Effect of pH and temperature on conformational equilibria and aggregation behaviour of curcumin in aqueous binary mixtures of ethanol†
Nidhi K. Bhatiaa,
Shyam Kishorb,
Nidhi Katyala,
Pankaj Gogoia,
Payal Naranga and
Shashank Deep*a
aDepartment of Chemistry, Indian Institute of Technology, Hauzkhas, Delhi, New Delhi 110016, India. E-mail: sdeep@chemistry.iitd.ac.in; Fax: +91 11 26581102; Tel: +91 11 26596596
bDepartment of Chemistry, J. V. College, Baraut, Uttar Pradesh 250611, India
First published on 18th October 2016
Abstract
Recently, curcumin has emerged as a potential therapeutic agent against many chronic diseases, due to some of its significant biological properties. However, the mechanism of its action is not properly understood, due to the lack of physicochemical knowledge on this compound in aqueous media. In the present article, we have investigated the effect of pH and temperature on the conformational equilibria and aggregation behavior of curcumin in an almost completely aqueous solution and in solutions containing different binary mixtures of ethanol and water. Keto formation was favored at acidic pH, whereas neutral and anionic forms of curcumin were predominant at neutral and alkaline pH, respectively. Moreover, the curcumin solutions were found to be more heterogenous at acidic and neutral pH, due to the presence of different aggregated forms of curcumin, as characterized by fluorescence and DLS studies. Temperature was also found to significantly influence the keto–enol–enolate equilibrium, as well as the aggregation of curcumin. Keto–enol tautomerization was observed to be enthalpy driven at both acidic and neutral pH.
1. Introduction
Curcumin is a polyphenol that is present as one of the major components of turmeric. It exhibits many useful biological and pharmacological properties and thus has been used as a therapeutic agent against many chronic diseases like skin, cardio-vascular, inflammatory and ocular diseases associated with aging.1–3 Recently, it has also emerged as a potential anti-tumor and anti-amyloidogenic agent and therefore has drawn the attention of many researchers from multiple disciplines, such as chemistry, biology, medicine and physics.4–10 Unfortunately, the mechanism of action of curcumin as a potential therapeutic agent is not completely understood, due to the lack of spectral data for curcumin in aqueous media.The IUPAC name of curcumin is (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione and the molecule has two ortho methoxy phenol groups linked by an α,β unsaturated β-diketone. It exists in different tautomeric (keto–enol) and isomeric forms, as depicted in Scheme S1,† depending on the solvent and other environmental conditions.1,11 The conjugation between two aryl groups via a β-diketone moiety imparts tautomerism and interesting photophysical and photochemical properties to curcumin.11 The absorption maximum of curcumin ranges from 408 to 430 nm in most organic solvents.11 Blue-shifted and sharp peaks have been observed in non-polar solvents, whereas red-shifted and broad peaks have been observed in polar solvents.11 X-ray diffraction,12 NMR13 and DFT14–16 analyses of curcumin suggest that the cis-enolic form of curcumin is the most stable form in a vacuum, in solid form and in solution, owing to its intramolecular hydrogen bonds. NMR studies have also revealed that keto formation may become enhanced under certain conditions, such as low pH, or in protic solvents where intramolecular hydrogen bonding competes with intermolecular hydrogen bonding with the solvent.13 Steady state fluorescence studies of curcumin in various organic solvents have revealed that larger Stokes shifts occur in hydrogen bond donating or accepting solvents, when compared to aprotic and non-polar solvents. This is because the rigidity of the molecule decreases due to the perturbations caused by intramolecular hydrogen bonding in hydrogen bonding solvents.11,17 Numerous data are also available regarding the excited state photophysics of curcumin in non-polar and polar organic solvents.18–22 Curcumin is known to decay through different excited state processes, such as excited state proton transfer (ESPT), solvation and cis–trans isomerization in various organic solvents.18,23
Curcumin is reported to show varying pKa values depending on the solvent and the method of estimation.11 Its conformational equilibria depend on many factors, such as pH, temperature, and solvent polarity, etc.1,24 The keto form acts as a potent proton donor, whereas the enolate form of curcumin acts as a potent electron donor.1 Recently, binding studies of curcumin with Aβ aggregates have shown that the different tautomeric structures of curcumin have different binding affinities for the aggregates. These studies suggest that the keto form of curcumin derivatives has a weaker binding for Aβ aggregates than the enol form and the equilibrium of curcumin shifts towards the enol form during its binding to Aβ aggregates.25 The physicochemical properties and antioxidant activities of curcumin are also governed by the relative keto–enol–enolate concentrations.26 Therefore, it is important to know what factors modulate the keto–enol tautomerism and what are their relative concentrations in aqueous solution? To the best of our knowledge, little is known about the conformational equilibria of curcumin in aqueous medium.
Curcumin has a low solubility in aqueous media. Recently, temperature dependent solubility and stability studies of curcumin in aqueous media have been reported, in which the mechanism of aggregation and disaggregation and its dependence on temperature were investigated.27 Unfortunately, these studies were carried out at a very high concentration of curcumin, which is not generally used for biomedical purposes. Curcumin exists as soluble aggregates even at lower concentrations. The size and nature of these aggregates might change with a change in the pH, concentration and temperature. This may lead to a change in its physico-chemical properties and hence, its pharmacological properties. Therefore, to understand the mechanism of action of curcumin, a thorough understanding of its soluble aggregates at low concentration is required.
In the present work, we investigated the effect of temperature, pH and solvent composition on the conformational equilibria of curcumin at low concentration. We used three different pH conditions and investigated the effect of temperature and solvent composition on the equilibrium populations of curcumin by examining its absorption and emission behavior in binary mixtures of ethanol and water. The presence of different conformations was predicted using DFT calculations. For this, extensive calculations on the absorption and emission spectra of different conformations of curcumin were carried out. Although DFT studies on the absorption behaviour of the keto and enol forms of curcumin in non-aqueous and gaseous phase have been reported previously,15 its absorption behaviour in binary mixtures of ethanol and water has not been reported. We also examined the nature and size of the curcumin aggregates in different binary mixtures and under different conditions.
2. Methods and materials
Curcumin of highest purity was purchased from Sigma-Aldrich and was used without further purification. Potassium di-hydrogen phosphate, di-potassium hydrogen phosphate and ethanol of analytical grade were obtained from Merck. Stock solutions of curcumin were prepared in 100% ethanol and were protected from light. The concentration was determined by measuring the absorbance of curcumin at 429 nm and using the molar absorption coefficient of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. The ionic strength of the phosphate buffer used was 50 mM in all binary mixtures of curcumin. The buffers were prepared by mixing 50 mM of H3PO4 and 50 mM of KH2PO4 in an appropriate ratio to achieve the desired pH value in the range of 2.0–3.0. To achieve a pH range of 6.0–9.0, 50 mM of KH2PO4 and 50 mM of K2HPO4 were mixed in an appropriate ratio. Different binary mixtures of curcumin were prepared by mixing a buffer of desired pH and ethanol in an appropriate volume. The chosen concentration of curcumin was 25 μM, at which curcumin is reported to be soluble in aqueous medium.27 UV-visible spectra were recorded by using a Varian Cary-100 spectrophotometer equipped with a Cary Dual Cell Peltier Accessory. Fluorescence experiments were performed on a Varian Cary Eclipse fluorimeter. The excitation and emission slit widths were fixed at 10 nm each for all measurements. Unless mentioned, the excitation wavelength was kept at 429 nm and the emission spectra were acquired from 450 to 800 nm. Thermal studies were carried out by varying the temperature from 25 °C to 60 °C, with an interval of 5 °C, and the samples were incubated for 10 minutes at each temperature before acquisition of the spectra. The particle size distributions of curcumin in 5% ethanolic solutions at different temperatures and pH were measured using a Malvern Zetasizer ZS 90 unit fitted with a 633 nm ‘red’ laser. A glass cuvette with a round aperture was used for the measurements. The sizes reported are an average of 25 runs per measurement, with a correlation time of 10 seconds for each run. For each sample, three measurements were taken. Analysis of the data was performed using in-built Zetasizer software 6.01.
