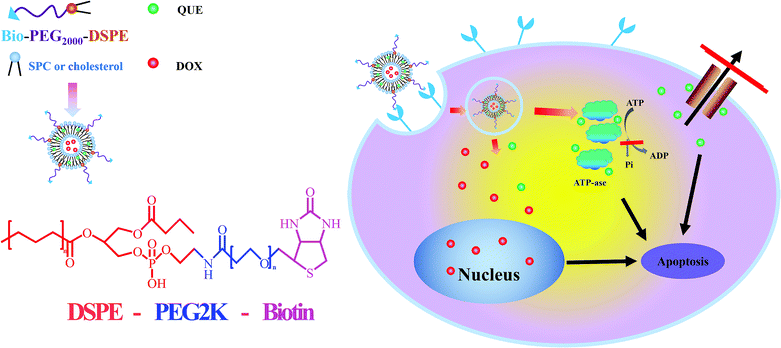
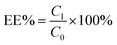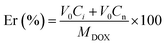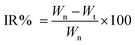Co-delivery of doxorubicin and the traditional Chinese medicine quercetin using biotin–PEG2000–DSPE modified liposomes for the treatment of multidrug resistant breast cancer
Jiulong Zhanga,
Yue Luob,
Xiufeng Zhaob,
Xiaowei Lib,
Kexin Lib,
Dawei Chenb,
Mingxi Qiaob,
Haiyang Hub and
Xiuli Zhao*b
aDepartment of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China. E-mail: zjl1160@163.com; Fax: +86 24 23986306
bDepartment of Pharmaceutics, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China. E-mail: raura3687yd@163.com
First published on 27th October 2016
Abstract
At present, multidrug resistance (MDR) in cancer therapy is an international problem, which is caused mostly by the overexpressed P-glycoprotein (P-gp) efflux pump. To address this issue and effectively deliver chemotherapeutic drugs to cancer cells, a liposomal drug delivery system (DOX/QUE BPL) has been designed for the co-delivery of the antitumor drug doxorubicin (DOX) and the traditional Chinese medicine quercetin (QUE). The MTT assay demonstrated that DOX/QUE BPL showed the highest cytotoxicity of all formulations tested against MCF-7/adr cell lines due to the inhibition of P-gp caused by QUE. The same result could also be confirmed by cellular uptake assay. To investigate the mechanism by which QUE reverses the MDR effect, DOX accumulation and efflux, P-gp expression and ATP content determination were measured and the results indicated that QUE could downregulate the expression of P-gp and facilitate drug accumulation in the cytoplasm, thereby reversing the MDR effect. In vivo antitumor activity studies demonstrated that DOX/QUE BPL could reach higher antitumor activity than other reference preparations for MCF-7/adr solid tumors. Histological assays indicated that this preparation could decrease the cardio toxicity arising from DOX and induce apoptosis in solid tumors. Meanwhile, this preparation could also downregulate the expression of P-gp in vivo. All this evidence demonstrated that this liposomal formulation is a suitable carrier for co-delivery of chemotherapeutic drugs to overcome MDR.
1 Introduction
Breast cancer remains the second leading cause of cancer-related mortality in women.1–3 Cancer progression is greatly dependent on the age, race and body mass index (BMI) of the patient.4,5 Despite many advances in the treatment of solid tumors, including surgery and radiation, chemotherapy still remains one of the most widely used approaches and approximately 60% of all patients with breast cancer undergo chemotherapy, eventually.6–8 Among chemotherapy drugs, doxorubicin (DOX) is one of the most widely used cytotoxic drugs for the treatment of breast cancer.9,10 It is well known that DOX can intercalate into the DNA and react with topoisomerase II to produce maximal toxicity in the S phase and G2/M phase of the cell cycle.11–13 However, there are many obstacles that compromise its clinical application. One is the severe cardio toxicity arising from the side effects of DOX due to its distribution in the heart.14 Another major problem is the development of multidrug resistance (MDR) during the chemotherapy.9,15 Only a minority of patients during chemotherapy show long-term remission and most of the patients fail to show this mainly due to the MDR of tumor tissue. Therefore, significant effort should be made to solve MDR in breast cancer.There are multiple mechanisms that could explain the occurrence of MDR, such as tumor vascularization,16,17 deregulation of apoptosis,18,19 the altering of the activities of specific enzyme systems,20 activation of ATP-binding cassette proteins (ABC transporters),21–23 etc. Among all of these phenomena, the main mechanism is the overexpression of ABC transporters, particularly P-glycoprotein (P-gp), which is the most significant factor in the failure of chemotherapy. P-gp can efflux drugs from the cells and decrease their intracellular concentration, resulting in insufficient therapeutical efficacy.24,25 Therefore, methods to decrease the efflux of drugs and so increase the drug accumulation in tumor cells could play a significant role for chemotherapy, and many attempts have been made to inhibit the function of P-gp. Li's group26 used verapamil as a P-gp inhibitor and incorporated it with DOX in nanoparticles for the treatment of cancerous cells. This drug delivery system showed significant reversal of the MDR effect in MCF-7/adr cell lines, but the severe cardiovascular side effects from verapamil limited its further application. In comparison, due to the low toxicity and high therapeutical efficacy of small interference RNA (siRNA), some researchers were interested in the co-delivery of antitumor drugs and siRNA to tumor cells to knockdown the P-gp. For example, Shen's group27 prepared β-cyclodextrin-based nanoparticles for the co-delivery of DOX and MDR1 siRNA to reverse the MDR effect. The results showed that this drug delivery system displayed excellent knockdown ability for P-gp and excellent antitumor activities against MDR cell lines. However, the process of preparation was very complex and siRNA was not stable in the blood system.28 In addition, the low delivery efficiency of nanoparticles (median 0.7% of the injected dose is accumulated in the tumour) is another barrier for the success of chemotherapy. Some researchers are interested in changing the shape of the nanocarriers to overcome this drawback and this method is supporting new ideas for the success of chemotherapy.29–31 Therefore, it is necessary to find an effective approach to reverse the MDR effect (Scheme 1).
