Multispectroscopic analysis and molecular modeling to investigate the binding of beta lactoglobulin with curcumin derivatives†
Abstract
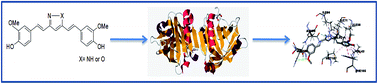
Bovine beta lactoglobulin (β-lg), the major whey protein, has a great affinity for a wide range of organic compounds like fatty acids, retinol etc. Curcumin, a polyphenolic antioxidant present in turmeric and its isoxazole (IOC) and pyrazole (PY) derivatives have been elicited worldwide for their therapeutic activities. However, the nature of interaction of β-lg with these derivatives remains unexplored. Fluorescence quenching studies suggest a static quenching mechanism for both the compounds. The average distances of 7.28 nm and 7.33 nm have been determined for IOC and PY respectively for energy transfer based on FRET which have application in many biological and biophysical fields. Circular dichroism spectra (CD) and Fourier transform infrared spectroscopy (FTIR) have been utilized to analyze the influence on the secondary structure of the protein. Docking simulation reveals a possible mechanism for different quenching behaviours and modes of binding preferred by the two compounds. Our findings will be helpful in the design of the drugs and other biologically active molecules that bind more strongly to β-lg and have the ability to show FRET.

 Please wait while we load your content...
Please wait while we load your content...