Highly conductive graphene-bonded polyimide yarns for flexible electronics†
Abstract
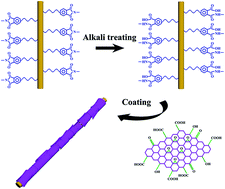
Recently there has been a strong interest in flexible and conductive fibers to meet the demands of wearable electronics. However, how to combine high conductivity, good durability and low cost in one fiber is still a big challenge. Here, we fabricate graphene-bonded polyimide yarns through a large-scale dip-reduction process with an initial alkali treatment. The role of interfacial bonding on conductivity and durability is investigated. Resultantly, conductive yarns of 1.02 × 103 S m−1 are obtained and possess outstanding stability after bending up to 100 times, water wash, and even Scotch tape test. Furthermore, the graphene-bonded polyimide yarns can serve as an effective flexible conductor wire. Supercapacitors made from two conductive yarns show a high specific capacitance of 22.89 F cm−3. These highly conductive yarns are demonstrated to have a great potential in flexible electronics.

 Please wait while we load your content...
Please wait while we load your content...