A nanoporous carbon material derived from pomelo peels as a fiber coating for solid-phase microextraction†
Wenchang Wang,
Lihong Zhang,
Zhi Li,
Shuaihua Zhang*,
Chun Wang and
Zhi Wang*
Department of Chemistry, College of Science, Agricultural University of Hebei, Baoding 071001, China. E-mail: zhangshuaihua@hebau.edu.cn; wangzhi@hebau.edu.cn; Fax: +86-312-7521513; Tel: +86-312-7521513
First published on 1st December 2016
Abstract
In this work, we present a facile and economical approach to synthesize a nanoporous carbon via a KOH chemical activation process using waste pomelo peels as the biomass precursor. The nanoporous carbon derived from pomelo peels exhibits a nanoporous structure (pore size, 0.69 nm) and high surface area (1770 m2 g−1). To evaluate the extraction performance of the nanoporous carbon, it was applied as a solid-phase microextraction coating for the enrichment of benzene homologues from water and soil samples prior to their determination by gas chromatography-mass spectrometry. The main influencing experimental parameters for the extraction were investigated and optimized. Under the optimum conditions, a good linearity for the signal response was achieved in the concentration range of 0.4–100.0 ng L−1 for water samples and 5.0–1000.0 ng kg−1 for soil samples with determination coefficients (r2) larger than 0.9960. The limits of detection (S/N = 3) of the method ranged from 0.05 to 0.18 ng L−1 for water samples and from 0.11 to 0.18 ng kg−1 for soil samples, respectively. The recoveries of the analytes were in the range of 81.3–112.0% with the relative standard deviations (RSDs) ranging from 1.5% to 11.4% (n = 5). The single fiber repeatability and fiber-to-fiber reproducibility values expressed as RSDs were in the range of 2.4–8.1% and 5.9–9.9%, respectively. The method was simple, convenient and feasible for the determination of benzene homologues in real samples.
1. Introduction
Nanoporous carbons (NPCs),1,2 with their advantageous characteristics of high surface area and tunable structures, are promising for a variety of applications, such as gaseous or liquid adsorption,2 electric double layer capacitors,3 and catalyst supports.4 Recently, NPCs derived from biomass sources have gained exceptional attention due to their high adsorption capacity, renewable, low-cost and eco-friendly properties.5 Up to now, a wide variety of biomass source materials have been utilized as carbon precursors to prepare NPCs, including celtuce leaves,6 tea leaves,7 corn grains,8 sunflower seed shells,9 cashew nut shells,10 coconut shells11 and pineapple,12 etc. NPCs derived from biomass source materials with different structures and morphologies have been prepared via a variety of methods, such as, pyrolysis,13 activation,6 hydrothermal carbonization,14 ionothermal carbonization15 and molten salt carbonization.16 In activation method, the activation process, either physical or chemical activation is vital to enhance the properties of the NPCs. The raw material in physical activation is first paralyzed under a temperature above 400 °C, followed by the activation in the presence of suitable agents (O2, CO2 or steam) to develop the porosity, usually in the temperature range of 600–1200 °C.17 Chemical activation is a two-step process which involves the carbonization of carbon precursors, in the temperature range of 400–900 °C, to release the volatile matters and the activation of the carbon char in the presence of suitable chemical activators such as ZnCl2, K2CO3 and KOH. Compared with physical activation, chemical activation has superior advantages such as higher yields and higher specific surface area.17 In previous studies, NPCs by KOH chemical activation have been widely used in many fields, such as for the adsorption of CO2,6 and the removal of dyes.18 The NPCs with a high adsorption capacity have endowed them with a great application potential as adsorbent in sample pretreatment.Solid-phase microextraction (SPME), first introduced by Arthur and Pawliszyn in 1990s,19 has been considered as one of the most advanced sample preparation methods. It integrates sampling, extraction, pre-concentration and sample introduction into one step20 and widely applied for the analysis of food,21 environmental,22 pharmaceutical,23 biological24 and medicinal samples.25 SPME is generally based on the partition of the target analytes between the sample and fiber coating, and therefore, the selection of a suitable fiber coating is the key for a particular extraction.26 Although several different commercial SPME fibers are available, such as nonpolar polydimethylsiloxane (PDMS), semi-polar PDMS/divinylbenzene (PDMS/DVB) and polar polyacrylate (PA), etc. However, the variety of the commercial SPME fibers is still limited. In the past decades, many efforts have been made to develop the novel SPME coating materials, such as ionic liquid/polymeric ionic liquids,27,28 molecularly imprinted polymer,29,30 and metal–organic frameworks (MOFs).31,32 However, as far as we know, the applications of the NPCs derived from biomass source as the SPME coating for the extraction of organic pollutants have rarely been reported.
Pomelo peel is mainly composed of cellulose, hemicellulose and lignin,33 which can be a good biomass source material for the production of NPCs. In this paper, we used waste pomelo peel to prepare a NPC by KOH activation approach. The prepared pomelo peel-based nanoporous carbon (PP-NPC) was then immobilized onto a stainless-steel wire through physical coating method to prepare the SPME fiber. The extraction performance of the PP-NPC coated fiber was evaluated with some benzene homologues as the model analytes. The effects of the main experimental parameters on the extraction efficiency, including the extraction temperature and time, salt addition, stirring rate, desorption temperature and time, were investigated. In the end, a headspace SPME (HS-SPME) with the newly prepared fiber coupled with gas chromatography-mass spectrometry (GC-MS) was developed and applied for the determination of benzene homologues in water and soil samples.
2. Experimental
2.1. Reagents and materials
The ten benzene homologues standards, chlorobenzene, ethylbenzene, m-xylene, p-xylene, o-xylene, m-dichlorobenzene, p-dichlorobenzene, o-dichlorobenzene, 1,3,5-trichlorobenzene and 1,2,4-trichlorobenzene were obtained from Sinopharm Chemical Reagent (Shanghai, China). Other chemical reagents including hexane, toluene, NaCl and KOH, all of analytical grade, were from Kemiou Chemical Reagent (Tianjin, China). Neutral multifunctional silicone sealant was purchased from Guangzhou Baiyun Chemical Industry Co., Ltd. Double-distilled water was obtained from Yarong SZ-93A automatic double-distilled system (Shanghai, China). The 5 μL GC microsyringe and stainless steel wires were obtained from Shanghai Gaoge Industrial and Trading Co., Ltd (Shanghai, China).The standard stock mixture solution of benzene homologues were prepared in hexane at a concentration of 1.0 mg mL−1 for each of the analytes and stored at 4 °C in the dark for further use. For the method optimization, the standard mixture solutions of different concentrations of the benzene homologues were prepared by a serial dilution of the corresponding stock solutions with hexane.
2.2. Apparatus and characterization
An Agilent 7820A gas chromatography-5977E mass spectrometry system (GC-MS) (Santa Clara, CA, USA) was employed for all the experiments. The mass spectrometer was operated in the electron ionization mode (70 eV). All the separations were performed on a HP-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness, Agilent J&W Scientific, CA, USA). The helium of high purity (>99.999%) was used as the carrier and the make-up gas at 1.2 mL min−1 and 25 mL min−1, respectively. The GC chromatographic conditions for benzene homologues were as follows: injector temperature, 270 °C; the oven temperature program: initial oven temperature at 40 °C (held for 5 min), then increased to 90 °C at a rate of 5 °C min−1 (held for 1 min), and finally increased to 250 °C at a rate of 40 °C min−1. The total running time was 20 min. The GC-MS interface, ion source and quadrupole temperatures were kept at 280, 230, and 150 °C, respectively. The MS was operated in full-scan mode in the m/z range from 35 to 300 for the determination of the analytes. To gain the highest possible selectivity and sensitivity, the data acquisition was performed in the selected ion monitoring (SIM) mode.The morphologies of the PP-NPC coated fiber were observed by scanning electron microscopy (SEM) using a Hitachi S4800 field emission electron microscope (Tokyo, Japan). The XRD patterns of the materials were measured with a Tongda TD-3500× X-ray diffractometer (Dandong, China) using Cu Kα radiation (40 kV, 150 mA) in the range of 2θ = 2–50°. The specific surface area of the PP-NPC was measured by Brunauer–Emmett–Teller (BET) method using nitrogen adsorption and desorption isotherms on a V-Sorb 2800P volumetric adsorption equipment (Gold APP Instruments Corporation, Beijing, China).
2.3. Preparation of PP-NPC
The PP-NPC was prepared by KOH activation strategy as described previously34 with some modifications. Typically, pomelo peel was dried in the air and ground into powder. The pomelo peel powder was pre-carbonized in a conventional furnace at 450 °C for 2 h under nitrogen flow. Subsequently, the resulting black powder was further ground and thoroughly mixed with different volumes of 1 mol L−1 KOH aqueous solution for 3 h. The obtained products were labeled as PP-NPC-1, PP-NPC-2 and PP-NPC-3 when the weight ratio of KOH to the pre-carbonized product was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Then, the mixed solution was dried at 100 °C overnight, and then was ground to a fine black powder. This mixed powder was then placed in a nickel crucible and activated in a conventional furnace at 450 °C for 1.5 h, then at 800 °C for 2.5 h with a heating rate of 5 °C min−1 under nitrogen atmosphere. After being cooled to room temperature, the obtained product was immersed into 1 mol L−1 HCl solution for about 12 h. Afterwards, the obtained sample was washed with double-distilled water until the pH of the solution became 6–7. Finally, the sample was dried at 80 °C in an oven overnight.