000 M−1 cm−1. The ionic strength of the phosphate buffer used was 50 mM in all binary mixtures of curcumin. The buffers were prepared by mixing 50 mM of H3PO4 and 50 mM of KH2PO4 in an appropriate ratio to achieve the desired pH value in the range of 2.0–3.0. To achieve a pH range of 6.0–9.0, 50 mM of KH2PO4 and 50 mM of K2HPO4 were mixed in an appropriate ratio. Different binary mixtures of curcumin were prepared by mixing a buffer of desired pH and ethanol in an appropriate volume. The chosen concentration of curcumin was 25 μM, at which curcumin is reported to be soluble in aqueous medium.27 UV-visible spectra were recorded by using a Varian Cary-100 spectrophotometer equipped with a Cary Dual Cell Peltier Accessory. Fluorescence experiments were performed on a Varian Cary Eclipse fluorimeter. The excitation and emission slit widths were fixed at 10 nm each for all measurements. Unless mentioned, the excitation wavelength was kept at 429 nm and the emission spectra were acquired from 450 to 800 nm. Thermal studies were carried out by varying the temperature from 25 °C to 60 °C, with an interval of 5 °C, and the samples were incubated for 10 minutes at each temperature before acquisition of the spectra. The particle size distributions of curcumin in 5% ethanolic solutions at different temperatures and pH were measured using a Malvern Zetasizer ZS 90 unit fitted with a 633 nm ‘red’ laser. A glass cuvette with a round aperture was used for the measurements. The sizes reported are an average of 25 runs per measurement, with a correlation time of 10 seconds for each run. For each sample, three measurements were taken. Analysis of the data was performed using in-built Zetasizer software 6.01.
First principles calculations were carried out within the framework of density functional theory (DFT), using the B3LYP functional and the 6-311++G (d,p) basis set, as implemented in the Gaussian 09 software package.28 The structure of the molecule was optimized in water and binary solvent (5% ethanol) via direct inversion in iterative subspace (DIIS), until the largest component of the ionic forces attained a value of 0.00045 a.u. The polarisable continuum model (PCM), using the integral equation formalism variant (PEFPCM), was employed to investigate the effect of the solvent. The solvent descriptors were assumed to vary linearly with the molar fraction of each component of the homogenous mixture. In order to confirm that the stationary point obtained corresponded to a local minimum, frequency calculations were performed at the same level of the theory.
In order to compute the absorption spectrum, time dependent density functional theory (TDDFT) calculations were carried out on the optimized structure. The vertical excitation energies excluding zero point energies and oscillator strengths were calculated at the ground state geometries. The emission spectra were also calculated at the same level of accuracy as that for absorption. For this, the TDDFT excited states with a large oscillator strength in the absorption spectrum were chosen and their structures were geometry optimized in these states. In order to further characterize the absorption spectrum, Franck–Condon (FC) analyses were performed. For this, computationally expensive frequency calculations were performed on the optimized excited states whose oscillator strengths were greater than 0.4 in the TDDFT calculation.
3. Results and discussion
3.1 DFT calculations of absorption and emission behaviour of different forms of curcumin
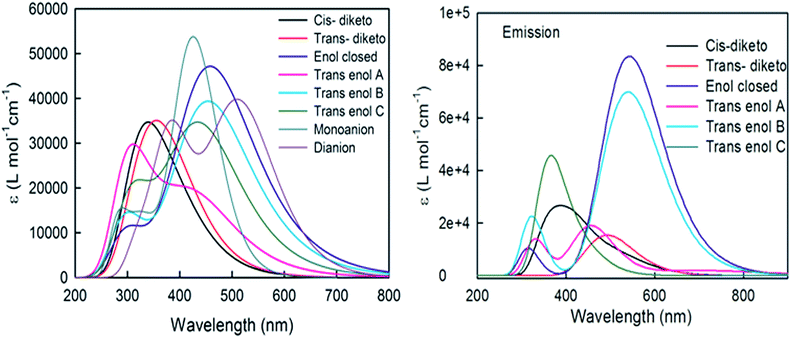
As discussed earlier, curcumin exhibits tautomerism and can exist in different isomeric forms and aggregated forms, depending on the solution conditions (Schemes S1 and S2†).11,23 We employed a DFT approach to determine the relative stability and to understand the spectral behaviour of these conformations and the possible dimeric forms of curcumin in binary mixtures of ethanol and water. The relative energies of the different forms of curcumin were calculated and the closed enol form was found to be the most stable. This stability can be attributed to the presence of intramolecular hydrogen bonding (Table 1). Fig. 1 (left panel) depicts the absorption spectra of different isomers and ionic forms of curcumin in 5% ethanol obtained using DFT calculations. The calculated wavelength maximum of each form of curcumin in 5% ethanol and their oscillator strengths are listed in Table 2. The closed enol form has the highest oscillator strength with a maximum absorption at 460 nm. For comparison, the FC incorporated absorption spectra of the isomers whose TDDFT frequency converged correctly are given in Fig. S1.† We also calculated the emission behaviour of different monomeric forms of curcumin using a DFT approach in binary mixtures of ethanol and water (Fig. 1, right panel). Maximum oscillator strength was observed in the case of the closed enol form of curcumin. Emission maxima of all the conformers are reported in Table 3. In a few selected cases it became impossible to track the desired excited state during geometry optimization. This arises due to frequent hopping in the ground state orbitals due to the closeness in energy of several excited electronic states, as has also been observed in previous studies.29| System | Energy (a.u.) in water | Relative energy (kcal mol−1) in water | Energy (a.u.) in binary solvent | Relative energy (kcal mol−1) in binary solvent |
|---|---|---|---|---|
| a Relative energies of the monoanion and dianion were computed based on stoichiometric considerations. | ||||
| Diketo (cis) | −1263.635079 | 12.45 | −1263.635064 | 7.18 |
| Diketo (trans) | −1263.643464 | 7.18 | −1263.643459 | 12.45 |
| Enol (cis closed) | −1263.654914 | 0.0 | −1263.654910 | 0.0 |
| Enol (trans-A) | −1263.627016 | 17.50 | −1263.627007 | 17.50 |
| Enol (trans-B) | −1263.641115 | 8.65 | −1263.641106 | 8.66 |
