T. Tatsumi, J. Phys. Chem. C, 2015, 119, 15303–15315 CAS.
- B. Yang, J.-g. Jiang, H. Xu, P. Ji and P. Wu, Microporous Mesoporous Mater., 2015, 203, 54–62 CrossRef CAS.
- K. Suzuki, T. Watanabe and S. Murahashi, Angew. Chem., 2008, 47, 2079–2081 CrossRef CAS PubMed.
- J. S. Reddy and A. Sayari, Catal. Lett., 1994, 28, 263–267 CrossRef CAS.
- M. Kidwai and S. Bhardwaj, Synth. Commun., 2011, 41, 2655–2662 CrossRef CAS.
- D. H. Olson, G. T. Kokotailo, S. L. Lawton and W. M. Meier, J. Phys. Chem., 1981, 85, 2238–2243 CrossRef CAS.
- W. P. Ding, G. D. Meitzner, D. O. Marler and E. Iglesia, J. Phys. Chem. B, 2001, 105, 3928–3936 CrossRef CAS.
- A. J. Jones and E. Iglesia, ACS Catal., 2015, 5, 5741–5755 CrossRef CAS.
- A. D. McLean and G. S. Chandler, J. Chem. Phys., 1980, 72, 5639–5648 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- B. Miehlich, A. Savin, H. Stoll and H. Preuss, Chem. Phys. Lett., 1989, 157, 200–206 CrossRef CAS.
- M. J. T. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Journal, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- A. Heidari, H. Younesi and Z. Mehraban, Chem. Eng. J., 2009, 153, 70–79 CrossRef CAS.
- S. Zhang, M. Cui, Y. Zhang, Z. Yu and C. Meng, J. Sol-Gel Sci. Technol., 2016, 80, 215–225 CrossRef CAS.
- S. Sang, F. Chang, Z. Liu, C. He, Y. He and L. Xu, Catal. Today, 2004, 93–95, 729–734 CrossRef CAS.
- H. Yu, X.-q. Wang and Y.-c. Long, Microporous Mesoporous Mater., 2006, 95, 234–240 CrossRef CAS.
- Y. Wang, T. Lv, H. Wang, Y. Zhao, C. Meng and H. Liu, Microporous Mesoporous Mater., 2015, 208, 66–71 CrossRef CAS.
- C. T. G. Knight, J. Phys. Chem. B, 2002, 106, 3329–3332 CrossRef CAS.
- J. C. Jansen, F. J. V. D. Gaag and H. V. Bekkum, Zeolites, 1984, 4, 369–372 CrossRef CAS.
- S. Cheng, A. Jengning Tzeng and B. Y. Hsu, Chem. Mater., 1997, 9, 1788–1796 CrossRef CAS.
- D. L. Felix, M. Strauss, L. C. Ducati and H. O. Pastore, Microporous Mesoporous Mater., 2009, 120, 187–194 CrossRef CAS.
- C. Gieck, C. Bisio, L. Marchese, Y. Filinchuk, C. E. da Silva and H. O. Pastore, Angew. Chem., Int. Ed., 2007, 46, 8895–8897 CrossRef CAS PubMed.
- P. K. Dutta, K. M. Rao and J. Y. Park, J. Phys. Chem., 1991, 95, 6654–6656 CrossRef CAS.
- P. K. Dutta, D. C. Shieh and M. Puri, Zeolites, 1988, 8, 306–309 CrossRef CAS.
- S. Slang, K. Palka, L. Loghina, A. Kovalskiy, H. Jain and M. Vlcek, J. Non-Cryst. Solids, 2015, 426, 125–131 CrossRef CAS.
- J. J. C. Teixeira-Dias, Spectrochim. Acta, Part A, 1986, 42, 589–597 CrossRef.
- H. O. Pastore, É. C. de Oliveira, G. B. Superti, G. Gatti and L. Marchese, J. Phys. Chem. C, 2007, 111, 3116–3129 CAS.
- G. Engelhardt, D. Michel, G. Engelhardt and D. Michel, High-Resolution Solid-State NMR of Silicates and Zeolites, John Wiley & Sons, New York Ny, 1987, vol. 4 Search PubMed.
- S.-H. Lee, C.-H. Shin, G. J. Choi, T.-J. Park, I.-S. Nam, B. Han and S. B. Hong, Microporous Mesoporous Mater., 2003, 60, 237–249 CrossRef CAS.
- D. Lin-Vien, N. B. Colthup, W. G. Fateley and J. G. Grasselli, The handbook of infrared and Raman characteristic frequencies of organic molecules, Elsevier, 1991 Search PubMed.
- K. Hayamizu, M. Yanagisawa, O. Yamamoto, N. Wasada, K. Someno, K. Tanabe, T. Tamura and J. Hiraishi, National Institute of Standards and Technology, Tsukuba, Japan, 2001; SDBS No. 874.
- R. M. Silverstein, F. X. Webster and D. J. Kiemle, Spectometric Indentification of Organic Compounds, John Wiley & Sons, 2005, vol. 4 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |