Construction of a carbon fiber based layer-by-layer (LbL) assembly – a smart approach towards effective EMI shielding†
Abstract
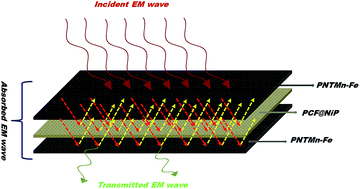
Construction of ultrathin multilayer polymer nanocomposite films by precise layer-by-layer (LbL) architectural assembly with tailor-made properties has been achieved here to block incoming EM radiation. To accomplish this, Mn (manganese)–ferrite nanoparticles were synthesized and incorporated in a thermoplastic matrix (PVDF, polyvinylidene fluoride) along with conductive MWNTs (PNTMn–Fe) by a facile solution blending process. These nanocomposite films were used as outer layers of the LbL assembly. In order to scavenge the transmitted radiation through PNTMn–Fe layers, PVDF films sandwiched with a Ni (nickel)-deposited woven carbon fiber (CF) mat (PCF@NiP), designed using a facile electroless deposition technique. These layers were used as inner layers of the LbL assembly. The different layers were then stacked and hot pressed into a composite structure. This ultrathin (0.60 mm) multilayer architecture showed an extraordinary (52 dB at 18 GHz) shielding effectiveness and thereby promises a smart approach to accomplish a lightweight, high performance, EMI shielding material.


 Please wait while we load your content...
Please wait while we load your content...