It is widely known that natural products from plants such as flavonoids can overcome MDR in many drug-resistant cell lines.32,33 Quercetin (QUE) is a typical flavonoid drug which is ubiquitously presented in vegetables and fruits. It has been reported that QUE can modulate drug efflux via interacting with ATP-binding sites and/or the substrate-binding sites directly, to reverse MDR effects.34 In addition, owing to its apoptosis-inducing effect, anti-angiogenesis effect and anti-proliferative effect, it is a potential anticancer agent. However, despite these many advantages of QUE, its poor water solubility (1 μg mL−1 in water), pharmacokinetic properties,35 permeability and tumor-targeting properties could limit its application, and a lack of available methods for efficiently delivering QUE and DOX to tumor sites is becoming a major obstacle for treatment of cancer.
Nowadays, various drug delivery systems have been developed which could decrease side effects and deliver chemotherapeutic drugs to tumor tissue in a targeted way.36,37 Liposome-based drug delivery systems have attracted considerable attention due to their biodegradable, non-toxic and biocompatible features.38–40 Liposomes are not only suitable carriers for chemical drugs, but could also interact with nucleic acids to prepare lipoplexes as non-viral gene vectors.41,42 In parallel, hydrophobic drugs could be inserted into the lipid membrane while hydrophilic drugs could be loaded into the internal hydrate phase. Thus, liposomes are appropriate carriers for the co-delivery of both hydrophobic and hydrophilic drugs. However, they could easily be recognized by the reticuloendothelial system (RES) which is their major problem. To solve this problem, polyethylene glycol (PEG) has been used for the surface modification of liposomes to prepare “stealth liposomes”; these PEGylated liposomes could avoid recognition by the RES, maintain longer circulation in the blood system, prevent drug leakage and offer increased stability.43 Due to the microenvironment of tumor tissue, “stealth liposomes” could also accumulate in the tumor site via the enhanced permeability and retention effect (EPR). Although the liposomes could accumulate in tumor tissue, the concentration of drug in the cytoplasm was limited. It has been reported that receptor-mediated endocytosis could increase drug accumulation in the cytoplasm.44 Therefore, we hypothesized that the drug concentration could be significantly increased via active-targetability. Biotin (Bio), a member of the vitamin family, is a growth promoter of cells, which has been widely expressed in most cells including tumor cells. Furthermore, the biotin receptor is overexpressed in tumor cells compared with normal cells. It has been reported that biotin-modified nanoparticles could display significantly increased cellular uptake and cell cytotoxicity against cancerous cells.45 Therefore, biotin was considered to be a promising ligand for active targetability.
In this paper, we constructed a liposomal preparation for the co-delivery of DOX and QUE in order to reverse the MDR effect. The hydrophilic antitumor drug DOX and the traditional Chinese medicine and P-gp inhibitor QUE were both incorporated into the liposomes. In order to increase the stability, circulation time and cellular uptake, Bio–DSPE–PEG2000 was used to modify the surface of the liposomes. This preparation remained stable in the blood system and was not recognized easily by the RES due to the PEG shell. When the liposomes accumulated in tumor tissue via the EPR effect, biotin receptor-mediated cell endocytosis could increase the cellular uptake and a greater amount of drugs could accumulate in the cytoplasm. QUE could inhibit the P-gp activity and DOX could exert its antitumor activity. The particle size, loading content, encapsulation efficiency and in vitro release behaviour were demonstrated. The cell cytotoxicity, cellular uptake, and mechanism of reversing the MDR effect were also evaluated. Furthermore, in vivo targetability and in vivo antitumor activity were investigated to evaluate the antitumor efficacy of this preparation.
2 Materials and methods
2.1 Materials
Doxorubicin (DOX) was purchased from Beijing HuaFeng United Technology Co., Ltd. (Beijing, PR China) and quercetin (QUE) was obtained from Shanghai ANPEL Laboratory Technologies (Shanghai) Inc. (Shanghai, PR China). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE–PEG2000) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methyl(polyethylene glycol)-2000]-biotin (Bio–PEG2000–DSPE) were purchased from Shanghai A.V.T. Medical Technology Co., Ltd. (China). Soy lecithin was obtained from Shanghai Tai Wei Pharmaceutical Co., Ltd (China) and cholesterol (biochemistry pure) was acquired from Tianjin Bodie Chemical Co., Ltd. (China). Sephadex G50 was purchased from Shanghai Biological Technology Development Company (Kyrgyzstan). Dialysis bags with an average cut-off molecular weight of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 daltons were obtained from Shanghai Green Bird Technology Development Co., Ltd. (China). Folate-deficient RPMI 1640 growth medium, fetal bovine serum (FBS) and penicillin streptomycin were obtained from Gibco (USA). Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO, USA). P-gp primary antibody (ab103477) was supplied from Abcam (England). Hoechst 33258, FITC-labelled goat-anti rabbit IgG, the hematoxylin–eosin (H&E) staining kit and the immunofluorescence staining kit with Cy3-labeled goat anti-rabbit IgG were purchased from Beyotime Biotechnology (Shanghai, China). The in situ death detection kit, POD, was purchased from Roche (Switzerland). All other chemicals were commercially available reagents of at least analytical grade.
000 daltons were obtained from Shanghai Green Bird Technology Development Co., Ltd. (China). Folate-deficient RPMI 1640 growth medium, fetal bovine serum (FBS) and penicillin streptomycin were obtained from Gibco (USA). Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO, USA). P-gp primary antibody (ab103477) was supplied from Abcam (England). Hoechst 33258, FITC-labelled goat-anti rabbit IgG, the hematoxylin–eosin (H&E) staining kit and the immunofluorescence staining kit with Cy3-labeled goat anti-rabbit IgG were purchased from Beyotime Biotechnology (Shanghai, China). The in situ death detection kit, POD, was purchased from Roche (Switzerland). All other chemicals were commercially available reagents of at least analytical grade.
The human breast cancer cell line MCF-7 and multidrug resistant cell line MCF-7 (MCF-7/adr) were obtained from the China Centre for Type Culture Collection (Wuhan University, China). The cells were cultured in Dulbecco's modified eagle medium (DMEM, for MCF-7 cells) or RPMI 1640 medium with 20% fetal bovine serum (for MCF-7/adr cells) (both from Gibco, USA) with 100 U mL−1 penicillin and 100 μg L−1 streptomycin in a humidified atmosphere with 5% CO2 at 37 °C. The medium was replaced every two days and all the experiments were performed on cells in the logarithmic phase of growth.