| Enol (trans-C) | −1263.636557 | 11.51 | −1263.636549 | 11.52 |
| Monoanion | −1263.182010 | {−1263.182010 + (−0.174563) − (−1263.654914)}a627.5095 = {(−1263.356573) − (−1263.654914)}a627.5095 = 187.21 | −1263.18183 + (−0.174534) = −1263.356517 | {−1263.18183 + (−0.174534) − (−1263.654910)}a627.5095 = {(−1263.356517) − (−1263.654910)}a627.5095 = 187.24 |
| Dianion | −1262.715336 | {−1262.715336 + 2(−0.174563) − (−1263.654914)}a627.5095 = {(−1263.064462) − (−1263.654914)}a627.5095 = 370.51 | −1262.715264 | {−1262.715264 + 2(−0.174534) − (−1263.654910)}a627.5095 = {(−1263.064332) − (−1263.654910)}a627.5095 = 370.59 |
| System | Solvents | nstates, E* (eV) | Excited states (@S0) | Excitation energy E (eV) | Wavelength (nm) | Oscillator strength |
|---|---|---|---|---|---|---|
| a E* is the maximum energy obtained in each case by using the maximum number of states given, nstates. | ||||||
| cis-Diketo | Water | 10, 4.49 | S0 → S1 | 3.08 | 402.39 | 0.3527 |
| S0 → S2 | 3.27 | 378.80 | 0.1535 | |||
| S0 → S5 | 3.68 | 337.28 | 0.2222 | |||
| S0 → S6 | 3.75 | 330.94 | 0.5936 | |||
| S0 → S8 | 4.03 | 307.73 | 0.1712 | |||
| Binary | 10, 4.49 | S0 → S1 | 3.08 | 402.65 | 0.3562 | |
| S0 → S2 | 3.27 | 378.95 | 0.1592 | |||
| S0 → S5 | 3.67 | 337.45 | 0.2216 | |||
| S0 → S6 | 3.74 | 331.35 | 0.5994 | |||
| S0 → S8 | 4.03 | 307.85 | 0.1669 | |||
| trans-Diketo | Water | 10, 4.28 | S0 → S1 | 3.09 | 401.29 | 0.4457 |
| S0 → S2 | 3.27 | 379.52 | 0.1773 | |||
| S0 → S4 | 3.57 | 347.13 | 0.4065 | |||
| S0 → S6 | 3.73 | 332.70 | 0.3243 | |||
| S0 → S7 | 3.86 | 321.31 | 0.1275 | |||
| Binary | 10, 4.28 | S0 → S1 | 3.09 | 401.55 | 0.4533 | |
| S0 → S2 | 3.26 | 379.72 | 0.1805 | |||
| S0 → S4 | 3.57 | 347.42 | 0.4067 | |||
| S0 → S6 | 3.72 | 332.99 | 0.3261 | |||
| S0 → S7 | 3.86 | 321.47 | 0.1260 | |||
| Enol-closed | Water | 10, 4.67 | S0 → S1 | 2.70 | 459.57 | 1.7027 |
| S0 → S8 | 4.24 | 292.39 | 0.1959 | |||
| Binary | 10, 4.67 | S0 → S1 | 2.69 | 460.58 | 1.7097 | |
| S0 → S8 | 4.24 | 292.52 | 0.1974 | |||
| Enol-trans-A | Water | 10, 4.62 | S0 → S1 | 2.80 | 442.59 | 0.5062 |
| S0 → S2 | 3.21 | 386.54 | 0.2063 | |||
| S0 → S5 | 3.88 | 319.11 | 0.4612 | |||
| S0 → S7 | 4.11 | 301.99 | 0.3013 | |||
| S0 → S8 | 4.19 | 296.13 | 0.2528 | |||
| Binary | 10, 4.62 | S0 → S1 | 2.80 | 443.15 | 0.5110 | |
| S0 → S2 | 3.21 | 386.78 | 0.2118 | |||
| S0 → S5 | 3.88 | 319.37 | 0.4697 | |||
| S0 → S7 | 4.10 | 302.17 | 0.3055 | |||
| S0 → S8 | 4.18 | 296.33 | 0.2466 | |||
| Enol-trans-B | Water | 10, 4.64 | S0 → S1 | 2.72 | 455.68 | 1.4162 |
| S0 → S7 | 4.07 | 304.30 | 0.1958 | |||
| S0 → S8 | 4.25 | 291.89 | 0.2225 | |||
| Binary | 10, 4.64 | S0 → S1 | 2.72 | 456.56 | 1.4235 | |
| S0 → S7 | 4.07 | 304.61 | 0.1985 | |||
| S0 → S8 | 4.24 | 292.02 | 0.2246 | |||
| Enol-trans-C | Water | 10, 4.66 | S0 → S1 | 2.80 | 441.93 | 1.1628 |
| S0 → S2 | 3.17 | 391.09 | 0.1150 | |||
| S0 → S5 | 3.79 | 326.91 | 0.1126 | |||
| S0 → S6 | 3.90 | 317.54 | 0.3504 | |||
| S0 → S7 | 4.08 | 303.92 | 0.1740 | |||
| S0 → S8 | 4.14 | 299.43 | 0.1011 | |||
| Binary | 10, 4.66 | S0 → S1 | 2.80 | 442.61 | 1.1706 | |
| S0 → S2 | 3.17 | 391.39 | 0.1173 | |||
| S0 → S5 | 3.79 | 327.00 | 0.1162 | |||
| S0 → S6 | 3.90 | 317.78 | 0.3450 | |||
| S0 → S7 | 4.08 | 304.12 | 0.1793 | |||
| S0 → S8 | 4.14 | 299.54 | 0.0995 | |||
| Monoanion (enol) | Water | 10, 4.37 | S0 → S2 | 2.91 | 425.54 | 1.3159 |
| S0 → S5 | 3.71 | 334.20 | 0.1369 | |||
| S0 → S6 | 3.82 | 324.18 | 0.1460 | |||
| S0 → S10 | 4.37 | 283.48 | 0.3309 | |||
| Binary | 10, 4.37 | S0 → S2 | 2.91 | 426.33 | 1.3207 | |
| S0 → S5 | 3.71 | 334.31 | 0.1384 | |||
| S0 → S6 | 3.82 | 324.54 | 0.1475 | |||
| S0 → S10 | 4.37 | 283.57 | 0.3305 | |||
| Dianion (enol) | Water | 10, 3.96 | S0 → S1 | 2.41 | 513.39 | 0.9614 |
| S0 → S3 | 3.17 | 390.95 | 0.6946 | |||
| S0 → S4 | 3.44 | 360.60 | 0.1949 | |||
| S0 → S9 | 3.85 | 321.64 | 0.2079 | |||
| Binary | 10, 3.96 | S0 → S1 | 2.42 | 512.45 | 0.9516 | |
| S0 → S3 | 3.17 | 390.57 | 0.6983 | |||
| S0 → S4 | 3.44 | 360.09 | 0.1919 | |||
| S0 → S9 | 3.86 | 321.45 | 0.2052 | |||
| System | Excited states | Excitation energy E (eV) | Wavelength (nm) | Oscillator strength |
|---|---|---|---|---|
| cis-Diketo | S0 ← S1(@S1) | 2.41 | 514.18 | 0.1822 |
| S0 ← S2(@S2) | 2.97 | 418.10 | 0.4683 | |
| S0 ← S5(@S5) | ||||
| S0 ← S6(@S6) | ||||
| S0 ← S8(@S8) | 3.45 | 359.60 | 0.4747 | |
| trans-Diketo | S0 ← S1(@S1) | 2.51 | 492.93 | 0.3808 |
| S0 ← S2(@S2) | ||||
| S0 ← S4(@S4) | ||||
| S0 ← S6(@S6) | ||||
| S0 ← S7(@S7) | ||||
| Enol-closed | S0 ← S1(@S1) | 2.28 | 542.95 | 2.0614 |
| S0 ← S8(@S8) | 3.94 | 314.70 | 0.2587 | |
| Enol-trans-A | S0 ← S1(@S1) | 1.74 | 711.37 | 0.0480 |
| S0 ← S2(@S2) | 2.72 | 456.23 | 0.4704 | |
| S0 ← S5(@S5) | ||||
| S0 ← S7(@S7) | 3.72 | 332.60 | 0.3136 | |
| S0 ← S8(@S8) | 3.86 | 320.86 | 0.0362 | |
| Enol-trans-B | S0 ← S1(@S1) | 2.30 | 540.19 | 1.7260 |
| S0 ← S7(@S7) | 3.74 | 331.26 | 0.2825 | |
| S0 ← S8(@S8) | 3.93 | 315.65 | 0.3070 | |
| Enol-trans-C | S0 ← S1(@S1) | |||
| S0 ← S2(@S2) | 2.80 | 443.33 | 0.2083 | |
| S0 ← S5(@S5) | 3.47 | 357.35 | 0.3014 | |
| S0 ← S6(@S6) | 3.23 | 384.32 | 0.4431 | |
| S0 ← S7(@S7) | 3.50 | 354.35 | 0.4763 | |
| Monoanion | S0 ← S2(@S2) | 2.38 | 519.75 | 1.4623 |
To understand the effect of aggregation on the spectral behaviour, two different dimeric forms of the closed enol form (Scheme S2†) were studied. The energies for the pi-stacked and H-bonded geometries were estimated to be −2527.9134 a.u. and −2527.9277 a.u. respectively (where 1 a.u. = 1 hartree = 2625.5 kJ mol−1 and H-bonded geometry is partially optimized). The H-bonded dimer is expected to be more stable than its pi-stacked form. The energy difference between them is 36.75 kJ mol−1. However, during optimization, the two fragments of the intermolecular H-bonded enol dimer form move away from each other as the energy decreases, whereas the π-stacked enol dimer is found to be stable.