1, respectively. Then, the mixed solution was dried at 100 °C overnight, and then was ground to a fine black powder. This mixed powder was then placed in a nickel crucible and activated in a conventional furnace at 450 °C for 1.5 h, then at 800 °C for 2.5 h with a heating rate of 5 °C min−1 under nitrogen atmosphere. After being cooled to room temperature, the obtained product was immersed into 1 mol L−1 HCl solution for about 12 h. Afterwards, the obtained sample was washed with double-distilled water until the pH of the solution became 6–7. Finally, the sample was dried at 80 °C in an oven overnight.
2.4. Fabrication of PP-NPC coated SPME fiber
Prior to coating, the stainless steel wire was first etched to generate a rough surface with a diameter of about 180 μm according to our previously report.35 The pretreated stainless steel wire was vertically immersed into silicone sealant, which was diluted with toluene (w/v: 500 mg mL−1) in an Eppendorf tube, then pulled out quickly. Then, the fiber was vertically inserted into the prepared PP-NPC powder, rotated for a few cycles and pulled out, and the NPC coated fiber was placed in an oven for conditioning at 70 °C for 30 min. The above process was repeated several times until a desired thickness was obtained. Subsequently, the wire was dipped into the silicone sealant again to form a thin film of polymer which could protect the whole coating and avoid the flaking of the powder.36 Finally, the obtained PP-NPC coated fiber was assembled into a 5 μL GC microsyringe by replacing the plunger to makeup a homemade SPME device. Before use, the fiber was aged in the GC injector under nitrogen at 270 °C until a stable GC baseline was obtained.2.5. Sample preparation and HS-SPME procedures
Two water samples were collected from local tap and pond (Baoding, China) and 10.0 mL of them was directly used for the following HS-SPME. Soil samples were collected from two farmlands, one close to our campus (soil I), one from the plough layer of the field at Wumazhuang, Baoding, China (soil II). The soil samples were air-dried at ambient temperature, and then powdered and passed through 350 μm sieves. Then, 2.0 g of the so-prepared soil samples were weighed and transferred into a 25 mL glass vial to which 10.0 mL water was added and mixed thoroughly for subsequent HS-SPME.For the HS-SPME process, the extraction was carried out with the above obtained water or soil sample solution plus 2.0 g of NaCl (20%, w/v) with the vial immediately capped with PTFE silicone septum under a magnetic stirring at 1200 rpm. Then, the needle was used to penetrate the septum of the vial, and the PP-NPC coated fiber was pushed out and exposed to the headspace above the sample solution at 30 °C for 40 min. After extraction, the fiber was pulled out and immediately inserted into GC inlet at 270 °C for 3 min for GC-MS analysis.
3. Results and discussion
3.1. Characterization
For chemical activation process, KOH plays an important role in the development of the pore structure of the PP-NPC. In particular, the surface area and the pore structure of the products were obviously influenced by the weight ratio of KOH to the pre-carbonization product.17 In this study, different weight ratios of KOH to the pre-carbonized PP-NPC product (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were investigated. The resulting products were labeled as PP-NPC-1, PP-NPC-2 and PP-NPC-3, respectively. The results from the nitrogen adsorption–desorption experiments are listed in Table 1.
1) were investigated. The resulting products were labeled as PP-NPC-1, PP-NPC-2 and PP-NPC-3, respectively. The results from the nitrogen adsorption–desorption experiments are listed in Table 1.
| Material | BET (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| PP-NPC-1 | 1184 | 0.63 | 2.08 |
| PP-NPC-2 | 1770 | 0.95 | 2.14 |
| PP-NPC-3 | 1168 | 0.59 | 2.06 |
The data in Table 1 show that the surface area of the PP-NPCs was remarkably increased from 1184 m2 g−1 to 1770 m2 g−1 with the weight ratio of KOH to the pre-carbonized product being changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then decreased from 1770 m2 g−1 to 1168 m2 g−1 when the weight ratio was changed from 2
1, and then decreased from 1770 m2 g−1 to 1168 m2 g−1 when the weight ratio was changed from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It can also be seen from the Table 1 that the PP-NPC-2 possesses the total pore volume of 0.95 cm3 g−1 and pore size of 2.14 nm, which are the highest among the three. Furthermore, the PP-NPCs exhibit a type-I isotherm with steep uptakes below P/P0 = 0.05, which suggested an intrinsic micropore characteristic. And the PP-NPC-2 has abundant micropores and a small amount of mesopores, which should be beneficial for the extraction performance (Fig. 1A).