Female nude mice (20 ± 2 g), supplied by the Department of Experimental Animals, Shenyang Pharmaceutical University (Shenyang, China), were acclimated at 25 °C and 55% humidity under natural light/dark conditions. All animal experiments were carried out in accordance with guidelines evaluated and approved by the ethics committee of Shenyang Pharmaceutical University.
2.2 Preparation of drug-loaded liposomes
Liposomes were prepared by the thin-film hydration and ammonium sulfate gradient drug-loading method. Briefly, for the DOX/QUE BPL sample (biotin-modified, PEGylated liposomes incorporating DOX and QUE), soy lecithin, cholesterol, Bio–PEG2000–DSPE and QUE (weight ratios: 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were dissolved in dehydrated alcohol. A lipid thin film was formed by gently evaporating the solvent at 40 °C using rotary evaporation. This film was hydrated with 10 mL of ammonium sulfate solution (100 mmol L−1) for 1 h at 60 °C. The resulting preparation was then sonicated at 400 W for 6 min with a probe sonicator in an ice bath, before being extruded through 0.2 μm pore size polycarbonate filters. The liposomes were dialyzed with deionized water overnight using a dialysis bag with a molecular weight cut off of 14.0 kDa to form the gradient of ammonium sulfate. To encapsulate DOX, pre-treated liposomes were incubated with DOX solution (5 mg mL−1) at 50 °C for 1 h.
1) were dissolved in dehydrated alcohol. A lipid thin film was formed by gently evaporating the solvent at 40 °C using rotary evaporation. This film was hydrated with 10 mL of ammonium sulfate solution (100 mmol L−1) for 1 h at 60 °C. The resulting preparation was then sonicated at 400 W for 6 min with a probe sonicator in an ice bath, before being extruded through 0.2 μm pore size polycarbonate filters. The liposomes were dialyzed with deionized water overnight using a dialysis bag with a molecular weight cut off of 14.0 kDa to form the gradient of ammonium sulfate. To encapsulate DOX, pre-treated liposomes were incubated with DOX solution (5 mg mL−1) at 50 °C for 1 h.
The DOX/QUE L sample (non-PEGylated liposomes incorporating DOX and QUE) was prepared using the same method as that for DOX/QUE BPL but without adding Bio–PEG2000–DSPE. The DOX L sample (non-PEGylated liposomes incorporating DOX) was prepared similarly to DOX/QUE L but without adding QUE. For the preparation of DOX/QUE PL (PEGylated liposomes incorporating DOX and QUE), an identical operation was conducted to that for DOX/QUE BPL except that the equivalent weight ratio of Bio–PEG2000–DSPE was replaced by DSPE–PEG2000.
The entrapment efficiency (EE%) of DOX and QUE by different liposomes was determined through HPLC assay. In brief, uncoupled drug molecules in the liposomes were separated by Sephadex-50 mini-columns and the liposomes were dissolved with methanol. The concentrations of QUE and/or DOX in the liposomes were measured by HPLC using a linear range from 1–20 μg mL−1 for DOX and QUE. (The mobile phase was methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile
acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphate buffered saline, 20
phosphate buffered saline, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v/v; pH 3.8.) The EE% was calculated by the following equation:
50, v/v/v; pH 3.8.) The EE% was calculated by the following equation:
2.3 Characterization of liposomes
The average size and zeta potential of the different formulations were determined by dynamic light scattering (Zetasizer Nano ZS, Malvern Instruments Ltd., UK). The morphologies of the liposomes were observed using transmission electron microscopy (TEM) (TecnaiG220, FEI, USA).2.4 In vitro drug release from liposomes
The in vitro release behaviour of different liposomes was investigated using a simple dialysis method in the presence of PBS (pH 7.4) with 5% Tween-80. In brief, liposomes were placed in the dialysis tubing (MW 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and incubated in 100 mL of PBS (pH 7.4) in a shaking incubator at 37 °C. At pre-determined time intervals, 2 mL of the aqueous solution was removed and replenished with 2 mL of fresh buffer solution. The accumulative percentage drug release (Er) was calculated using this formula:
000) and incubated in 100 mL of PBS (pH 7.4) in a shaking incubator at 37 °C. At pre-determined time intervals, 2 mL of the aqueous solution was removed and replenished with 2 mL of fresh buffer solution. The accumulative percentage drug release (Er) was calculated using this formula:MDOX represents the amount of DOX in the liposomes, V0 represents the total volume of the release medium and Ci represents the concentration of DOX in the ith aliquot removed.
2.5 In vitro cytotoxicity assay
In vitro cytotoxicity was assessed by MTT assay. The mass ratio of DOX and QUE was optimized using the MTT assay and the result showed that the optimized ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). The optimized ratio was used for subsequent MTT assays. MCF-7 or MCF-7/adr cells were seeded into 96-well plates (103 cells per well) and incubated overnight to allow attachment. Different formulations were added into each well and incubated for a further 48 h. Then, 20 μL of MTT solution (5 mg mL−1) was added to each well, and incubated with the cells for 4 h. At the end of the incubation period, the cell culture medium was blotted, and 150 μL of DMSO was added to each well. The 96-well plates were shaken for 10 min to solubilize the formazan crystals and subsequently were measured on the multifunctional microplate reader (Tecan, Austria) with an absorbance wavelength of 570 nm. Cell survival at the end of treatment was calculated from the absorbance readings as a percentage of the control. All assays were performed in triplicate.
1 (w/w). The optimized ratio was used for subsequent MTT assays. MCF-7 or MCF-7/adr cells were seeded into 96-well plates (103 cells per well) and incubated overnight to allow attachment. Different formulations were added into each well and incubated for a further 48 h. Then, 20 μL of MTT solution (5 mg mL−1) was added to each well, and incubated with the cells for 4 h. At the end of the incubation period, the cell culture medium was blotted, and 150 μL of DMSO was added to each well. The 96-well plates were shaken for 10 min to solubilize the formazan crystals and subsequently were measured on the multifunctional microplate reader (Tecan, Austria) with an absorbance wavelength of 570 nm. Cell survival at the end of treatment was calculated from the absorbance readings as a percentage of the control. All assays were performed in triplicate.