3.2 Effect of the increase in ethanol content on conformational equilibria of the curcumin
Fig. 2 shows the absorption spectra of curcumin in various binary mixtures of ethanol and phosphate buffer of pH 7.0 at 25 °C. A prominent band at 429 nm was observed, along with a weak shoulder at 357 nm, in an almost completely aqueous solution (i.e. a 5% ethanol solution), in agreement with a previous report.30 Our DFT calculations showed a peak at 331 nm for the diketo form (trans or cis) and peaks at ∼460 nm and 425 nm for the different enolic forms (closed, trans-B, trans-C) and the monoanionic form of curcumin respectively. Therefore, we speculate that the weak shoulder at 357 nm and the peak at 429 nm might be due to the keto and enolic or monoanionic forms, respectively. The weak shoulder was observed in solutions containing up to 20% ethanol, and it gradually diminished with an increase in the ethanol content. The peak intensity at ∼429 nm, due to the enol and monoanion forms, increased with an increase in the volume percentage of ethanol. This might be due to two factors: (i) an increase in the molar extinction coefficients (ε values) of the different conformations of curcumin in these different binary mixtures and/or (ii) the shifting of equilibria towards the enol form at higher ethanol percentages.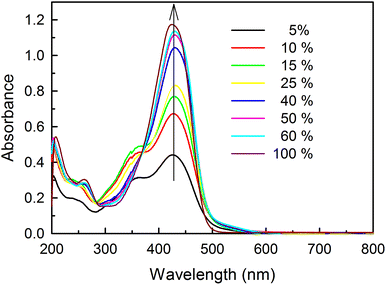 | ||
| Fig. 2 Absorption spectra of curcumin (25 μM) in solutions of different volume percentages of ethanol at pH 7.0. | ||
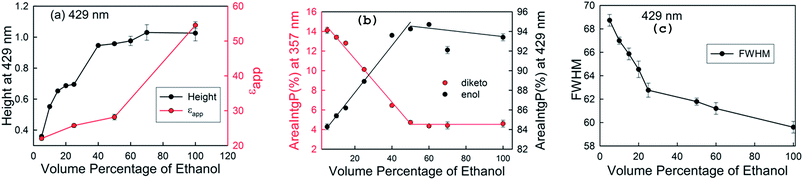
To examine the reason for the increase in the peak intensity at 429 nm, we calculated the εapp values of curcumin by measuring the absorbance of curcumin as a function of its concentration in aqueous solutions of ethanol. The εapp values were determined from the slope of the calibration plots in aqueous solutions (Fig. S2 and Table S2†). Fig. 3a depicts the comparative change in the εapp value of curcumin, and the absorbance (height) at 429 nm with an increase in the volume percentage of ethanol. Initially, there was a gradual increase in the εapp value with the increase in the volume percentage of ethanol. However, a sharp increase was observed at volume percentages of ethanol beyond 50%. We further investigated whether the increase in absorbance (height) was only due to the increase in εapp, or if it was also due to the conversion of the keto to the enol form. For this, we plotted the relative increase in the peak area at 429 nm and 357 nm as a function of ethanol concentration (Fig. 3b). The area under the peak was calculated using OriginPro 8.5 software by integrating the peaks at 429 and 357 nm. There was a sharp decrease in the percentage area corresponding to the diketo peak (357 nm) up to an ethanol volume percentage of 50%, and this value then remained almost constant upon further increasing the percentage of ethanol. Similarly, the enol content first increased and then became constant at volume percentages of ethanol beyond 50%, indicating that there is no significant change in equilibrium between the enol and keto forms at higher volume percentages of ethanol. Multivariate component resolution was also performed using Unscrambler software to obtain the pure spectra of the components and the concentration distribution of the components (Fig. S3†). There was a significant increase in the concentration of component 1 (427 nm, enol), whereas the concentration of component 2 (∼352 nm, keto) decreased with an increase in the volume percentage of ethanol. The MCR analyses were consistent with the analysis from the relative area distribution of the keto and enol forms. These results suggest that the conversion of the diketo form to the enol form is the predominant factor behind the increase in the absorbance at 429 nm in solutions with volume percentages of ethanol up to 50%. In solutions with volume percentages of ethanol more than 50%, the increase in absorbance is due to increase in the apparent epsilon value at 429 nm. We speculate that the anomalous behaviour of the ethanol–water binary mixture plays a significant role in keto–enol interconversion. It is well known that ethanol–water binary mixtures deviate from ideal behaviour at low and intermediate volume percentages of ethanol, due to the emergence of microheterogenity in such systems.31 Formation of such structures in binary mixtures has been shown to significantly affect the conformational distributions of various solutes, such as peptides32–34 and polymers31 etc. Wakisaka and Matsuura showed that in 5% ethanol solution, the hydrogen bonded clusters of water are conserved like those in bulk water.35 They also suggested that there is bicontinuous phase separation at the microscopic level in solutions containing 10 to 90% ethanol by volume, due to the coexistence of self-associated ethanol clusters and hydrogen bonded water clusters. Therefore, the presence of such ethanol clusters at higher volume percentages of ethanol increases the local concentration of ethanol around curcumin and thus favors enol formation, as the enol form is known to be more stable in non-polar solvents.36,37 However, at a lower volume percentage of ethanol, the presence of water clusters perturbs the intramolecular hydrogen bonding in the enol form and the equilibrium shifts towards the diketo form. We also observed a sharp decrease in the full width at half maximum (FWHM) with an increase in the volume percentage of ethanol, which suggests a decrease in the heterogeneity of the solution (Fig. 3c).
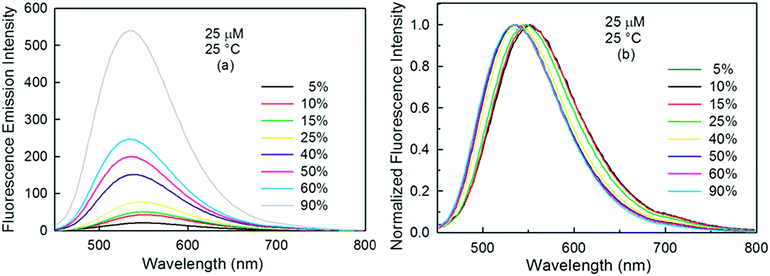
The effect of the volume percentage of ethanol on the excited state photophysics of curcumin was also examined by exciting it at 429 nm and measuring its fluorescence in different binary mixtures of ethanol and buffer (Fig. 4a). A broad peak was observed with a maximum fluorescence intensity at ∼534 nm in 90% ethanol solution, whose intensity gradually decreased with an increase in water (buffer) content. Our DFT results indicate that the peak at ∼540 nm is due to the enol form of curcumin, confirming that the absorption band at 429 nm is also due to the enol form. A bathochromic shift was observed with an increase in the polar solvent content (i.e. buffer), which indicates the intramolecular charge transfer character of the S1 state (Fig. 4b).17 It is known that the fluorescence signal is proportional to the product of the quantum yield and the epsilon value of the fluorophore.38 Therefore, the decrease in fluorescence intensity might be due to two reasons: the low εapp value of curcumin in solvent mixtures with a high water content, or the quenching of curcumin fluorescence by water. To understand the reason behind the decrease in fluorescence intensity at 548 nm, we compared the relative change in the area under the peak at 548 nm and the relative change in the εapp value of curcumin in different binary mixtures (Fig. S4†). The increase in the area under 548 nm was much higher than the increase observed in the εapp value of curcumin upon raising the volume percentage of ethanol. This observation suggests that some non-radiative process is involved in quenching the fluorescence intensity, along with decreasing the εapp value of curcumin with an increase in the water content.
