Single-stage PN/A technology treating saline ammonia-rich wastewater: finding the balance between efficient performance and less N2O and NO emissions†
Abstract
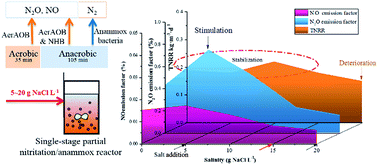
A single-stage partial nitrification/anammox (PN/A) process is suitable for treating high ammonia industrial wastewater with energy savings. However, little is known about nitrous oxide (N2O) and nitric oxide (NO) emissions from single stage nitrification/anammox (PN/A) process when treating ammonia-rich industrial wastewater which contains salt (NaCl). In this study, the effect of increasing salinity (0, 5, 10, 15 and 20 g NaCl per L) on N2O/NO emissions and total nitrogen removal rate (TNRR) were comprehensively examined in an intermittent aeration single-stage partial nitritation and anammox (PN/A) system, where anammox bacteria coexisted with ammonia oxidation bacteria and heterotrophic denitrifiers. Results showed that N2O emissions in aerobic stages accounted for at least 68% of the total emission at all salinities, and N2O was predominantly produced via hydroxylamine oxidation. When without NaCl addition, the N2O and NO emission factors (N2O & NO–Ngaseous/TNinfluent) were 0.43% and 0.015% respectively, and the TNRR stayed at 0.19 kg N per m3 per d. Low salinities of 5 and 10 g NaCl per L increased N2O emission factors to 0.75% and 0.52% respectively; while, relatively high salinities of 15 and 20 g NaCl per L decreased N2O emission factors to 0.23% and 0.16%. The TNRR remained over 0.18 kg N per m3 per d under 5–15 g NaCl per L, but deteriorated to 0.15 kg N per m3 per d under 20 g NaCl per L. Our results proved that single-stage PN/A system is a promising technology to treat ammonia-rich saline wastewater, where the best balance can be achieved between the efficient nitrogen removal performance and less N2O/NO emissions consideration.

 Please wait while we load your content...
Please wait while we load your content...