1. It can also be seen from the Table 1 that the PP-NPC-2 possesses the total pore volume of 0.95 cm3 g−1 and pore size of 2.14 nm, which are the highest among the three. Furthermore, the PP-NPCs exhibit a type-I isotherm with steep uptakes below P/P0 = 0.05, which suggested an intrinsic micropore characteristic. And the PP-NPC-2 has abundant micropores and a small amount of mesopores, which should be beneficial for the extraction performance (Fig. 1A).
XRD measurements were employed to investigate the crystalline and microstructure of the synthesized PP-NPC. As can be seen from Fig. 1B, the XRD pattern for the PP-NPC displays the presence of the weak and broad peaks at 2θ = 22.8° and 43.3°, which correspond to the diffraction of (002) and (100) planes of the graphite lattice, respectively, although disordered. The peak at 43.3° is very weak, suggesting that graphitic structure was only developed to a slight extent. In addition, the broad diffraction peak (002) at about 22.8° indicates a disturbed structure due to the randomly oriented aromatic carbon sheets in the PP-NPC, which is beneficial for generating high specific surface area.37
The morphological details of the PP-NPC coated fiber were identified by SEM images (Fig. 2). The low-magnification image (Fig. 2A) shows that the PP-NPC coating possessed a homogeneous and rough surface on the stainless steel substrate, while the high magnification SEM images in Fig. 2B and C further show that the PP-NPC had a large amount of irregular pore structure, which may be attributed to the porous nature of pomelo peels and KOH activation process.38
 | ||
Fig. 2 Scanning electron micrographs of the SPME fiber coated with PP-NPC: surface images at magnifications of (A) 200×, (B) 2000×, and (C) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. 000×. | ||
3.2. Optimization of the SPME procedure
In order to achieve the best extraction efficiency of the PP-NPC coated fiber for the benzene homologues, different experimental parameters that affect the extraction efficiency, including the extraction temperature and time, agitation speed, salt concentration, and desorption time and temperature, were investigated.Under the aforementioned optimized conditions, the durability of the PP-NPC coated fiber was investigated by evaluating the extraction performance of the PP-NPC coated after it was subjected to different extraction/desorption/conditioning cycles. As depicted in Fig. S1 in the ESI,† for the same fiber, no obvious deteriorations in its extraction performance for the benzene homologues were found after 90 replicate extractions under the same optimized extraction conditions, indicating a high durability of the PP-NPC coated fiber.
3.3. Method evaluation
In order to evaluate the performance of the developed method for the determination of the benzene homologues in water and soil samples, the features of the method in terms of linearity, repeatability, reproducibility and limits of detection (LODs) were evaluated under the optimized conditions. For water samples, a series of working solutions containing each of the benzene homologues at eight concentration levels of 0.4, 1.0, 2.0, 4.0, 10.0, 20.0, 40.0 and 100.0 ng L−1 were prepared for the construction of the calibration curves. For soil samples, a series of benzene homologues-free soil samples containing each of the ten benzene homologues at eight concentration levels of 5.0, 10.0, 20.0, 50.0, 100.0, 200.0, 500.0 and 1000.0 ng kg−1 were prepared. The calibration curves were evaluated by plotting peak areas of the analytes against their corresponding concentrations. The resulting analytical characteristics are listed in Table 2, Fig. S2 and S3.