3.3 Effect of pH on the absorption and emission behaviour of curcumin in 5% ethanolic solution
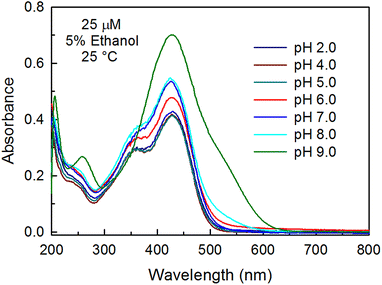
Fig. 5 depicts the absorption spectra of curcumin at different pH in 5% ethanolic solutions of curcumin at 25 °C. At acidic pH (pH 2.0), curcumin exhibits the signature bands: a prominent enolic peak at ∼429 nm and a weak shoulder due to the diketo form at 357 nm. At neutral pH, there is an increase in the intensity of band at ∼429 nm. We postulate that at pH 7.0, curcumin exists in the keto–enol–enolate form. This is also consistent with reported literature value of the pKa (8.3) for the enolic proton of curcumin.11 At pH 9.0, a significant change in the absorption spectrum was observed and the weak shoulder at 357 nm vanished. This suggests that at high pH, formation of the keto form is not favored. Deconvolution of the broad peak indicates the presence of three forms/conformations of curcumin (Fig. S5†). A new peak at 509 nm (∼29.8%) also appeared, which might be due to the presence of the dianion, according to our DFT results (Table 2). The increase in intensity at 429 nm with an increase in pH is probably due to the shifting of equilibrium towards the forms/species of curcumin which have higher εapp values at 429 nm, as it has been shown earlier that anionic forms of curcumin exhibit higher molar absorptivity.39Therefore, we calculated the εapp values at 429 nm from the calibration curves at different pH (Fig. S6†), which showed that there is an increase in εapp with an increase in pH (Fig. S7a†). Moreover, an increase in FWHM was also observed with the increase in pH, indicating an increase in the number of conformations of curcumin (heterogeneity) at higher pH (Fig. S7b†). From these observations we speculate that at higher pH, the emergence of new forms of curcumin takes place (anionic forms) which have higher ε values. A new peak at 260 nm was also observed, which may be due to the presence of degradation products at basic pH. It is already known that as the medium become basic, curcumin becomes more prone to degradation, leading to the formation of smaller aromatic compounds.40
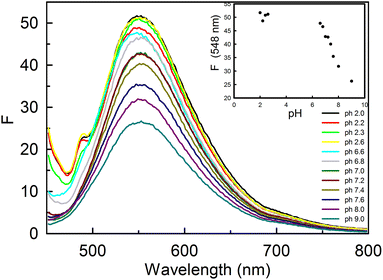
We also observed the emission behaviour of curcumin at different pH (Fig. 6) by exciting curcumin molecules at 429 nm (a higher wavelength at which only enol and enolate forms of curcumin become excited). At acidic pH (pH 2.0), a broad peak was observed at 548 nm with a weak shoulder at 488 nm. The weak shoulder completely vanished at increased pH. A decrease in the fluorescence intensity at 548 nm (enolic band) was also observed at increased pH. This trend is the opposite to that observed in the case of the absorbance of curcumin at 429 nm at varying pH. As mentioned earlier, deprotonated forms of curcumin have higher epsilon values than the neutral enolic form. Therefore, the decrease in intensity at 548 nm is not due to the formation of deprotonated forms of curcumin at higher pH, but might be due to some non-radiative process that becomes enhanced upon deprotonation. We speculate that the shoulder at 488 nm might be due to the formation of a higher order of curcumin aggregates, since curcumin is known to have lower solubility at acidic pH.27
3.4 Effect of temperature on the absorption and the emission behaviour of curcumin in 5% ethanolic solution
The temperature dependent absorption behaviours of curcumin at pH 2.0, 7.0 and 9.0 are shown in Fig. 7a–c. In acidic pH and at 25 °C, a prominent peak at 429 nm and a weak shoulder at ∼357 nm are observed. With an increase in temperature, the intensity of the peak at 429 nm gradually decreases, whereas the peak intensity at 357 nm increases. The decrease in intensity at 429 nm might be due to the shift of equilibrium towards the keto form of curcumin at higher temperatures. An isosbestic point at ∼382 nm indicates that these two species exist in equilibrium. A hypsochromic shift of ∼10 nm is also observed (from 357 nm to 347 nm) with an increase in temperature. The hypsochromic shift might be due to the weaker intermolecular interactions between the solvent and the excited state of curcumin at higher temperature.27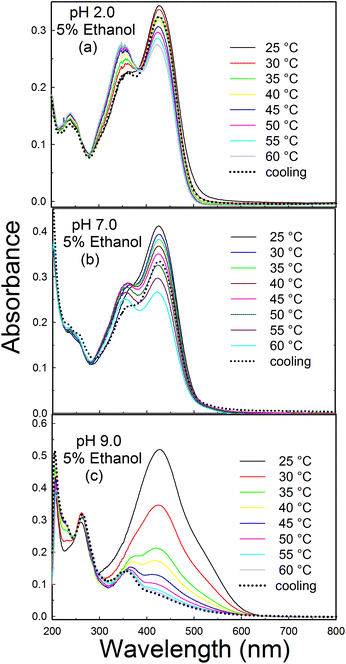 | ||
| Fig. 7 Effect of temperature on the absorption spectra of curcumin at (a) pH 2.0, (b) pH 7.0 and (c) pH 9.0 in 5% ethanolic solutions. | ||
The thermal profile of curcumin obtained at pH 7.0 is similar to the profile at pH 2.0, with a prominent peak at 429 nm and a weak shoulder at 357 nm (Fig. 7b). With an increase in temperature, the peak intensity at 429 nm decreases, whereas the ratio of A357/A429 increases with the shoulder at 357 nm becoming prominent (Fig. S8†). But there is an overall decrease in the absorption of both peaks, which may be due to the degradation of curcumin at neutral pH (Fig. S9†). This is consistent with previous reports on the decomposition of curcumin in neutral and basic buffer solutions.41 From the above results we conclude that at acidic and neutral pH, a rise in temperature shifts the keto–enol equilibrium towards the diketo form. The rise in temperature results in the rupturing of the intramolecular hydrogen bond of the cis-enolic form, thus favouring the formation of the diketo form. This process is reversible, since the diketo form reverts back to the enol form upon further cooling and the spectrum shifts to its original position.
The thermal profile of curcumin at pH 9.0 in 5% ethanol shows an additional peak at 260 nm, along with a broad peak at 429 nm and a weak shoulder at around 366 nm (Fig. 7c). The peak intensity at 429 nm decreases drastically with the increase in temperature and becomes negligible at 60 °C. The peak at 260 nm is prominent at all temperatures and there is a slight increase in its intensity at higher temperatures. The peaks at lower wavelengths (260 nm and 360 nm) are probably due to degradation products.42 The formation of degradation products at higher temperature was further confirmed by monitoring the absorption profile of curcumin with time at 25 °C (Fig. S10†). The degradation profile of curcumin obtained at 25 °C for about 2.5 h was found to be similar to the thermal profile of curcumin, where curcumin was incubated for 10 min at each temperature. This observation indicates that only the rate of degradation increases with a rise in temperature, but the degradation profile of curcumin is not affected by the temperature at pH 9.0. Also, upon cooling the curcumin solution from 60 °C to 25 °C, the spectrum did not shift to its original position. This confirms that the peaks at 260 and 350 nm are due to degradation products.
We further investigated the effect of the temperature dependence of curcumin spectra in binary mixtures with a higher ethanol content at different pH. In a 50% binary mixture, the thermal profiles of curcumin obtained in acidic and neutral pH solutions were similar (Fig. 8a and b) and no significant changes were observed with an increase in temperature. There was only a slight decrease in the intensity of the 429 nm peak at higher temperatures, indicating that in a binary mixture with a higher ethanol content, intramolecular hydrogen bonding of the cis-enolic form remains intact even at higher temperature. At pH 9.0, significant changes were observed in the curcumin absorption behaviour upon increasing the temperature (Fig. 8c). Therefore, from these studies, we conclude that the temperature dependent behaviour of curcumin strongly depends on the solvent composition.