The resistance index (RI) and reversal factor (RF) were measured to evaluate the MDR reversal effect of different formulations using these formulae:
| RI = IC50 (MCF-7/adr)/IC50 (MCF-7) |
| RF = IC50 (DOX)/IC50 (liposomes) |
2.6 Cellular uptake studies
The cellular uptake of the different formulations was measured using fluorescence microscopy and flow cytometry. Cells were seeded into 6-well plates at a density of 5 × 105 cells per well and incubated overnight to allow attachment. Then different formulations were added into each well such that the final concentration of DOX was 10 μg mL−1, and the plate was incubated for 4 h at 37 °C. The cells were washed in triplicate using PBS and fixed using 4% paraformaldehyde for 10 min at room temperature. The cells were washed with PBS and stained with Hoechst 33258 (10 μg mL−1, 15 min). The cells were observed using fluorescence microscopy.In order to quantitatively analyse the cellular uptake of different liposomes, flow cytometry was carried out. In brief, MCF-7 or MCF-7/adr cells were seeded in a 6-well plate at a density of 1 × 106 cells per well and incubated overnight to allow attachment. Then different liposomes were added into each well such that the final concentration of DOX was 10 μg mL−1, and the plate was incubated for 4 h at 37 °C. At the end of the incubation, the cells were washed with cold PBS, harvested, resuspended in 0.5 mL PBS and detected using flow cytometry (FCM, Becton Dickinson).
2.7 Mechanism of MDR reversal effect
For the DOX efflux assay, cells were pre-treated with DOX for 2 h and different DOX-free liposomes were added into each well. The cells were incubated for a further 4 h and detected using flow cytometry.
2.8 Animal study
where Wn represent the tumor weight of the saline control group and Wt represents a drug-treated group.
2.9 Statistical analysis
All the experiments were repeated at least three times. Results are presented as the mean ± SD. Statistical comparisons were performed using a one-way analysis of variance (ANOVA). Pair-wise comparisons between treatments were made using the Student's t-test (two-tailed) at a confidence level P < 0.05 (*) or P < 0.01 (**).3 Results and discussion
3.1 Characterization of the liposomes
Particle size and size distribution are important parameters for the development of suitable nanomedicines for therapeutic purposes; they affect the in vivo distribution, biological fate, toxicity and targeting ability of the nanoparticle systems. In addition, they can influence the drug release and the stability of the drug inside the nanoparticles.46–48 As shown in Table 1, the particle sizes of the different liposomes were within the range of 100–150 nm and they had relatively low polydispersity index (PDI) values (Fig. 1A). Compared with blank liposomes, there was no significant increase in particle size for DOX L; this was probably because DOX was loaded in the internal hydrate phase of the liposomes and did not influence the structure of the lipid layer. However, QUE L showed a significant increase in particle size; this phenomenon was attributed to the insertion of the hydrophobic drug QUE into the bilayer of the liposomes which changed the structure and increased the particle size. Both PEGylated liposomes (DOX/QUE PL and DOX/QUE BPL) showed a slight increase in particle size; this demonstrated that a PEG shell was successfully coated on the surface of the liposomes. The introduction of a PEG shell should increase the stability of the liposomes and avoid their elimination by the RES. TEM images demonstrated that the liposomes were spherical with narrow size distributions.| Formulations | Size (nm) | PDI | Zeta potential (mV) | EE (%) | |
|---|---|---|---|---|---|
| DOX | QUE | ||||
| Blank L | 104.7 ± 3.1 | 0.247 ± 0.02 | −20.1 ± 0.9 | — | — |
| DOX L | 106.7 ± 4.5 | 0.218 ± 0.02 | −19.6 ± 1.7 | 95.6 ± 0.3 | — |
| QUE L | 134.2 ± 3.4 | 0.219 ± 0.03 | −23.1 ± 2.1 | — | 70.4 ± 2.9 |
| DOX/QUE L | 139.4 ± 2.4 | 0.157 ± 0.04 | −27.7 ± 1.2 | 93.9 ± 0.7 | 66.8 ± 1.6 |
| DOX/QUE PL | 149.0 ± 2.1 | 0.203 ± 0.02 | −23.3 ± 1.4 | 92.6 ± 0.9 | 66.4 ± 2.1 |
| DOX/QUE BPL | 148.5 ± 3.3 | 0.127 ± 0.02 | −22.7 ± 1.1 | 93.2 ± 0.2 | 69.7 ± 2.1 |
 | ||
| Fig. 1 Particle sizes and TEM image of DOX/QUE BPL (A); in vitro DOX release from different liposomes at 37 °C, n = 3 (B). | ||
The zeta potential of the nanoparticle is one of the most important factors if the formulation is for intravenous administration in the blood system.44 A positive charge existing on the surface of the nanoparticle would result in non-specific interactions with serum proteins and increase the systemic toxicity. For this reason, we measured the zeta potential of the different liposomes and the results are shown in Table 1. From the results we find that all the liposomes showed a negative zeta potential. The negative charge on the surface of the liposomes should prevent the reorganization of the RES and interactions with serum proteins. Meanwhile, the negative charge could increase the stability of the liposomes via electronic repulsion.
Encapsulation efficiency (EE%) is an indispensable factor for the evaluation of nanoparticles. The EE% of the different liposomes is shown in Table 1. Both DOX and QUE showed a relatively high EE%. The higher EE% of DOX was directly attributed to the ammonium sulfate gradient drug-loading method, while the high EE% for QUE was probably because of the hydrophobic features of QUE.
3.2 In vitro DOX release
The in vitro DOX release behaviour of the different liposomes is shown in Fig. 1B. The different liposomes all showed a rapid release of DOX over about 24 h, followed by a sustained release during later time periods.The accumulative DOX release values from DOX L and DOX/QUE L were 68.25% and 59.21%, respectively. The relatively lower release profile for the DOX/QUE L group was probably because the introduction of QUE into the phospholipid membrane increased the stability of the membrane liposomes making it more difficult for DOX to diffuse from the inner core of the liposomes. Compared with the DOX/QUE L group, DOX/QUE PL showed a slightly lower accumulative release (55.25%); this was probably because the PEG shell coated on the liposomes increased the thickness of the liposomes such that there was a relatively “longer distance” from the inner core to the surface of the liposomes.49 There was no significant difference between DOX/QUE PL and DOX/QUE BPL (accumulative DOX release rates for both were 55.87%), demonstrating that the biotin on the surface of the PEG did not influence the thickness of the PEG shell. All these results demonstrate that the liposomes showed significant sustained release behaviour and that this advantage could decrease the time required for administration, and maintain the drug concentration in the blood system.