† For water samples, the linear response for the analytes was observed in the concentration range from 0.4–100.0 ng L−1 with r2 all larger than 0.9960. For soil samples, the linear range for the benzene homologues existed in the range from 5.0 to 1000.0 ng kg−1 with r2 all larger than 0.9979. Based on a signal-to-noise ratio of 3, the LODs were estimated to be 0.05–0.18 ng L−1 for water samples, 0.11–0.18 ng kg−1 for soil samples, respectively. For repeatability studies, the same fiber was used for five replicate extractions under the same conditions and the relative standard deviations (RSDs) for single fiber repeatability were below 8.1%. Five PP-NPC coated fibers prepared in the same batch were used to evaluate the fiber-to-fiber reproducibility and the RSDs were lower than 9.9%. In addition, the batch-to-batch reproducibility of three different fibers prepared in three different batches (fiber batch 1, fiber batch 2 and fiber batch 3) was also investigated under the same SPME conditions and the RSDs were range from 10.1% to 16.9% (Table 2 and Fig. S4†).| Compounds | Water samples | Soil samples | RSD% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LRa (ng L−1) | LODs (ng L−1) | r2 | LR (ng kg−1) | LODs (ng kg−1) | r2 | Repeatability (n = 5, %) | Reproducibility | ||
| Fiber-to-fiber (n = 5, %) | Batch-to-batch (n = 3, %) | ||||||||
| a LR, linear range. | |||||||||
| Chlorobenzene | 0.4–100.0 | 0.18 | 0.9960 | 5.0–1000.0 | 0.13 | 0.9979 | 2.4 | 7.1 | 15.4 |
| Ethylbenzene | 0.4–100.0 | 0.13 | 0.9982 | 5.0–1000.0 | 0.13 | 0.9981 | 4.9 | 6.1 | 10.5 |
| m,p-Xylene | 0.4–100.0 | 0.05 | 0.9983 | 5.0–1000.0 | 0.12 | 0.9993 | 5.1 | 5.9 | 11.7 |
| o-Xylene | 0.4–100.0 | 0.16 | 0.9980 | 5.0–1000.0 | 0.17 | 0.9995 | 5.3 | 6.8 | 16.9 |
| m-Dichlorobenzene | 0.4–100.0 | 0.15 | 0.9999 | 5.0–1000.0 | 0.14 | 0.9993 | 5.4 | 7.6 | 10.1 |
| p-Dichlorobenzene | 0.4–100.0 | 0.06 | 0.9993 | 5.0–1000.0 | 0.18 | 0.9984 | 8.1 | 7.5 | 11.6 |
| o-Dichlorobenzene | 0.4–100.0 | 0.17 | 0.9995 | 5.0–1000.0 | 0.11 | 0.9995 | 5.5 | 8.0 | 13.0 |
| 1,3,5-Trichlorobenzene | 0.4–100.0 | 0.08 | 0.9997 | 5.0–1000.0 | 0.12 | 0.9991 | 6.5 | 9.7 | 11.3 |
| 1,2,4-Trichlorobenzene | 0.4–100.0 | 0.08 | 0.9992 | 5.0–1000.0 | 0.14 | 0.9990 | 6.7 | 9.9 | 13.4 |
3.4. Analysis of the benzene homologues in water and soil samples
The developed HS-SPME-GC-MS method was applied to determine the ten benzene homologues in water and soil samples. The water and soil samples were treated according to the procedures described in the section of “Sample preparation and HS-SPME procedures”. The results indicated that none of the benzene homologues were detected in the water samples. To test the accuracy of the method, the recoveries of the method for the ten benzene homologues were investigated by spiking the standard solution of the benzene homologues into the water samples at the concentrations of 2.0 and 10.0 ng L−1, then analysing the spiked samples with the developed method. For each concentration, five replicate determinations were performed. As shown in Table 3, for water samples, the determined recoveries of the benzene homologues were in the range from 81.3% to 112.0%, with RSDs between 1.5% and 11.4%.| Compound | Spiked/ng L−1 | Tap water | Lake water | Spiked/ng kg−1 | Soil I | Soil II | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Found/ng L−1 | Ra (%) | RSD (%) | Found/ng L−1 | R (%) | RSD (%) | Found/ng kg−1 | R (%) | RSD (%) | Found/ng kg−1 | R (%) | RSD (%) | |||
| a R, recovery.b nd, not detected or lower than LOD. | ||||||||||||||
| Chlorobenzene | 0.0 | ndb | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 1.63 | 81.3 | 11.3 | 2.18 | 109.0 | 4.6 | 10.0 | 9.7 | 97.7 | 3.2 | 8.5 | 84.7 | 10.3 | |