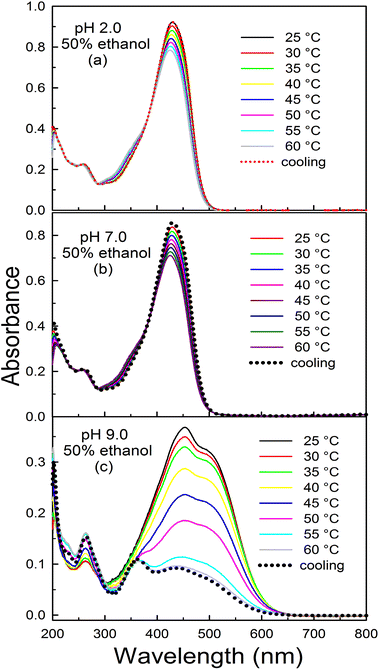 | ||
| Fig. 8 Effect of temperature on the absorption spectra of curcumin at (a) pH 2.0, (b) pH 7.0 and (c) pH 9.0 in 50% ethanolic solutions. | ||
We also observed the effect of temperature on the fluorescence spectra of curcumin, to gain insight into the conformational equilibria of curcumin in the excited state. Fig. 9 demonstrates the emission spectra of curcumin at pH 2.0 obtained at two different excitation wavelengths: 429 nm (absorption maximum for enol or monoanionic form) (Fig. 9a) and 350 nm (absorption maximum for keto form) (Fig. 9b) at various temperatures. Upon exciting curcumin at 429 nm, a weak shoulder was observed at 488 nm, which completely vanished at high temperature and the intensity of the prominent peak at 548 nm decreased with the rise in temperature. Upon cooling to 25 °C, the shoulder appeared again, which suggests that this shoulder might be due to aggregated species of curcumin. Interestingly, both excitation wavelengths (429 nm and 357 nm) resulted in an emission maximum at ∼548 nm at 25 °C. There was no significant change in the fluorescence intensity, but a hypsochromic shift (∼40 nm) was observed with an increase in temperature when curcumin was excited at 350 nm. Furthermore, upon cooling to 25 °C, the peak shifted back to ∼540 nm. Our DFT results suggest that the diketo form has its emission maximum at ∼490 nm with a low oscillator strength. Therefore, we speculate that both the diketo and enol forms of curcumin become excited at 350 nm and the peak at 548 nm is due to the enol form. However, with an increase in temperature, the formation of the diketo form is favoured, as observed by our thermal studies on the absorption behaviour of curcumin, and this increases the ground state population of the diketo form. The peak at lower wavelength (∼500 nm) and high temperature might be due to the greater formation of the diketo form. At pH 7.0, with λex = 429 nm, there was a decrease in the fluorescence intensity at 548 nm with an increase in temperature and no shoulder appeared at 488 nm at lower temperature. The absence of the shoulder suggests that no aggregation of curcumin occurred at this concentration of curcumin at pH 7.0. With λex = 350 nm, the same trend was observed in the emission behaviour of curcumin as that seen at pH 2.0.
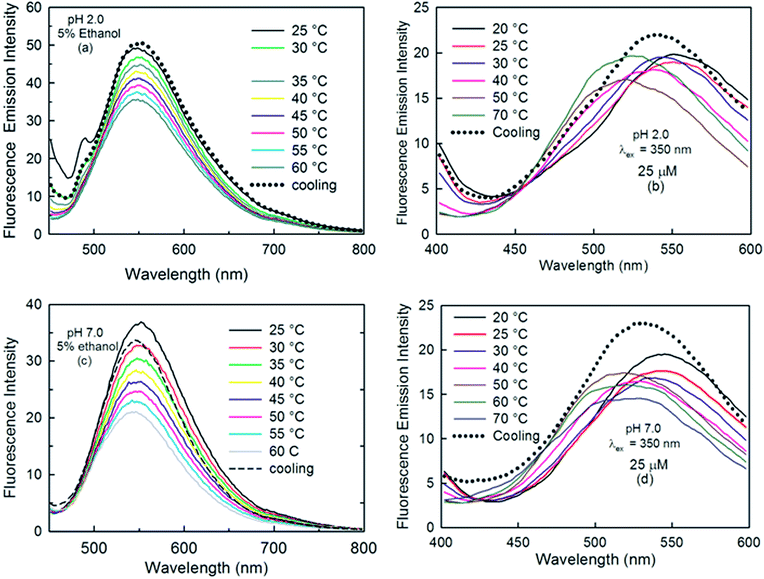 | ||
| Fig. 9 Effect of temperature on the emission spectra of curcumin at pH 2.0 (a and b) and pH 7.0 (c and d) in 5% ethanolic solutions with λex = 429 nm (left panel) and 350 nm (right panel). | ||
3.5 Effect of concentration on the physiochemical properties of curcumin in 5% ethanolic solution
To further characterize the shoulder at 488 nm, we studied the effect of curcumin concentration on the emission spectra of curcumin at pH 2.0 and pH 7.0 at 25 °C in 5% ethanolic solutions (Fig. 10, upper and lower panel). At pH 2.0, drastic changes in the emission spectra were observed with an increase in concentration. The shoulder intensity at 488 nm increased initially with the increase in concentration and then decreased at concentrations above 35 μM. The decrease in the shoulder intensity at 488 nm might be due to the emergence of a new shoulder at 590 nm at higher concentrations. Unlike at pH 2.0, no shoulder was observed for the solution at pH 7.0 at a low concentration of curcumin (i.e. 25 μM), but with an increase in the concentration, a similar trend in the emission spectrum to that of the pH 2.0 solution was observed. The appearance of a new shoulder and the changes in the emission spectrum might be due to the formation of new species/aggregates, thereby increasing the heterogeneity of the solution.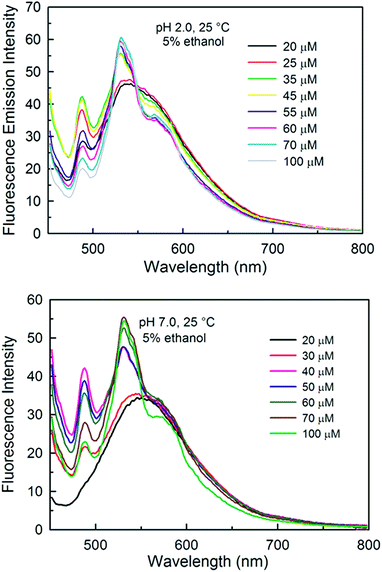 | ||
| Fig. 10 Effect of concentration on the emission spectra of curcumin at pH 2.0 (upper panel) and pH 7.0 (lower panel) in 5% ethanol solution at 25 °C. | ||
To obtain further insight into the changes in the emission behaviour of curcumin due to its aggregation, we carried out thermal studies of curcumin at high concentrations (70 μM) in acidic (Fig. 11a) and neutral (Fig. 11b) solutions. At pH 2.0, both the shoulders at 488 nm and 590 nm vanished at high temperature. However, the emission spectra almost reverted back to its original shape and position upon cooling the solution back to 25 °C. This observation indicates that some reversible phenomenon is taking place upon heating and cooling. Therefore, we suggest that curcumin exists in more than one aggregated form at lower temperature and upon heating, disaggregation takes place and the solution becomes more homogenous. There was a reappearance of these aggregates upon lowering the temperature again. The decrease in intensity at higher temperature might be due to some non-radiative process. In a neutral solution of curcumin, a similar trend was observed as both the shoulders (488 nm and 590 nm) disappeared upon heating and reappeared upon cooling.
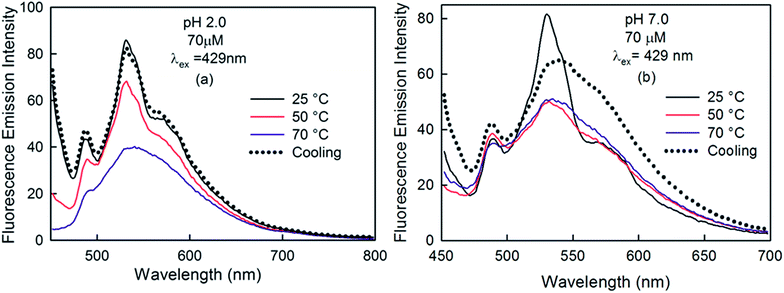 | ||
| Fig. 11 Effect of temperature on the emission spectra of curcumin (70 μM) at (a) pH 2.0 and (b) pH 7.0 in 5% ethanolic solutions with λex = 429 nm. | ||
Fig. S11† shows the effect of temperature on the particle size distribution of curcumin at three different concentrations (25 μM, 70 μM and 100 μM) in acidic solutions of pH 2.0. It can be clearly seen from Fig. S11† that at each concentration of curcumin, the particle size decreased with an increase in temperature. Interestingly at 25 °C and 50 °C, particle size was observed to be dependent on the curcumin concentration, whereas at 70 °C, the particle size was ∼95 nm at all concentrations of curcumin. Also, the peak became sharper with the increase in temperature, indicating that at higher temperature, the curcumin solution became more homogenous. All of these observations indicate that at pH 2.0, curcumin exists in an aggregated form and these aggregates dissociate at higher temperature.