3.3 In vitro cytotoxicity assay
For evaluation of cytotoxicity against MCF-7 cells and MCF-7/adr cells with overexpressed P-gp, the standard MTT method was used in this study. The IC50 values of the different groups are listed in Table 2. Meanwhile, RI and RF values were also calculated. For free DOX, the RI value was 14.7, which suggests that the cells showed good drug resistance. However, the DOX + QUE group showed a significant decrease in RI value, demonstrating the synergistic effect of DOX and QUE in increasing the cytotoxicity against MCF-7/adr cells. This result was probably because the reversal of the MDR effect by QUE inhibited the P-gp efflux and increased the concentration of DOX in the cytoplasm. Compared with the DOX + QUE group, the DOX/QUE L group showed a lower IC50 value in both cell lines, demonstrating that liposomes as drug carriers could efficiently deliver drugs into cells and show higher cytotoxicity. However, the DOX/QUE PL group showed a slightly lower cytotoxicity in both cell lines, suggesting that the existence of the PEG shell could form a weak exclusion between the liposomes and cell membrane. In fact, it is necessary to use PEG to modify the surface of liposomes, because the existence of PEG enhances the circulation time and increases the stability of the liposomes. However, the lower cytotoxicity of the PEGylated liposomes compared with the non-PEGylated liposomes is a major barrier. To overcome this drawback, we used Bio–PEG2k–DSPE to modify the liposomes (DOX/QUE BPL) and achieve active targetability. The results showed that this group had the highest cytotoxicity among all drug-treated groups; this was attributed to receptor-mediated endocytosis which could increase the cellular uptake.| Formulations | IC50 (μg mL−1) | RI | RF | |
|---|---|---|---|---|
| MCF-7 | MCF-7/adr | |||
| DOX | 20.1 | 296.3 | 14.7 | 1.00 |
| DOX + QUE | 18.6 | 40.2 | 2.16 | 6.80 |
| DOX/QUE L | 10.2 | 28.6 | 2.80 | 5.25 |
| DOX/QUE PL | 12.1 | 30.1 | 2.48 | 5.92 |
| DOX/QUE BPL | 9.5 | 18.5 | 1.94 | 7.58 |
3.4 Cellular uptake assay
In this study, cellular uptake was qualitatively and quantitatively analysed using fluorescence microscopy and flow cytometry, respectively. As shown in Fig. 2A, red fluorescence indicates DOX, while blue fluorescence denotes the nucleus. The DOX group showed a weak fluorescence intensity in both MCF-7 and MCF-7/adr cells, demonstrating that limited DOX could accumulate in the cytoplasm. In comparison, for the DOX + QUE group, the fluorescence intensity in the MCF-7/adr cells was stronger than that for the DOX group, demonstrating that QUE could inhibit P-gp efflux and increase the DOX accumulation in the cytoplasm; this result was consistent with the MTT assay results. The DOX/QUE L group showed a higher fluorescence intensity compared with the DOX + QUE group, demonstrating that the liposome-based drug delivery system has a high affinity to the cell membrane and increases the cellular uptake. The DOX/QUE BPL showed the strongest fluorescence intensity among all groups, demonstrating that biotin receptor-mediated endocytosis could increase the cellular uptake and accumulation of DOX in the cytoplasm. All these results were in alignment with those from the MTT assay.In order to quantitatively analyse the cellular uptake displayed by the different groups, flow cytometry was carried out (Fig. 2B). The fluorescence intensity of the DOX + QUE group was 1.45-fold higher than that of the free DOX group in MCF-7/adr cells, while there was no significant difference for MCF-7 cells. This interesting result demonstrated that the introduction of QUE could efficiently reverse the MDR effect and increase the drug accumulation in the cytoplasm. The fluorescence intensity of the DOX/QUE L group was 1.34-fold and 1.35-fold higher than that of the DOX + QUE group in MCF-7 cells and MCF-7/adr cells respectively, demonstrating that the high affinity between the liposomes' membranes and cell membranes could increase the DOX accumulation in the cytoplasm. DOX/QUE BPL showed the highest cellular uptake among all groups, demonstrating that the existence of biotin on the surface of the liposomes plays an important role for efficient cellular uptake.