| 10.0 | 10.36 | 103.6 | 3.0 | 8.44 | 84.4 | 3.1 | 50.0 | 49.0 | 98.0 | 5.6 | 42.0 | 84.0 | 7.6 | |
| Ethylbenzene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 1.91 | 95.3 | 4.5 | 1.84 | 92.1 | 4.3 | 10.0 | 10.1 | 101.1 | 7.5 | 9.2 | 91.9 | 11.4 | |
| 10.0 | 9.39 | 93.9 | 3.9 | 8.85 | 88.5 | 3.7 | 50.0 | 46.8 | 93.5 | 9.6 | 49.1 | 98.2 | 4.2 | |
| m,p-Xylene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 2.18 | 109.0 | 7.4 | 2.02 | 101.4 | 1.5 | 10.0 | 9.9 | 99.0 | 8.6 | 9.3 | 92.7 | 10.3 | |
| 10.0 | 8.45 | 84.5 | 3.9 | 9.68 | 96.8 | 3.2 | 50.0 | 53.0 | 106.0 | 9.3 | 50.3 | 100.6 | 5.5 | |
| o-Xylene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 2.04 | 102.2 | 5.4 | 2.24 | 112.0 | 11.4 | 10.0 | 10.0 | 99.6 | 5.3 | 9.2 | 91.8 | 5.6 | |
| 10.0 | 9.86 | 98.6 | 2.7 | 9.53 | 95.4 | 3.1 | 50.0 | 46.8 | 93.5 | 8.4 | 45.2 | 90.4 | 3.5 | |
| m-Dichlorobenzene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 2.08 | 103.8 | 4.1 | 2.02 | 101.0 | 3.1 | 10.0 | 10.0 | 100.3 | 7.6 | 9.4 | 93.8 | 8.7 | |
| 10.0 | 9.58 | 95.8 | 4.7 | 9.21 | 92.1 | 5.5 | 50.0 | 52.0 | 103.9 | 10.7 | 49.0 | 97.9 | 4.1 | |
| p-Dichlorobenzene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 1.97 | 98.4 | 3.1 | 1.81 | 90.7 | 5.9 | 10.0 | 10.0 | 99.9 | 9.3 | 9.8 | 97.9 | 6.3 | |
| 10.0 | 8.84 | 88.4 | 11.1 | 8.77 | 87.7 | 9.8 | 50.0 | 45.2 | 90.4 | 10.8 | 50.2 | 100.3 | 5.6 | |
| o-Dichlorobenzene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 2.00 | 100.0 | 5.3 | 1.95 | 97.6 | 6.1 | 10.0 | 9.9 | 98.6 | 8.5 | 9.3 | 92.6 | 5.2 | |
| 10.0 | 9.48 | 84.9 | 3.4 | 8.99 | 89.9 | 4.3 | 50.0 | 48.7 | 97.4 | 10.8 | 48.8 | 97.5 | 2.6 | |
| 1,3,5-Trichlorobenzene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 1.90 | 95.0 | 6.8 | 2.13 | 106.5 | 8.2 | 10.0 | 9.9 | 99.4 | 8.1 | 9.7 | 96.7 | 4.6 | |
| 10.0 | 9.23 | 92.3 | 6.4 | 9.41 | 94.1 | 6.8 | 50.0 | 44.0 | 88.0 | 6.9 | 41.0 | 82.0 | 6.3 | |
| 1,2,4-Trichlorobenzene | 0.0 | nd | nd | 0.0 | nd | nd | ||||||||
| 2.0 | 2.19 | 109.6 | 4.3 | 1.93 | 96.3 | 6.2 | 10.0 | 10.1 | 100.5 | 7.3 | 9.4 | 94.3 | 7.9 | |
| 10.0 | 9.07 | 90.8 | 4.3 | 8.96 | 89.6 | 9.4 | 50.0 | 49.6 | 99.2 | 8.2 | 54.0 | 108.0 | 4.1 | |
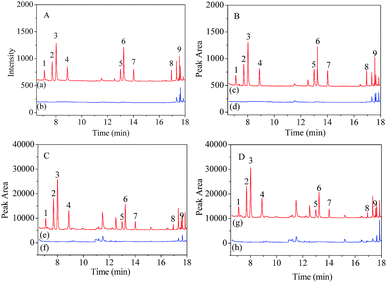
For soil samples, no benzene homologues were detected. The recoveries obtained at the spiking level of 10.0 and 50.0 ng kg−1 each of the benzene homologues in soil samples ranged from 82.0% to 108.0%, with RSDs between 2.6% and 11.4%. These results demonstrate a good applicability of the established method for the analysis of benzene homologues from water and soil samples. Chromatograms of the benzene homologues in water and soil samples and their corresponding spiked samples are shown in Fig. 4.
3.5. Comparison with other SPME coatings
The performance of the current HS-SPME method based on PP-NPC coated fiber for the determination of benzene homologues was compared with the other reported SPME methods with different coatings including vinyl-SBA-15,40 carbon,41 Poly(o-anisidine)/graphene oxide nanosheets,42 MIL-101(Cr)43 and PDMS-DVB.44 As listed in Table 4, the current method was better than the reported methods from the viewpoint of linearity and LODs (S/N = 3). For the repeatability, the method developed here has a comparable performance with the previously reported SPME methods.40–44| Methods | Coating materials | Sample | Linearity (ng mL−1) | LODs (S/N = 3, ng L−1) | Repeatability (RSDs, %) | Ref. |
|---|---|---|---|---|---|---|
| SPME-GC-MS | Vinyl-SBA-15 | Water | 10–2000 | 10–77 | 0.2–10.2 | 40 |
| SPME-GC-MS | Carbon | Sea water | 1–1500 | 8–47 | 3.9–4.6 | 41 |
| SPME-GC-MS | Poly(o-anisidine)/graphene oxide nanosheets | Water | 0.1–500 | 10–60 | 4.9–7.3 | 42 |