Aggregation of curcumin was also observed at pH 7.0 (Fig. S12†). At higher concentrations of curcumin (70 μM and 100 μM), the solution became more heterogeneous with larger aggregates. At this pH, no effects of temperature were observed at lower concentration. However, at higher concentrations of curcumin (70 μM and 100 μM), there was a reduction in particle size and heterogeneity with an increase in temperature.
3.6 Estimation of thermodynamic parameters
Principal component analysis (PCA) was carried out to determine the number of principal components (PCs) leading to the observed absorption spectral variation (Fig. S13†). Using PCA, two PCs account for 99.4% of the total variance. Fig. S13† shows the coefficients of the loadings with temperature. The coefficient for the loading of PC-1 drops with increasing temperature, resulting in a decrease in the overall absorption at 429 nm. The loading for PC-2 at 350 nm and 500 nm shows the opposite trend. Therefore, a decrease in its score indicates an increase in the absorption at 350 nm and a decrease in the absorption at 500 nm with a rise in temperature. A further number of components and pure spectra for each component were extracted by employing a multivariate curve resolution (MCR) approach, using the Unscrambler software package. Three components contributed to the overall spectral behaviour of curcumin, as shown in Fig. S14,† where component 1, 2 and 3 can be attributed to the keto (∼350 nm), dianion (408 and 490 nm) and enol/monoanion (352 nm and 430 nm) forms, respectively. This is consistent with our DFT results. The concentration variation of each component with temperature was also calculated at pH 2.0, 7.0 and 9.0 (Fig. S15a–c†) and the variation correlated well with our previous analysis. The equilibrium constants for the keto–enol tautomerization (K = [enol]/[keto]) of curcumin were also calculated at pH 2 and 7, for estimated concentrations of the keto and enol forms (Fig. S15 and S16, Tables S3 and S4†). The decrease in the K value with temperature at both pH 2 and 7 suggests that the enol form is favoured at lower temperatures. As K at pH 2 is greater than K at pH 7 at 25 °C, the enolic form is favoured in acidic solution. Moreover, the K values at pH 2 and 7 approach the same values at higher temperatures, indicating that the keto–enol equilibrium becomes independent of pH at higher temperatures. K values for the keto–enol tautomerisation could not be calculated at pH 9 due to the degradation of curcumin at alkaline pH. ΔG at different temperatures was calculated from the corresponding K value by using eqn (1):
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K
| (1) |
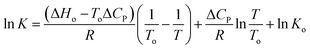 | (2) |
ΔH and ΔS were determined using eqn (3) and (4) respectively (Tables S3 and S4†).
| ΔH = ΔHo + ΔCP(T − To) | (3) |
| ΔG = ΔH − TΔS | (4) |
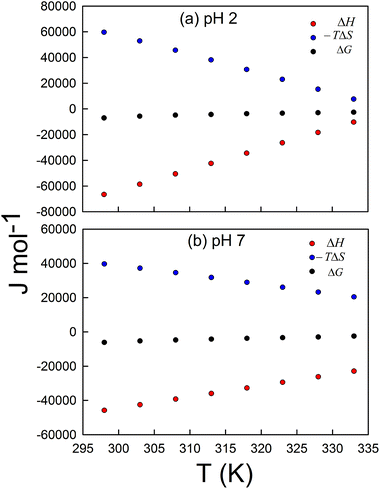
Fig. 12a and b depict the variation of ΔG, ΔH, and −TΔS with temperature at pH 2 and 7, respectively. Keto–enol tautomerization is enthalpy driven at all temperatures at both acidic and neutral pH. The increase in enthalpy is more prominent than the increase in the Gibbs free energy of the keto–enol tautomerization, which indicates that there is some compensation by the decrease in the entropy factor, i.e. −TΔS. The entropy change (i.e. ΔS = Senol − Sketo) of the keto–enol tautomerization is negative at all temperatures, but approaches zero at higher temperatures. From this observation, we speculate that initially there is a higher level of solvent organization around the enol form that becomes perturbed due to the increase in temperature, thereby decreasing the overall order of the system.
Moreover, as K at pH 2 is greater than K at pH 7 at 25 °C (Fig. S16†), the enolic form is favoured in acidic solution, because at lower temperature, the enthalpy of the keto–enol tautomerization (ΔH = Henol − Hketo) is more negative in acidic pH compared to that in neutral pH, which might be due to the more favourable non-covalent interactions (electrostatic or hydrogen bonding) between the solvent and/H+ ions with the enolic form of curcumin. At higher temperatures, the K values at pH 2 and 7 approach the same value, indicating that the keto–enol equilibrium becomes independent of pH at higher temperatures. This might be due to entropy and enthalpy compensation, as shown in Fig. S17a and b.† At higher temperatures, the stabilizing interactions might become perturbed, and there is also a release of ordered solvent molecules around the enolic form with a rise in temperature.
4. Conclusions
In summary, our findings suggest that the physiochemical properties of curcumin are significantly influenced by various factors, such as its concentration, composition, solution pH and temperature. Curcumin exhibits different acid–base equilibria as well as keto–enol tautomerism in various binary mixtures of ethanol and buffer. The solvent composition notably influences the keto–enol tautomerism as well as the excited state of curcumin, as demonstrated using UV absorption and fluorescence studies, respectively. Not much of a significant change was observed in its conformational equilibria in solutions with volume percentages of ethanol more than 50%. Lower pH and higher temperature favours keto formation and at alkaline pH, only the rate of degradation is enhanced with an increase in temperature.Comprehensive studies were also carried out on the aggregation behaviour of curcumin in aqueous media. Fluorescence spectra showed three bands at a higher concentration of curcumin that merged into one band at higher temperature, indicating the heterogeneity of the curcumin solution at higher concentration and lower temperature. Curcumin aggregates form at an even lower concentration (i.e. 25 μM) in acidic medium, and these aggregates dissociate at higher temperature, whereas at neutral pH, larger aggregates are observed at higher concentration (>25 μM). The overall behaviour of curcumin in binary mixtures of ethanol and water under different conditions is schematically represented in Fig. 13.
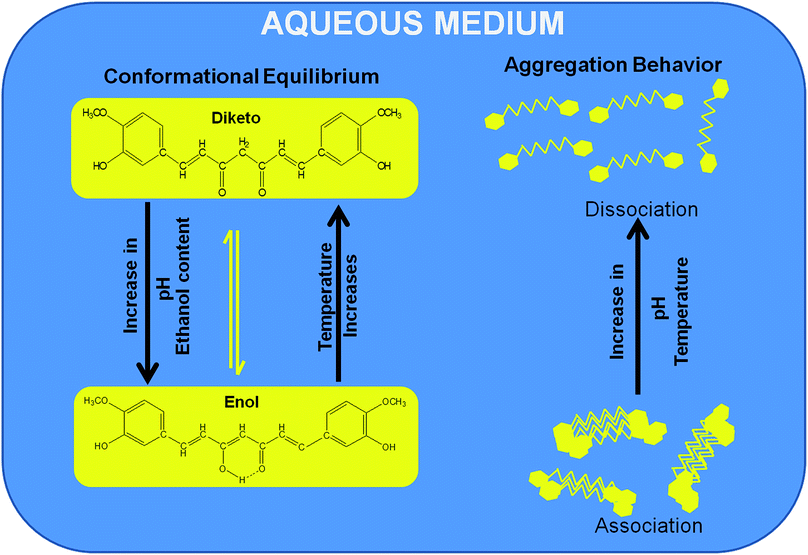 | ||
| Fig. 13 Schematic representation of the overall behaviour of curcumin in aqueous media under different conditions. | ||
These findings may have utility in terms of the enhancement of the bioavailability and therapeutic potential of curcumin, as all our experiments were performed in almost completely aqueous solution and binary mixtures.