3.5 Mechanism of reversal of the MDR effect
In the previous experiments we found that the existence of QUE could dramatic increase the cytotoxicity and cellular uptake of DOX in MCF-7/adr cells, demonstrating that QUE could successfully reverse the MDR effect. However, the mechanism of reversal of the MDR effect caused by QUE was not clearly demonstrated. In this section a series of studies were carried out to investigate the mechanism of reversal of the MDR effect. DOX accumulation and efflux were investigated first using flow cytometry. As shown in Fig. 3A and B, the control showed a higher accumulation and lower efflux compared with QUE-free groups, demonstrating that QUE plays a positive role in the reversal of the MDR effect. This phenomenon was probably because QUE could inhibit the efflux of drugs caused by P-gp. We supposed that the inhibition of P-gp by QUE was probably due to inhibition of P-gp expression, thus we measured the P-gp expression levels of the different drug-treated groups. As shown in Fig. 3C, compared with free DOX, DOX + QUE showed a significant decrease in P-gp expression, with levels 0.30-fold lower than that in the DOX group. P-gp expression of DOX/QUE L, DOX/QUE PL and DOX/QUE BPL were 0.48, 0.57 and 0.29-fold lower than that of the DOX group. Since P-gp is an energy-dependent efflux pump, we were interested to investigate the ATP levels in the different drug-treated groups and the results are shown in Fig. 3D. The ATP content of DOX + QUE, DOX/QUE L, DOX/QUE PL and DOX/QUE BPL were respectively 0.85, 0.61, 0.69 and 0.44-fold lower than that of the DOX group.From the results above we can conclude that QUE can downregulate P-gp expression and reverse the MDR effect. It has been reported that QUE can inhibit the upregulation of P-gp and mRNA in response to hyperthermia. This effect may be via inhibition of HSF (heat shock factor) DNA-binding activity or downregulation of the β-catenin signalling pathway.34
3.6 In vivo antitumor activities
From the results above we found that the synergistic effects of DOX and QUE show significant cytotoxicity to MDR cells in vitro. However, DOX/QUE PL showed a relatively lower cytotoxicity and cellular uptake compared with the DOX/QUE L group due to the existence of the PEG shell. It seems that the PEG shell could decrease the therapeutical efficiency of the liposomes. However, it is necessary to use PEG modifications on the liposomes to increase circulation time in vivo and increase the in vivo stability.50,51 To demonstrate this viewpoint, an in vivo antitumor activity study was carried out using MCF-7/adr bearing nude mice. As shown in Fig. 4A–C, compared with the control group, the DOX group showed a moderate antitumor efficiency (IR% = 10.2%), suggesting that DOX could inhibit the growth of tumor tissue. The DOX L group showed a relatively higher antitumor activity compared with the DOX group (IR% = 21.3%) demonstrating that liposomes, as drug carriers, could increase the drug accumulation in tumor tissue via the EPR effect. The DOX/QUE L group showed a stronger antitumor activity compared with the DOX L group, suggesting that the synergistic effect of DOX and QUE could increase the cytotoxicity against MDR solid tumors; this result was consistent with those from the in vitro assay. The DOX/QUE PL group showed more significant inhibition than all the groups above (IR% = 60.4%); this result demonstrated that the PEG shell could avoid the elimination of the liposomes by the RES and increase the circulation time in vivo. From all the drug-treated groups, DOX/QUE BPL group showed the highest antitumor efficiency (IR% = 70.5%), suggesting that the liposomes could accumulate in the cytoplasm via receptor-mediated endocytosis and achieve higher therapeutical effects. All these results indicate that this drug delivery system can increase therapeutical effects both in vitro and in vivo. | ||
| Fig. 4 The mean tumor volume (A), inhibition rate (B), tumor graph (C) and body weight (D) of nude mice bearing MCF-7/adr cells, on intravenous administration of the different formulations (n = 6). | ||
The body weights of animals in the different groups were also measured to evaluate the systemic toxicity of the treatments. As shown in Fig. 4D, all the drug-treated groups showed a slight increase in body weight, suggesting that the liposomal drug delivery system is a suitable carrier for the delivery of chemotherapeutic drugs to tumor sites. However, the DOX group showed a significant decrease in body weight, which can probably be attributed to the cardio toxicity of DOX when it is intravenously administered in the blood system.
All these in vivo antitumor activity assays indicate that DOX/QUE BPL can specifically accumulate in tumor tissue and accumulate in the cytoplasm via receptor-mediated endocytosis. QUE can inhibit the P-gp efflux via downregulating the expression of P-gp and increasing the DOX concentration, leading to a higher therapeutical effect.
3.7 H&E staining of heart tissue
Since it has been reported that the intravenous administration of DOX results in severe cardio toxicity,14 H&E stained sections of heart were examined. As shown in Fig. 5, compared with the control group, the myocardial architecture in the DOX group was altered obviously, displaying myofibrillar disorientation, and demonstrating that the loss of body weight was partially attributed to the cardio toxicity. However, no obvious haemorrhage, inflammatory cell infiltration or other lesions could be observed for any of the liposome groups which suggested that liposomes could specifically accumulate in the tumor site via EPR-mediated passive targetability and/or biotin-mediated active targetability, decreasing their non-specific biodistribution and reducing their non-specific toxicity. | ||
| Fig. 5 H&E staining of heart tissue from nude mice treated with different formulations: (a) control; (b) DOX solution; (c) DOX L; (d) DOX/QUE L; (e) DOX/QUE PL; (f) DOX/QUE BPL. | ||
3.8 Histological evaluation of tumor tissue
In the in vivo antitumor study, DOX/QUE BPL showed the highest tumor inhibition rate against MCF-7/adr solid tumors among all the drug-treated groups. Based on these studies, we were interested to observe the behaviour of tumor tissue after treatment with different liposomes. In this section, H&E staining and TUNEL staining were carried out. As shown in Fig. 6A, the histological aspects of the post-treated tumor tissue were revealed by H&E staining. The control group (a) showed a high density of cancerous cells with relatively larger nuclei (blue pixel dot). Significant histological changes could be observed for the DOX/QUE BPL group (f), including the shrivelling of the nucleolus and loss of the cytoplasm (pink pixel dot); this was probably because DOX/QUE BPL could efficiently deliver drugs to the cytoplasm and induce apoptosis in cancerous cells. The presence of voids was probably due to the loss of dead cells.To evaluate whether the loss of tumor cells was attributed to apoptosis or necrosis, TUNEL staining was also used in this section. As shown in Fig. 6B, green fluorescence indicates the apoptotic cells while blue fluorescence indicates the nucleus. There was no obvious green fluorescence in the control group, suggesting that the tumor tissue kept a high proliferation status. However, the green fluorescence increased when the mice were administrated with different formulations. Among all of the groups, the DOX/QUE BPL group showed the highest green fluorescence, demonstrating that most of the tumor cells were apoptotic. This result indicated that DOX could induce cell apoptosis with the help of QUE in MDR solid tumors, which was consistent with the in vivo antitumor activity and H&E staining results.
From the in vitro study we came to the conclusion that QUE can reverse the MDR effect via downregulating P-gp expression. It is well known that in vitro results may not completely represent the in vivo result. Therefore, we also measured the in vivo P-gp expression using immunofluorescence. As shown in Fig. 7, red fluorescence indicates the P-gp while blue fluorescence indicates the nucleus. The control group, DOX group and DOX L group all showed a high P-gp expression level, demonstrating that the tumor tissue kept a good resistance. However, DOX/QUE L showed relatively lower P-gp expression, suggesting that QUE could downregulate the P-gp expression. Furthermore, DOX/QUE PL showed significantly lower P-gp expression, suggesting that the introduction of a PEG shell on the surface of the liposomes increased the circulation time and efficiently delivered drug to the tumor tissue. The DOX/QUE BPL group showed the best P-gp inhibition activity, demonstrating that receptor-mediated endocytosis could increase the cellular uptake and drug accumulation, leading to higher antitumor activity.