| SPME-GC-MS | MIL-101(Cr) | Water | 0.01–20 | 0.32–1.7 | 2.1–7.1 | 43 |
| SPME-GC-MS | PDMS/DVB | Water | 0.03–1 | 70–300 | 3.2–11.5 | 44 |
| SPME-GC-MS | PP-NPC | Water | 0.004–0.1 | 0.05–0.18 | 2.4–8.1 | This method |
4. Conclusions
In summary, we prepared a nanoporous carbon by carbonizing low-cost agricultural waste pomelo peel followed by KOH activation. The prepared PP-NPC was immobilized onto a stainless steel wire via a physical coating approach to obtain SPME fibers. The PP-NPC coated SPME fiber was applied for the extraction of benzene homologues in water and soil samples prior to their determination by GC-MS. The results suggest that the developed HS-SPME-GC-MS method was suitable for the analysis of the benzene homologues in real water and soil samples. This study demonstrates the possibility of using nanoporous carbon derived from biomass sources as a coating absorbent for benzene homologues enrichment, and opens a new way for the design and fabrication of new types of coating material for the application in SPME.Acknowledgements
The authors acknowledge the financial support of the National Natural Science Foundation of China (31471643, 31671930 and 31571925), Hebei “Double First Class Discipline” Construction Foundation for the Discipline of Food Science and Engineering of Hebei Agricultural University (2016SPGCA18), the Innovation Research Program of the Department of Education of Hebei for Hebei Provincial Universities (LJRC009), the Natural Science Foundation of Hebei Province (B2016204136, B2016204146), the Scientific and Technological Research Foundation of the Department of Education of Hebei Province (ZD2016085), respectively.References
- M. Hu, J. Reboul, S. Furukawa, N. L. Torad, Q. Ji, P. Srinivasu, K. Ariga, S. Kitagawa and Y. Yamauchi, J. Am. Chem. Soc., 2012, 134, 2864–2867 CrossRef CAS PubMed.
- A. Aijaz, N. Fujiwara and Q. Xu, J. Am. Chem. Soc., 2014, 136, 6790–6793 CrossRef CAS PubMed.
- H. Itoi, H. Nishihara, T. Kogure and T. Kyotani, J. Am. Chem. Soc., 2011, 133, 1165–1167 CrossRef CAS PubMed.
- Y. Meng, D. Voiry, A. Goswami, X. Zou, X. Huang, M. Chhowalla, Z. Liu and T. Asefa, J. Am. Chem. Soc., 2014, 136, 13554–13557 CrossRef CAS PubMed.
- J. Deng, M. Li and Y. Wang, Green Chem., 2016, 18, 4824–4854 RSC.
- R. Wang, P. Wang, X. Yan, J. Lang, C. Peng and Q. Xue, ACS Appl. Mater. Interfaces, 2012, 4, 5800–5806 CAS.
- B. H. Hameed, J. Hazard. Mater., 2009, 161, 753–759 CrossRef CAS PubMed.
- X. Hou, Q. Deng, T. Ren and Z. Yuan, Environ. Sci. Pollut. Res., 2013, 20, 8521–8534 CrossRef CAS PubMed.
- X. Li, W. Xing, S. Zhuo, J. Zhou, F. Li, S.-Z. Qiao and G.-Q. Lu, Bioresour. Technol., 2011, 102, 1118–1123 CrossRef CAS PubMed.
- G. F. Coelho, A. C. Gonçalves Jr, C. R. T. Tarley, J. Casarin, H. Nacke and M. A. Francziskowski, Ecol. Eng., 2014, 73, 514–525 CrossRef.
- L. Sun, C. Tian, M. Li, X. Meng, L. Wang, R. Wang, J. Yin and H. Fu, J. Mater. Chem. A, 2013, 1, 6462–6470 CAS.
- M. N. Mahamad, M. A. A. Zaini and Z. A. Zakaria, Int. Biodeterior. Biodegrad., 2015, 102, 274–280 CrossRef CAS.
- M. Noked, A. Soffer and D. Aurbach, J. Solid State Electrochem., 2011, 15, 1563–1578 CrossRef CAS.
- C. Falco, N. Baccile and M. M. Titirici, Green Chem., 2011, 13, 3273–3281 RSC.
- Z. L. Xie, R. J. White, J. Weber, A. Taubert and M. M. Titirici, J. Mater. Chem., 2011, 21, 7434 RSC.
- X. Zheng, W. Lv, Y. Tao, J. Shao, C. Zhang, D. Liu, J. Luo, D. W. Wang and Q. H. Yang, Chem. Mater., 2014, 26, 6896–6903 CrossRef CAS.
- J. Wang and S. Kaskel, J. Mater. Chem., 2012, 22, 23710–23725 RSC.
- L. Chen, T. Ji, L. Brisbin and J. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 12230–12237 CAS.
- C. L. Arthur and J. Pawliszyn, Anal. Chem., 1990, 62, 2145–2148 CrossRef CAS.