Acknowledgements
This work was supported by grants to S. D. from the Council of Scientific and Industrial Research (CSIR). N. K. B. would like to thank Prof. B. Jayaram for providing the computational facility at the Supercomputing Facility for Bioinformatics and Computational Biology Center, Indian Institute of Technology, Delhi.References
- W.-H. Lee, C.-Y. Loo, M. Bebawy, F. Luk, R. S. Mason and R. Rohanizadeh, Curr. Neuropharmacol., 2013, 11, 338–378 CrossRef CAS PubMed
.
- R. A. Sharma, A. J. Gescher and W. P. Steward, Eur. J. Cancer, 2005, 41, 1955–1968 CrossRef CAS PubMed
.
- N. Pescosolido, R. Giannotti, A. M. Plateroti, A. Pascarella and M. Nebbioso, Planta Med., 2014, 80, 249–254 CAS
.
- G. Bar-Sela, R. Epelbaum and M. Schaffer, Curr. Med. Chem., 2010, 17, 190–197 CrossRef CAS PubMed
.
- R. Wilken, M. S. Veena, M. B. Wang and E. S. Srivatsan, Mol. Cancer, 2011, 10, 12 CrossRef CAS PubMed
.
- A. Goel, A. B. Kunnumakkara and B. B. Aggarwal, Biochem. Pharmacol., 2008, 75, 787–809 CrossRef CAS PubMed
.
- P. K. Singh, V. Kotia, D. Ghosh, G. M. Mohite, A. Kumar and S. K. Maji, ACS Chem. Neurosci., 2012, 4, 393–407 CrossRef PubMed
.
- B. Ahmad and L. J. Lapidus, J. Biol. Chem., 2012, 287, 9193–9199 CrossRef CAS PubMed
.
- N. K. Bhatia, A. Srivastava, N. Katyal, N. Jain, M. A. Khan, B. Kundu and S. Deep, Biochim. Biophys. Acta, Gen. Subj., 2015, 1854, 426–436 CrossRef CAS PubMed
.
- K. Ono, K. Hasegawa, H. Naiki and M. Yamada, J. Neurosci. Res., 2004, 75, 742–750 CrossRef CAS PubMed
.
- K. I. Priyadarsini, J. Photochem. Photobiol., C, 2009, 10, 81–95 CrossRef CAS
.
- S. P. Parimita, Y. V. Ramshankar, S. Suresh and T. N. Guru Row, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o860–o862 CAS
.
- F. Payton, P. Sandusky and W. L. Alworth, J. Nat. Prod., 2007, 70, 143–146 CrossRef CAS PubMed
.
- J. S. Wright, J. Mol. Struct.: THEOCHEM, 2002, 591, 207–217 CrossRef CAS
.
- L. Shen and H. F. Ji, Spectrochim. Acta, Part A, 2007, 67, 619–623 CrossRef PubMed
.
- K. Balasubramanian, J. Agric. Food Chem., 2006, 54, 3512–3520 CrossRef CAS PubMed
.
- S. M. Khopde, K. I. Priyadarsini, D. K. Palit and T. Mukherjee, Photochem. Photobiol., 2000, 72, 625–631 CrossRef CAS PubMed
.
- R. K. Saini and K. Das, J. Phys. Chem. B, 2012, 116, 10357–10363 CrossRef CAS PubMed
.
- R. K. Saini and K. Das, J. Lumin., 2014, 145, 832–837 CrossRef CAS
.
- Y. Erez, I. Presiado, R. Gepshtein and D. Huppert, J. Phys. Chem. A, 2011, 115, 10962–10971 CrossRef CAS PubMed
.
- Y. Erez, R. Simkovitch, S. Shomer, R. Gepshtein and D. Huppert, J. Phys. Chem. A, 2014, 118, 872–884 CrossRef CAS PubMed
.
- Y. Erez, I. Presiado, R. Gepshtein and D. Huppert, J. Phys. Chem. A, 2012, 116, 2039–2048 CrossRef CAS PubMed
.
- L. Nardo, R. Paderno, A. Andreoni, M. Másson, T. Haukvik and H. H. TØnnesen, Spectroscopy, 2008, 22, 187–198 CrossRef CAS
.
- D. Nie, Z. Bian, A. Yu, Z. Chen, Z. Liu and C. Huang, Chem. Phys., 2008, 348, 181–186 CrossRef CAS
.
- D. Yanagisawa, N. Shirai, T. Amatsubo, H. Taguchi, K. Hirao, M. Urushitani, S. Morikawa, T. Inubushi, M. Kato, F. Kato, K. Morino, H. Kimura, I. Nakano, C. Yoshida, T. Okada, M. Sano, Y. Wada, K.-N. Wada, A. Yamamoto and I. Tooyama, Biomaterials, 2010, 31, 4179–4185 CrossRef CAS PubMed
.
- S. V. Jovanovic, S. Steenken, C. W. Boone and M. G. Simic, J. Am. Chem. Soc., 1999, 121, 9677–9681 CrossRef CAS
.
- R. Jagannathan, P. M. Abraham and P. Poddar, J. Phys. Chem. B, 2012, 116, 14533–14540 CrossRef CAS PubMed
.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed
.
- K. Jain, S. Kaniyankandy, S. Kishor, I. Josefsson, H. N. Ghosh, K. S. Singh, S. Mookerjee, M. Odelius and L. M. Ramaniah, Phys. Chem. Chem. Phys., 2015, 17, 28683–28696 RSC
.
- D. Bagchi, S. Chaudhuri, S. Sardar, S. Choudhury, N. Polley, P. Lemmens and S. K. Pal, RSC Adv., 2015, 5, 102516–102524 RSC
.
- S. Banerjee, R. Ghosh and B. Bagchi, J. Phys. Chem. B, 2012, 116, 3713–3722 CrossRef CAS PubMed
.
- J. T. Gerig, J. Phys. Chem. B, 2013, 117, 4880–4892 CrossRef CAS PubMed
.
- A. Hagarman, T. J. Measey, D. Mathieu, H. Schwalbe and R. Schweitzer-Stenner, J. Am. Chem. Soc., 2010, 132, 540–551 CrossRef CAS PubMed
.
- B. Milorey, S. Farrell, S. E. Toal and R. Schweitzer-Stenner, Chem. Commun., 2015, 51, 16498–16501 RSC
.
- A. Wakisaka and K. Matsuura, J. Mol. Liq., 2006, 129, 25–32 CrossRef CAS
.
- K. I. Priyadarsini, J. Photochem. Photobiol., C, 2009, 10, 81–95 CrossRef CAS
.
- K. I. Priyadarsini, Molecules, 2014, 19, 20091–20112 CrossRef PubMed
.
- L. F. Mottram, S. Forbes, B. D. Ackley and B. R. Peterson, Beilstein J. Org. Chem., 2012, 8, 2156–2165 CrossRef CAS PubMed
.
- M. Bernabe-Pineda, M. T. Ramirez-Silva, M. Romero-Romo, E. Gonzadlez-Vergara and A. Rojas-Hernandez, Spectrochim. Acta, Part A, 2004, 60, 1091–1097 CrossRef
.
- M. V. Canamares, J. V. Garcia-Ramos and S. Sanchez-Cortes, Appl. Spectrosc., 2006, 60, 1386–1391 CrossRef CAS PubMed
.
- H. H. Tonnesen and J. Karlsen, Z. Lebensm.-Unters. Forsch., 1985, 180, 402–404 CrossRef CAS
.
- M. V. Canamares, J. V. Garcia-Ramos and S. Sanchez-Cortes, Appl. Spectrosc., 2006, 60, 1386–1391 CrossRef CAS PubMed
.
- H. Naghibi, A. Tamura and J. M. Sturtevant, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 5597–5599 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24256a |
| This journal is © The Royal Society of Chemistry 2016 |