4 Conclusions
In this study, biotin modified PEGylated liposomes with long circulation times were prepared for the “smart” co-delivery of the hydrophilic drug DOX and the hydrophobic drug QUE to overcome the MDR effect in MCF-7/adr solid tumors. QUE could increase the cytotoxicity of DOX via downregulating P-gp expression, and biotin could increase the cellular uptake via receptor-mediated endocytosis. Meanwhile, PEGylated liposomes displayed high therapeutical effects and overcame the MDR effect in vivo. All this evidence demonstrates that this liposomal drug delivery system is a potential nanocarrier for co-delivery of hydrophobic and hydrophilic drugs to overcome MDR.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (81202483), the Liaoning Natural Science Foundation for Excellent Talents in university (LR 2015020543), the Scientific Research Foundation for the Returned Overseas Chinese Scholars by the State Education Ministry (201303003), and the Science and Technology project of Shenyang (F15-139-9-06, F15-199-1-24).Notes and references
- N. K. Garg, B. Singh, A. Jain, P. Nirbhavane, R. Sharma, R. K. Tyagi, V. Kushwah, S. Jain and O. P. Katare, Colloids Surf., B, 2016, 146, 114–126 CrossRef CAS PubMed.
- A. Pramanik, D. Laha, S. K. Dash, S. Chattopadhyay, S. Roy, D. K. Das, P. Pramanik and P. Karmakar, Mater. Sci. Eng., C, 2016, 68, 327–337 CrossRef CAS PubMed.
- A. Sarkar, S. Ghosh, S. Chowdhury, B. Pandey and P. C. Sil, Biochim. Biophys. Acta, Gen. Subj., 2016, 1860, 2065–2075 CrossRef CAS PubMed.
- R. A. Medairos, J. Clark, S. Holoubek, J. C. Kubasiak, R. Pithadia, F. Hamid, G. W. Chmielewski, W. H. Warren, S. Basu, J. A. Borgia, M. J. Liptay and C. W. Seder, J. Thorac. Cardiovasc. Surg., 2016, 152, 55–61 CrossRef CAS PubMed.
- D. M. Moreira, N. E. Fleshner and S. J. Freedland, J. Urol., 2015, 194, 1258–1263 CrossRef PubMed.
- A. Charehbili, N. A. T. Hamdy, V. T. H. B. M. Smit, L. Kessels, A. van Bochove, H. W. van Laarhoven, H. Putter, E. Meershoek-Klein Kranenbarg, A. E. van Leeuwen-Stok, J. J. M. van der Hoeven, C. J. H. van de Velde, J. W. R. Nortier and J. R. Kroep, Breast, 2016, 25, 69–74 CrossRef CAS PubMed.
- S. Marguet, C. Mazouni, B. L. T. Ramaekers, A. Dunant, R. Kates, V. R. Jacobs, M. A. Joore, N. Harbeck and J. Bonastre, Eur. J. Cancer, 2016, 63, 168–179 CrossRef CAS PubMed.
- G. von Minckwitz, M. Rezai, H. Tesch, J. Huober, B. Gerber, D. M. Zahm, J. Hilfrich, S. D. Costa, P. Dubsky, J. U. Blohmer, C. Denkert, C. Hanusch, C. Jackisch, S. Kümmel, P. A. Fasching, A. Schneeweiss, S. Paepke, M. Untch, N. Burchardi, K. Mehta and S. Loibl, Eur. J. Cancer, 2016, 64, 12–21 CrossRef CAS PubMed.
- G. Chakravarty, A. Mathur, P. Mallade, S. Gerlach, J. Willis, A. Datta, S. Srivastav, A. B. Abdel-Mageed and D. Mondal, Biochimie, 2016, 124, 53–64 CrossRef CAS PubMed.
- Y. Guo, Y. Ding, T. Zhang and H. An, Phytomedicine, 2016, 23, 267–273 CrossRef CAS PubMed.
- E. Koziolová, O. Janoušková, L. Cuchalová, Z. Hvězdová, J. Hraběta, T. Eckschlager, L. Sivák, K. Ulbrich, T. Etrych and V. Šubr, J. Controlled Release, 2016, 233, 136–146 CrossRef PubMed.
- X. Wang, Y. Jia, X. Su, P. Wang, K. Zhang, X. Feng and Q. Liu, Ultrasound. Med. Biol., 2015, 41, 2731–2739 CrossRef PubMed.
- Y.-Z. Zhao, C.-Z. Sun, C.-T. Lu, D.-D. Dai, H.-F. Lv, Y. Wu, C.-W. Wan, L.-J. Chen, M. Lin and X.-K. Li, Cancer Lett., 2011, 311, 187–194 CrossRef CAS PubMed.
- L. Qiu, M. Qiao, Q. Chen, C. Tian, M. Long, M. Wang, Z. Li, W. Hu, G. Li, L. Cheng, L. Cheng, H. Hu, X. Zhao and D. Chen, Biomaterials, 2014, 35, 9877–9887 CrossRef CAS PubMed.
- Y. Wu, Y. Zhang, W. Zhang, C. Sun, J. Wu and J. Tang, Colloids Surf., B, 2016, 138, 60–69 CrossRef CAS PubMed.
- Q. Chen, Q. Yu, Y. Liu, D. Bhavsar, L. Yang, X. Ren, D. Sun, W. Zheng, J. Liu and L.-M. Chen, Nanomedicine, 2015, 11, 1773–1784 CAS.
- T. Leitha, C. Glaser and S. Lang, Nucl. Med. Biol., 1998, 25, 539–541 CrossRef CAS PubMed.
- P. S. Souza, J. P. Madigan, J.-P. Gillet, K. Kapoor, S. V. Ambudkar, R. C. Maia, M. M. Gottesman and K. Leung Fung, Exp. Cell Res., 2015, 336, 318–328 CrossRef CAS PubMed.
- L. Wang, B. Chen, M. Lin, Y. Cao, Y. Chen, X. Chen, T. Liu and J. Hu, Immunobiology, 2015, 220, 331–340 CrossRef CAS PubMed.
- M. S. Kim, M. J. Haney, Y. Zhao, V. Mahajan, I. Deygen, N. L. Klyachko, E. Inskoe, A. Piroyan, M. Sokolsky, O. Okolie, S. D. Hingtgen, A. V. Kabanov and E. V. Batrakova, Nanomedicine, 2016, 12, 655–664 CAS.