- X. Liu, Z. Sun, G. Chen, W. Zhang, Y. Cai, R. Kong, X. Wang, Y. Suo and J. You, J. Chromatogr. A, 2015, 1409, 46–52 CrossRef CAS PubMed.
- Z. Zhang, Y. Ma, Q. Wang, A. Chen, Z. Pan and G. Li, J. Chromatogr. A, 2013, 1290, 27–35 CrossRef CAS PubMed.
- S.-H. Huo, J. Yu, Y.-Y. Fu and P.-X. Zhou, RSC Adv., 2016, 6, 14042–14048 RSC.
- J. K. Lokhnauth and N. H. Snow, Anal. Chem., 2005, 77, 5938–5946 CrossRef CAS PubMed.
- H. Yuan, W. M. Mullett and J. Pawliszyn, Analyst, 2001, 126, 1456–1461 RSC.
- N. Li, C. Deng, Y. Li, H. Ye and X. Zhang, J. Chromatogr. A, 2006, 1133, 29–34 CrossRef CAS PubMed.
- H. L. Xu, Y. Li, D. Q. Jiang and X. P. Yan, Anal. Chem., 2009, 81, 4971–4977 CrossRef CAS PubMed.
- H. Kang, Y. Mao, X. Wang, Y. Zhang, J. Wu and H. Wang, RSC Adv., 2015, 5, 41934–41940 RSC.
- M. Wu, L. Wang, F. Zhao and B. Zeng, RSC Adv., 2015, 5, 99483–99490 RSC.
- E. Turiel, J. L. Tadeo and A. Martin-Esteban, Anal. Chem., 2007, 79, 3099–3104 CrossRef CAS PubMed.
- E. H. M. Koster, C. Crescenzi, W. D. Hoedt, K. Ensing and G. J. D. Jong, Anal. Chem., 2001, 73, 3140–3145 CrossRef CAS PubMed.
- H. B. Shang, C. X. Yang and X. P. Yan, J. Chromatogr. A, 2014, 1357, 165–171 CrossRef CAS PubMed.
- Y. Hu, H. Lian, L. Zhou and G. Li, Anal. Chem., 2015, 87, 406–412 CrossRef CAS PubMed.
- W. Chai, X. Liu, J. Zou, X. Zhang, B. Li and T. Yin, Carbohydr. Polym., 2015, 132, 245–251 CrossRef CAS PubMed.
- H. Li, Z. Sun, L. Zhang, Y. Tian, G. Cui and S. Yan, Colloids Surf., A, 2016, 489, 191–199 CrossRef CAS.
- S. Zhang, Q. Yang, W. Wang, C. Wang and Z. Wang, J. Agric. Food Chem., 2016, 64, 2792–2801 CrossRef CAS PubMed.
- S. Zhang, Q. Yang, Z. Li, W. Wang, C. Wang and Z. Wang, Analyst, 2016, 141, 1127–1135 RSC.
- Y. Zhao, M. Lu, P. Tao, Y. Zhang, X. Gong, Z. Yang, G. Zhang and H. Li, J. Power Sources, 2016, 307, 391–400 CrossRef CAS.
- N. Bader and A. Ouederni, J. Energy Storage, 2016, 5, 77–84 CrossRef.
- G. Zhang, X. Zang, Z. Li, C. Wang and Z. Wang, Talanta, 2014, 129, 600–605 CrossRef CAS PubMed.
- F. Zhu, Y. Liang, L. Xia, M. Rong, C. Su, R. Lai, R. Li and G. Ouyang, J. Chromatogr. A, 2012, 1247, 42–48 CrossRef CAS PubMed.
- F. Zhu, J. Guo, F. Zeng, R. Fu, D. Wu, T. Luan, Y. Tong, T. Lu and G. Ouyang, J. Chromatogr. A, 2010, 1217, 7848–7854 CrossRef CAS PubMed.
- M. Behzadi and M. Mirzaei, J. Chromatogr. A, 2016, 1443, 35–42 CrossRef CAS PubMed.
- L. Xie, S. Liu, Z. Han, R. Jiang, H. Liu, F. Zhu, F. Zeng, C. Su and G. Ouyang, Anal. Chim. Acta, 2015, 853, 303–310 CrossRef CAS PubMed.
- J. N. Bianchin, G. Nardini, J. Merib, A. N. Dias, E. Martendal and E. Carasek, J. Chromatogr. A, 2012, 1233, 22–29 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The durability of the PP-NPC coated fiber during the SPME, the extract performance of the PP-NPC coated fibers prepared in three different batches, and the calibration curves for benzene homologues from water and soil samples. See DOI: 10.1039/c6ra24225a |
| This journal is © The Royal Society of Chemistry 2016 |