- L. Jiang, L. Li, X. He, Q. Yi, B. He, J. Cao, W. Pan and Z. Gu, Biomaterials, 2015, 52, 126–139 CrossRef CAS PubMed.
- R. V. Kutty, S. L. Chia, M. I. Setyawati, M. S. Muthu, S. S. Feng and D. T. Leong, Biomaterials, 2015, 63, 58–69 CrossRef CAS PubMed.
- J. Lu, W. Zhao, H. Liu, R. Marquez, Y. Huang, Y. Zhang, J. Li, W. Xie, R. Venkataramanan, L. Xu and S. Li, J. Controlled Release, 2014, 196, 272–286 CrossRef CAS PubMed.
- A. Suo, J. Qian, Y. Zhang, R. Liu, W. Xu and H. Wang, Mater. Sci. Eng., C, 2016, 62, 564–573 CrossRef CAS PubMed.
- B. Tang, G. Fang, Y. Gao, Y. Liu, J. Liu, M. Zou, L. Wang and G. Cheng, Asian J. Pharm. Sci., 2015, 10, 363–371 CrossRef.
- N. Li, C. Huang, Y. Luan, A. Song, Y. Song and S. Garg, J. Colloid Interface Sci., 2016, 472, 90–98 CrossRef CAS PubMed.
- J. Shen, Q. Wang, Q. Hu, Y. Li, G. Tang and P. K. Chu, Biomaterials, 2014, 35, 8621–8634 CrossRef CAS PubMed.
- N. P. Truong, W. Gu, I. Prasadam, Z. Jia, R. Crawford, Y. Xiao and M. J. Monteiro, Nat. Commun., 2013, 4, 1902 CrossRef PubMed.
- N. P. Truong, J. F. Quinn, M. R. Whittaker and T. P. Davis, Polym. Chem., 2016, 7, 4295–4312 RSC.
- N. P. Truong, J. F. Quinn, A. Anastasaki, D. M. Haddleton, M. R. Whittaker and T. P. Davis, Chem. Commun., 2016, 52, 4497–4500 RSC.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nature Reviews Materials, 2016, 1, 16014 CrossRef.
- S. Borska, M. Chmielewska, T. Wysocka, M. Drag-Zalesinska, M. Zabel and P. Dziegiel, Food Chem. Toxicol., 2012, 50, 3375–3383 CrossRef CAS PubMed.
- M. K. Kim, K.-S. Park, H. Choo and Y. Chong, Phytomedicine, 2015, 22, 778–785 CrossRef CAS PubMed.
- C. Chen, J. Zhou and C. Ji, Life Sci., 2010, 87, 333–338 CrossRef CAS PubMed.
- P. Ader, A. Wessmann and S. Wolffram, Free Radicals Biol. Med., 2000, 28, 1056–1067 CrossRef CAS PubMed.
- Y. Ge, Y. Ma and L. Li, Colloids Surf., B, 2016, 146, 482–489 CrossRef CAS PubMed.
- D. Zhang, J. Wang and D. Xu, J. Controlled Release, 2016, 229, 130–139 CrossRef CAS PubMed.
- P. Pathak, V. Dhawan, A. Magarkar, R. Danne, S. Govindarajan, S. Ghosh, F. Steiniger, P. Chaudhari, V. Gopal, A. Bunker, T. Róg, A. Fahr and M. Nagarsenker, Int. J. Pharm., 2016, 509, 149–158 CrossRef CAS PubMed.
- Y. Tian, J. Xia, L. Zhang, J. Zhang, Y. Jiang, Y. Zhang, L. Yang, Q. Zhang and L. Xia, Bioelectrochemistry, 2016, 111, 41–48 CrossRef CAS PubMed.
- Y. Zhao, J. Zhu, H. Zhou, X. Guo, T. Tian, S. Cui, Y. Zhen, S. Zhang and Y. Xu, Colloids Surf., B, 2016, 145, 454–461 CrossRef CAS PubMed.
- N. Golkar, S. M. Samani and A. M. Tamaddon, Int. J. Pharm., 2016, 510, 30–41 CrossRef CAS PubMed.
- S. Stremersch, R. E. Vandenbroucke, E. Van Wonterghem, A. Hendrix, S. C. De Smedt and K. Raemdonck, J. Controlled Release, 2016, 232, 51–61 CrossRef CAS PubMed.
- G. Kibria, H. Hatakeyama, Y. Sato and H. Harashima, Int. J. Pharm., 2016, 509, 178–187 CrossRef CAS PubMed.
- L. Qiu, Q. Hu, L. Cheng, L. Li, C. Tian, W. Chen, Q. Chen, W. Hu, L. Xu, J. Yang, L. Cheng and D. Chen, Acta Biomater., 2016, 30, 285–298 CrossRef CAS PubMed.
- J. H. Kim, Y. Li, M. S. Kim, S. W. Kang, J. H. Jeong and D. S. Lee, Int. J. Pharm., 2012, 427, 435–442 CrossRef CAS PubMed.
- L. Han and X. Zhou, Appl. Surf. Sci., 2016, 376, 252–260 CrossRef CAS.
- Y. Shen, J. Guo, G. Chen, C. T. Chin, X. Chen, J. Chen, F. Wang, S. Chen and G. Dan, Ultrasound. Med. Biol., 2016, 42, 1499–1511 CrossRef PubMed.
- M. Ullrich, J. Haša, J. Hanuš, M. Šoóš and F. Štěpánek, Powder Technol., 2016, 295, 115–121 CrossRef CAS.
- Q. Chen, H. Ding, J. Zhou, X. Zhao, J. Zhang, C. Yang, K. Li, M. Qiao, H. Hu, P. Ding and X. Zhao, RSC Adv., 2016, 6, 17782–17791 RSC.
- M. R. Vijayakumar, R. Kosuru, P. R. Vuddanda, S. K. Singh and S. Singh, J. Drug Delivery Sci. Technol., 2016, 33, 125–135 CrossRef CAS.
- L. Zhang, L. Han, X. Sun, D. Gao, J. Qin and J. Wang, Fitoterapia, 2012, 83, 678–689 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |